The Effect of Subinhibitory Concentration of Metronidazole on the Growth and Biofilm Formation on Toxigenic Clostridioides difficile Strains Belonging to Different Ribotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Determination of Minimal Inhibitory Concentration of Metronidazole
2.3. Testing the Ability of C. difficile to Produce Biofilm In Vitro
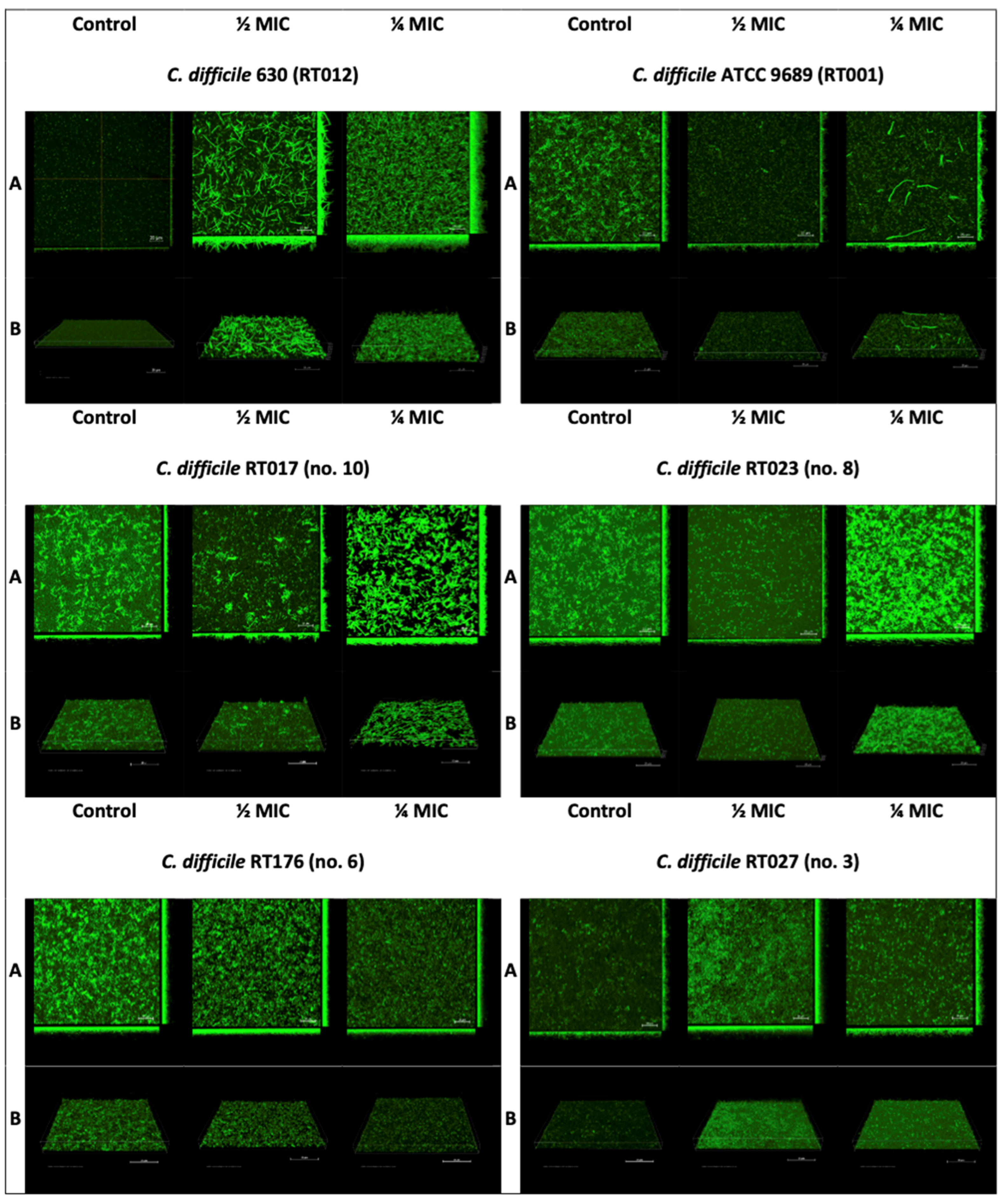
2.4. Confocal Laser Microscopy
2.5. Statistical Analysis
3. Results
3.1. Minimal Inhibitory Concentration of Metronidazole
3.2. The Effect of Sub-Inhibitory Concentrations of Metronidazole on C. difficile Biofilm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef]
- Valiente, E.; Cairns, M.D.; Wren, B.W. The Clostridium difficile PCR ribotype 027 lineage: A pathogen on the move. Clin. Microbiol. Infect. 2014, 20, 396–404. [Google Scholar] [CrossRef]
- McDonald, L.C.; Killgore, G.E.; Thompson, A.; Owens, R.C., Jr.; Kazakova, S.V.; Sambol, S.P.; Johnson, S.; Gerding, D.N. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 2005, 353, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, M.; Karpiński, P.; Pituch, H.; van Belkum, A.; Obuch-Woszczatyński, P. Antimicrobial effects of Manuka honey on in vitro biofilm formation by Clostridium difficile. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1661–1664. [Google Scholar] [CrossRef]
- Pituch, H.; Obuch-Woszczatyński, P.; Lachowicz, D.; Wultańska, D.; Karpiński, P.; Młynarczyk, G.; van Dorp, S.M.; Kuijper, E.J. Hospital-based Clostridium difficile infection surveillance reveals high proportions of PCR ribotypes 027 and 176 in different areas of Poland, 2011 to 2013. Euro Surveill. 2015, 20, 30025. [Google Scholar] [CrossRef]
- Normington, C.; Moura, I.B.; Bryant, J.A.; Ewin, D.J.; Clark, E.V.; Kettle, M.J.; Harris, H.C.; Spittal, W.; Davis, G.; Henn, M.R.; et al. Biofilms harbour Clostridioides difficile, serving as a reservoir for recurrent infection. NPJ Biofilms Microbiomes 2021, 7, 16. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, Y.S. Recurrent Clostridium difficile Infection: Risk Factors, Treatment, and Prevention. Gut Liver. 2019, 13, 16–24. [Google Scholar] [CrossRef]
- Weir, C.B.; Le, J.K. Metronidazole. 26 June 2023. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pankuch, G.A.; Jacobs, M.R.; Appelbaum, P.C. Susceptibilities of 428 gram-positive and -negative anaerobic bacteria to Bay y3118 compared with their susceptibilities to ciprofloxacin, clindamycin, metronidazole, piperacillin, piperacillin-tazobactam, and cefoxitin. Antimicrob. Agents Chemother. 1993, 37, 1649–1654. [Google Scholar] [CrossRef]
- Löfmark, S.; Edlund, C.; Nord, C.E. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin. Infect. Dis. 2010, 50 (Suppl. S1), S16–S23. [Google Scholar] [CrossRef]
- Edwards, D.I. Reduction of nitroimidazoles in vitro and DNA damage. Biochem. Pharmacol. 1986, 35, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Tocher, J.H.; Edwards, D.I. The interaction of reduced metronidazole with DNA bases and nucleosides. Int. J. Radiat. Oncol Biol. Phys. 1992, 22, 661–663. [Google Scholar] [CrossRef]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27 (Suppl. S2), S1–S21. [Google Scholar] [CrossRef]
- Davies, J.; Spiegelman, G.B.; Yim, G. The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 2006, 9, 445–453. [Google Scholar] [CrossRef]
- Narimisa, N.; Amraei, F.; Kalani, B.S.; Mohammadzadeh, R.; Jazi, F.M. Effects of sub-inhibitory concentrations of antibiotics and oxidative stress on the expression of type II toxin-antitoxin system genes in Klebsiella pneumoniae. J. Glob. Antimicrob. Resist. 2020, 21, 51–56. [Google Scholar] [CrossRef]
- Doan, T.H.; Bernet-Camard, M.F.; Hoys, S.; Janoir, C.; Péchiné, S. Impact of Subinhibitory Concentrations of Metronidazole on Morphology, Motility, Biofilm Formation and Colonization of Clostridioides difficile. Antibiotics 2022, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Moura, I.; Barbanti, F.; Donelli, G.; Spigaglia, P. Subinhibitory concentrations of metronidazole increase biofilm formation in Clostridium difficile strains. Pathog. Dis. 2016, 74, ftv114. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Renzoni, A.; Estoppey, T.; Bisognano, C.; Francois, P.; Kelley, W.L.; Lew, D.P.; Schrenzel, J.; Vaudaux, P. Induction of fibronectin adhesins in quinolone-resistant Staphylococcus aureus by subinhibitory levels of ciprofloxacin or by sigma B transcription factor activity is mediated by two separate pathways. Antimicrob. Agents Chemother. 2005, 49, 916–924. [Google Scholar] [CrossRef]
- Wultańska, D.; Piotrowski, M.; Pituch, H. The effect of berberine chloride and/or its combination with vancomycin on the growth, biofilm formation, and motility of Clostridioides difficile. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1391–1399. [Google Scholar] [CrossRef]
- Waack, U.; Nicholson, T.L. Subinhibitory Concentrations of Amoxicillin, Lincomycin, and Oxytetracycline Commonly Used to Treat Swine Increase Streptococcus suis Biofilm Formation. Front. Microbiol. 2018, 9, 2707. [Google Scholar] [CrossRef]
- Piotrowski, M.; Wultańska, D.; Obuch-Woszczatyński, P.; Pituch, H. Fructooligosaccharides and mannose affect Clostridium difficile adhesion and biofilm formation in a concentration-dependent manner. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1975–1984. [Google Scholar] [CrossRef]
- Johnson, S.; Louie, T.J.; Gerding, D.N.; Cornely, O.A.; Chasan-Taber, S.; Fitts, D.; Gelone, S.P.; Broom, C.; Davidson, D.M. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: Results from two multinational, randomized, controlled trials. Clin. Infect. Dis. 2014, 59, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, J.R.; Marcus, J.; Storm, M.; Sitko, J.; Kennedy, K.; Gerber, G.K.; Bry, L. Clinical Predictors of Recurrence After Primary Clostridioides difficile Infection: A Prospective Cohort Study. Dig. Dis. Sci. 2020, 65, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.N.; Donkor, E.S.; Brown, C.A. Biofilm formation of Clostridium difficile and susceptibility to Manuka honey. BMC Complement. Altern. Med. 2014, 14, 329. [Google Scholar] [CrossRef]
- Doan, T.H.; Yen-Nicolay, S.; Bernet-Camard, M.F.; Martin-Verstraete, I.; Péchiné, S. Impact of subinhibitory concentrations of metronidazole on proteome of Clostridioides difficile strains with different levels of susceptibility. PLoS ONE 2020, 15, e0241903. [Google Scholar] [CrossRef]
- Gerber, M.; Walch, C.; Löffler, B.; Tischendorf, K.; Reischl, U.; Ackermann, G. Effect of sub-MIC concentrations of metronidazole, vancomycin, clindamycin and linezolid on toxin gene transcription and production in Clostridium difficile. J. Med. Microbiol. 2008, 57, 776–783. [Google Scholar] [CrossRef]
- Ðapa, T.; Leuzzi, R.; Ng, Y.K.; Baban, S.T.; Adamo, R.; Kuehne, S.A.; Scarselli, M.; Minton, N.P.; Serruto, D.; Unnikrishnan, M. Multiple factors modulate biofilm formation by the anaerobic pathogen Clostridium difficile. J. Bacteriol. 2013, 195, 545–555. [Google Scholar] [CrossRef] [PubMed]

| No. of Strain | Ribotype | Profile of Toxigenicity | MIC (mg/L) Metronidazole |
|---|---|---|---|
| 1 | 027 | TcdA+ TcdB+ CDT+ | 0.094 |
| 2 | 027 | TcdA+ TcdB+ CDT+ | 0.032 |
| 3 | 027 | TcdA+ TcdB+ CDT+ | 0.50 |
| 4 | 176 | TcdA+ TcdB+ CDT+ | 0.032 |
| 5 | 176 | TcdA+ TcdB+ CDT+ | 0.064 |
| 6 | 176 | TcdA+ TcdB+ CDT+ | 0.094 |
| 7 | 023 | TcdA+ TcdB+ CDT+ | 0.032 |
| 8 | 023 | TcdA+ TcdB+ CDT+ | 0.016 |
| 9 | 023 | TcdA+ TcdB+ CDT+ | 0.016 |
| 10 | 017 | TcdA− TcdB+ CDT− | 0.064 |
| 11 | 017 | TcdA− TcdB+ CDT− | 0.064 |
| 12 | 017 | TcdA− TcdB+ CDT− | 0.047 |
| 630 | 012 | TcdA+ TcdB+ CDT− | 0.094 |
| ATCC 9896 | 001 | TcdA+ TcdB+ CDT− | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wultańska, D.; Karpiński, P.; Piotrowski, M.; Pituch, H. The Effect of Subinhibitory Concentration of Metronidazole on the Growth and Biofilm Formation on Toxigenic Clostridioides difficile Strains Belonging to Different Ribotypes. Pathogens 2023, 12, 1244. https://doi.org/10.3390/pathogens12101244
Wultańska D, Karpiński P, Piotrowski M, Pituch H. The Effect of Subinhibitory Concentration of Metronidazole on the Growth and Biofilm Formation on Toxigenic Clostridioides difficile Strains Belonging to Different Ribotypes. Pathogens. 2023; 12(10):1244. https://doi.org/10.3390/pathogens12101244
Chicago/Turabian StyleWultańska, Dorota, Paweł Karpiński, Michał Piotrowski, and Hanna Pituch. 2023. "The Effect of Subinhibitory Concentration of Metronidazole on the Growth and Biofilm Formation on Toxigenic Clostridioides difficile Strains Belonging to Different Ribotypes" Pathogens 12, no. 10: 1244. https://doi.org/10.3390/pathogens12101244






