Taking a Promising Vaccine Candidate Further: Efficacy of ASFV-G-ΔMGF after Intramuscular Vaccination of Domestic Pigs and Oral Vaccination of Wild Boar
Abstract
:1. Introduction
2. Results
2.1. Clinical Signs and Pathological Lesions
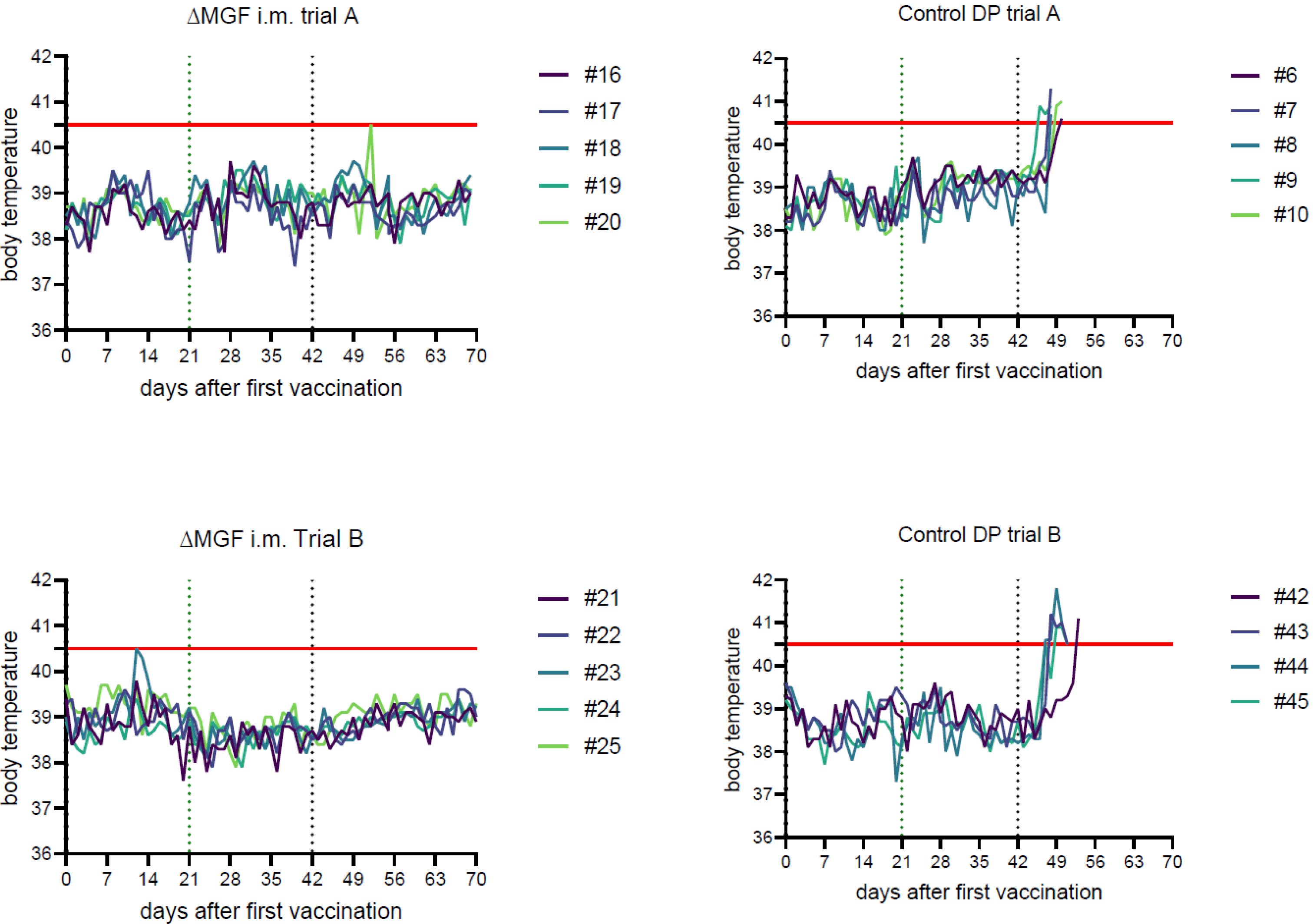
2.1.1. Domestic Pig Trial A
“ASFV-G-∆MGF”, Intramuscular
Challenge Controls
2.1.2. Domestic Pig Trial B
“ASFV-G-∆MGF”, Intramuscular
Challenge Controls
2.1.3. Wild Boar Trial
“ASFV-G-∆MGF”, Oral
Control Group
2.2. Genetic Characterization of the Vaccine Virus
2.3. ASFV Genome Detection
2.3.1. Domestic Pig Trial A
“ASFV-G-∆MGF”, Intramuscular
Challenge Control
2.3.2. Domestic Pig Trial B
“ASFV-G-∆MGF”, Intramuscular
Challenge Control
2.3.3. Wild Boar Trial
“ASFV-G-∆MGF”, Oral
Controls
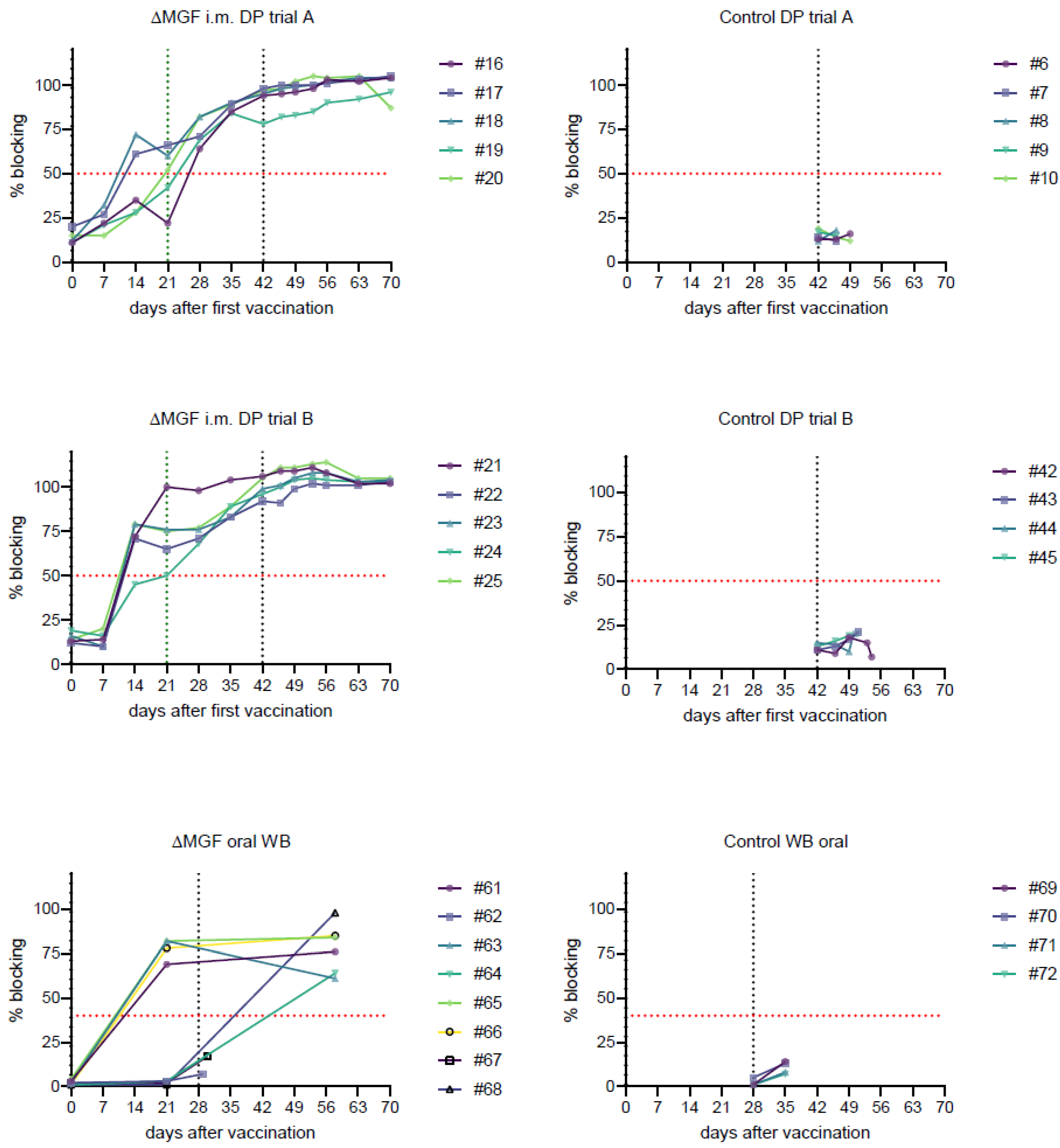
2.4. Detection of ASFV-Specific Antibodies
2.4.1. Domestic Pig Trial A
2.4.2. Domestic Pig Trial B
2.4.3. Wild Boar Trial
3. Discussion
4. Materials and Methods
4.1. Experimental Settings and Animals
4.2. Cells
4.3. Vaccine and Challenge Viruses
4.3.1. Vaccine viruses
4.3.2. Challenge Virus
4.4. Laboratory Investigations
4.4.1. Processing of Samples
4.4.2. Virus Detection
4.4.3. Antibody Detection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, M.P.; Tian, K.; Nowotny, N. African Swine Fever, the forgotten pandemic. Transbound. Emerg. Dis. 2021, 68, 2637–2639. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, H.; Hou, L.; Yang, C.; Wen, Y. Advance of African swine fever virus in recent years. Res. Veter-Sci. 2021, 136, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African Swine Fever Epidemiology and Control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef]
- Sánchez, E.G.; Pérez-Núñez, D.; Revilla, Y. Development of vaccines against African swine fever virus. Virus Res. 2019, 265, 150–155. [Google Scholar] [CrossRef]
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Gladue, D.P.; Borca, M.V. Recombinant ASF Live Attenuated Virus Strains as Experimental Vaccine Candidates. Viruses 2022, 14, 878. [Google Scholar] [CrossRef]
- Bosch-Camós, L.; López, E.; Rodriguez, F. African swine fever vaccines: A promising work still in progress. Porc. Health Manag. 2020, 6, 17. [Google Scholar] [CrossRef]
- O’Donnell, V.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Carlson, J.; Sanford, B.; Alfano, M.; Kramer, E.; Lu, Z.; Arzt, J.; et al. African Swine Fever Virus Georgia 2007 with a Deletion of Virulence-Associated Gene 9GL (B119L), when Administered at Low Doses, Leads to Virus Attenuation in Swine and Induces an Effective Protection against Homologous Challenge. J. Virol. 2015, 89, 8556–8566. [Google Scholar] [CrossRef]
- O’Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.A.; Gladue, D.P.; Borca, M.V. Simultaneous Deletion of the 9GL and UK Genes from the African Swine Fever Virus Georgia 2007 Isolate Offers Increased Safety and Protection against Homologous Challenge. J. Virol. 2017, 91, e01760-16. [Google Scholar] [CrossRef]
- Monteagudo, P.L.; Lacasta, A.; López, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; et al. BA71ΔCD2: A New Recombinant Live Attenuated African Swine Fever Virus with Cross-Protective Capabilities. J. Virol. 2017, 91, e01058-17. [Google Scholar] [CrossRef] [Green Version]
- Teklue, T.; Wang, T.; Luo, Y.; Hu, R.; Sun, Y.; Qiu, H.-J. Generation and Evaluation of an African Swine Fever Virus Mutant with Deletion of the CD2v and UK Genes. Vaccines 2020, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Gladue, D.P.; Ramirez-Medina, E.; Vuono, E.; Silva, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Borca, M.V. Deletion of the A137R Gene from the Pandemic Strain of African Swine Fever Virus Attenuates the Strain and Offers Protection against the Virulent Pandemic Virus. J. Virol. 2021, 95, e01139-21. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J. Virol. 2020, 94, e02017-19. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. J. Virol. 2015, 89, 6048–6056. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef]
- Blome, S.; Moß, C.; Reimann, I.; König, P.; Beer, M. Classical swine fever vaccines—State-of-the-art. Vet. Microbiol. 2017, 206, 10–20. [Google Scholar] [CrossRef]
- McVicar, J.W. Quantitative aspects of the transmission of African swine fever. Am. J. Vet. Res. 1984, 45, 1535–1541. [Google Scholar]
- Carrasco, L.; de Lara, F.C.-M.; Mulas, J.M.D.L.; Gómez-Villamandos, J.; Sierra, M.; Villeda, C.; Wilkinson, P. Ultrastructural changes related to the lymph node haemorrhages in acute African swine fever. Res. Veter. Sci. 1997, 62, 199–204. [Google Scholar] [CrossRef]
- Elmore, S.A. Histopathology of the lymph nodes. Toxicol. Pathol. 2006, 34, 425–454. [Google Scholar] [CrossRef]
- Pietschmann, J.; Guinat, C.; Beer, M.; Pronin, V.; Tauscher, K.; Petrov, A.; Keil, G.; Blome, S. Course and transmission characteristics of oral low-dose infection of domestic pigs and European wild boar with a Caucasian African swine fever virus isolate. Arch. Virol. 2015, 160, 1657–1667. [Google Scholar] [CrossRef]
- Barasona, J.A.; Gallardo, C.; Cadenas-Fernández, E.; Jurado, C.; Rivera, B.; Rodríguez-Bertos, A.; Arias, M.; Sánchez-Vizcaíno, J.M. First Oral Vaccination of Eurasian Wild Boar Against African Swine Fever Virus Genotype II. Front. Veter. Sci. 2019, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, C.; Soler, A.; Rodze, I.; Nieto, R.; Cano-Gómez, C.; Fernandez-Pinero, J.; Arias, M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound. Emerg. Dis. 2019, 66, 1399–1404. [Google Scholar] [CrossRef]
- Rossi, S.; Staubach, C.; Blome, S.; Guberti, V.; Thulke, H.H.; Vos, A.; Koenen, F.; Le Potier, M.F. Controlling of CSFV in European wild boar using oral vaccination: A review. Front. Microbiol. 2015, 6, 1141. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Gay, C.G.; Gladue, D.P. ASFV-G-I177L as an Effective Oral Nasal Vaccine against the Eurasia Strain of Africa Swine Fever. Viruses 2021, 13, 765. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Rai, A.; Ramirez-Medina, E.; Silva, E.; Velazquez-Salinas, L.; Vuono, E.; Pruitt, S.; Espinoza, N.; Gladue, D.P. A Cell Culture-Adapted Vaccine Virus against the Current African Swine Fever Virus Pandemic Strain. J. Virol. 2021, 95, e0012321. [Google Scholar] [CrossRef]
- Pikalo, J.; Schoder, M.E.; Sehl-Ewert, J.; Breithaupt, A.; Cay, A.B.; Lhoëst, C.; van Campe, W.; Mostin, L.; Deutschmann, P.; Roszyk, H.; et al. Towards Efficient Early Warning: Pathobiology of African Swine Fever Virus "Belgium 2018/1" in Domestic Pigs of Different Age Classes. Animals 2021, 11, 2602. [Google Scholar] [CrossRef]
- Galindo-Cardiel, I.; Ballester, M.; Solanes, D.; Nofrarías, M.; López-Soria, S.; Argilaguet, J.M.; Lacasta, A.; Accensi, F.; Rodríguez, F.; Segalés, J. Standardization of pathological investigations in the framework of experimental ASFV infections. Virus Res. 2013, 173, 180–190. [Google Scholar] [CrossRef]
- Gabriel, C.; Blome, S.; Malogolovkin, A.; Parilov, S.; Kolbasov, D.; Teifke, J.P.; Beer, M. Characterization of African Swine Fever Virus Caucasus Isolate in European Wild Boars. Emerg. Infect. Dis. 2011, 17, 2342–2345. [Google Scholar] [CrossRef]
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.; Drew, T.W. Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 2002, 107, 53–61. [Google Scholar] [CrossRef]
- Fischer, M.; Mohnke, M.; Probst, C.; Pikalo, J.; Conraths, F.J.; Beer, M.; Blome, S. Stability of African swine fever virus on heat-treated field crops. Transbound. Emerg. Dis. 2020, 67, 2318–2323. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Spearman, C. The method of right and wrong cases (constant stimuli) without Gauss’s formulae. Br. J. Psychol. 1908, 2, 227. [Google Scholar] [CrossRef]
| Trial | Group | Pig # | d0 | d7 | d14 | d21 | d28 | d35 | d0pc | d4pc | d7pc | d10pc | d14pc | d21pc | Necr. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DP A | 16 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 17 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| MGF i.m. B | 18 | n.d. | 2.8 × 100 | n.d. | n.d. | n.d. | n.d. | n.d. | 2.0 × 10−1 | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 19 | n.d. | n.d. | n.d. | 2.0 × 102 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| 20 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.7 × 10−1 | 3.2 × 10−2 | n.d. | n.d. | ||
| 6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 4.8 × 103 | ✞ | 5.8 × 105 | ||||
| 7 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ✞ | 9.5 × 104 | |||||
| control A | 8 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ✞ | 3.5 × 104 | ||||
| 9 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 5.1 × 104 | ✞ | 1.1 × 106 | |||||
| 10 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.3 × 105 | ✞ | 2.7 × 105 | ||||
| DP B | 21 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 22 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| MGF i.m. B | 23 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |
| 24 | n.d. | n.d. | 1.0 × 100 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| 25 | n.d. | 3.5 × 100 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| control B | 42 | n.d. | n.d. | n.d. | n.d. | 1.1 × 103 | ✞ | 7.4 × 104 | |||||||
| 43 | n.d. | n.d. | n.d. | 4.9 × 103 | ✞ | 1.5 × 105 | |||||||||
| 44 | n.d. | n.d. | 9.7 × 100 | 3.7 × 104 | ✞ | 1.0 × 105 | |||||||||
| 45 | n.d. | n.d. | 4.5 × 104 | 6.2 × 104 | ✞ | 1.5 × 105 | |||||||||
| WB | MGF oral | 61 | n.d. | 9.7 × 101 | n.d. | ||||||||||
| 62 | n.d. | n.d. | 9.2 × 104 | ||||||||||||
| 63 | n.d. | 3.3 × 101 | n.d. | ||||||||||||
| 64 | n.d. | n.d. | n.d. | ||||||||||||
| 65 | n.d. | 1.2 × 100 | n.d. | ||||||||||||
| 66 | n.d. | n.d. | n.d. | ||||||||||||
| 67 | n.d. | n.d. | 2.7 × 105 | ||||||||||||
| 68 | n.d. | n.d. | n.d. | ||||||||||||
| control WB | 69 | n.d. | 4.4 × 105 | ||||||||||||
| 70 | n.d. | 4.1 × 105 | |||||||||||||
| 71 | n.d. | 1.9 × 105 | |||||||||||||
| 72 | n.d. | 2.8 × 105 |
| Trial | Group | Animal # | Lung | Spleen | Kidney | Liver | Hep. Ln. | Popl. Ln. | Mand. Ln. | Tonsil |
|---|---|---|---|---|---|---|---|---|---|---|
| DP A | 16 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |||
| 17 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||||
| MGF i.m. A | 18 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |||
| 19 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||||
| 20 | 1.1 × 100 | n.d. | n.d. | n.d. | n.d. | 8.7 × 101 | ||||
| 6 | 9.4 × 102 | 1.3 × 103 | 5.3 × 100 | 1.2 × 103 | 2.0 × 101 | 6.4 × 101 | ||||
| 7 | 1.8 × 102 | 7.3 × 102 | 1.3 × 100 | 8.8 × 101 | 9.4 × 10−1 | n.d. | ||||
| control A | 8 | 4.0 × 101 | 1.6 × 103 | 1.8 × 100 | 3.3 × 102 | 4.9 × 10−1 | 4.5 × 100 | |||
| 9 | 1.4 × 103 | 1.5 × 103 | 6.7 × 101 | 2.4 × 103 | 6.4 × 102 | 9.0 × 102 | ||||
| 10 | 2.1 × 102 | 8.9 × 102 | 6.2 × 100 | 2.4 × 102 | 3.8 × 101 | 9.5 × 101 | ||||
| DP B | 21 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |||
| 22 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||||
| MGF i.m. B | 23 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |||
| 24 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||||
| 25 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||||
| control B | 42 | 8.3 × 102 | 9.3 × 102 | 9.8 × 100 | 5.3 × 102 | 9.0 × 100 | 3.0 × 100 | |||
| 43 | 8.8 × 101 | 5.0 × 102 | 1.2 × 101 | 6.5 × 102 | 5.0 × 101 | 7.0 × 101 | ||||
| 44 | 3.6 × 102 | 6.9 × 102 | 1.8 × 101 | 4.8 × 102 | 6.7 × 102 | 3.6 × 102 | ||||
| 45 | 3.2 × 102 | 1.1 × 103 | 2.8 × 101 | 2.4 × 103 | 5.1 × 102 | 2.1 × 102 | ||||
| WB | MGF oral | 61 | n.d. | n.d. | n.d. | n.d. | 1.2 × 100 | 2.0 × 100 | 9.2 × 100 | |
| 62 | 1.6 × 104 | 3.5 × 104 | 3.0 × 103 | 7.7 × 103 | 1.1 × 105 | 4.0 × 103 | 6.1 × 104 | |||
| 63 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |||
| 64 | n.d. | 1.6 × 10−1 | n.d. | n.d. | n.d. | n.d. | n.d. | |||
| 65 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |||
| 66 | n.d. | n.d. | 2.7 × 10−1 | n.d. | n.d. | n.d. | 5.1 × 10−1 | |||
| 67 | 1.0 × 104 | 7.5 × 104 | 2.7 × 103 | 2.1 × 104 | 1.1 × 104 | 2.9 × 103 | 1.3 × 104 | |||
| 68 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | |||
| control WB | 69 | 4.4 × 104 | 1.0 × 105 | 3.4 × 103 | 3.9 × 104 | 4.1 × 104 | 1.4 × 104 | 2.4 × 104 | ||
| 70 | 1.3 × 104 | 7.0 × 104 | 2.4 × 103 | 1.6 × 104 | 2.7 × 104 | 1.1 × 104 | 1.9 × 104 | |||
| 71 | 6.8 × 103 | 5.4 × 104 | 1.1 × 103 | 2.4 × 104 | 1.2 × 104 | 1.4 × 103 | 4.7 × 102 | |||
| 72 | 1.3 × 104 | 5.6 × 104 | 1.6 × 103 | 5.4 × 104 | 1.8 × 104 | 1.1 × 103 | 1.9 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deutschmann, P.; Carrau, T.; Sehl-Ewert, J.; Forth, J.H.; Viaplana, E.; Mancera, J.C.; Urniza, A.; Beer, M.; Blome, S. Taking a Promising Vaccine Candidate Further: Efficacy of ASFV-G-ΔMGF after Intramuscular Vaccination of Domestic Pigs and Oral Vaccination of Wild Boar. Pathogens 2022, 11, 996. https://doi.org/10.3390/pathogens11090996
Deutschmann P, Carrau T, Sehl-Ewert J, Forth JH, Viaplana E, Mancera JC, Urniza A, Beer M, Blome S. Taking a Promising Vaccine Candidate Further: Efficacy of ASFV-G-ΔMGF after Intramuscular Vaccination of Domestic Pigs and Oral Vaccination of Wild Boar. Pathogens. 2022; 11(9):996. https://doi.org/10.3390/pathogens11090996
Chicago/Turabian StyleDeutschmann, Paul, Tessa Carrau, Julia Sehl-Ewert, Jan Hendrik Forth, Elisenda Viaplana, Jose Carlos Mancera, Alicia Urniza, Martin Beer, and Sandra Blome. 2022. "Taking a Promising Vaccine Candidate Further: Efficacy of ASFV-G-ΔMGF after Intramuscular Vaccination of Domestic Pigs and Oral Vaccination of Wild Boar" Pathogens 11, no. 9: 996. https://doi.org/10.3390/pathogens11090996






