Targeting Mitochondrial Therapy in the Regulation of HPV Infection and HPV-Related Cancers
Abstract
:1. Introduction
2. Mitochondria, Oxidative Stress (OS), Apoptosis, and the Development of HPV-Related Cancer
3. HR-HPV Proteins, Mitochondria, OS, and Apoptosis
4. HPV Proteins Sensitize Cancer Cell Death through Mitochondrial Apoptosis or Mitophagy
5. Mitochondrial Therapy in HPV-Related Cancers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human Papillomavirus Is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egawa, N.; Doorbar, J. The Low-Risk Papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 890–907. [Google Scholar] [CrossRef] [Green Version]
- de Villiers, E.-M. Cross-Roads in the Classification of Papillomaviruses. Virology 2013, 445, 2–10. [Google Scholar] [CrossRef] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human Papillomavirus (HPV) Infection; International Agency for Research on Cancer: Lyon, France, 2007. [Google Scholar]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef]
- Zheng, Z.-M.; Baker, C.C. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front. Biosci. 2006, 11, 2286–2302. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, J.; Bienkowska-Haba, M.; Ortega, M.E.; Patel, H.D.; Bodevin, S.; Spillmann, D.; Bishop, B.; Sapp, M.; Chen, X.S. Structural Basis of Oligosaccharide Receptor Recognition by Human Papillomavirus. J. Biol. Chem. 2011, 286, 2617–2624. [Google Scholar] [CrossRef] [Green Version]
- Gravitt, P.E.; Winer, R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses 2017, 9, 267. [Google Scholar] [CrossRef]
- Aksoy, P.; Gottschalk, E.Y.; Meneses, P.I. HPV Entry into Cells. Mutat. Res. Rev. Mutat. Res. 2017, 772, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.L.; Caodaglio, A.S.; Sichero, L. Regulation of HPV Transcription. Clinics 2018, 73, e486s. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, W.; Roman, A. The E7 Proteins of Low- and High-Risk Human Papillomaviruses Share the Ability to Target the PRB Family Member P130 for Degradation. Proc. Natl. Acad. Sci. USA 2006, 103, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Tomaić, V. Functional Roles of E6 and E7 Oncoproteins in HPV-Induced Malignancies at Diverse Anatomical Sites. Cancers 2016, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Venuti, A.; Paolini, F.; Nasir, L.; Corteggio, A.; Roperto, S.; Campo, M.S.; Borzacchiello, G. Papillomavirus E5: The Smallest Oncoprotein with Many Functions. Mol. Cancer 2011, 10, 140. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ramírez, I.; Carrillo-García, A.; Contreras-Paredes, A.; Ortiz-Sánchez, E.; Cruz-Gregorio, A.; Lizano, M. Regulation of Cellular Metabolism by High-Risk Human Papillomaviruses. Int. J. Mol. Sci. 2018, 19, 1839. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Pedraza-Chaverri, J. Human Papillomavirus-Related Cancers and Mitochondria. Virus Res. 2020, 286, 198016. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, G.; Lei, T.; Huang, C.; Song, T.; Si, L. Two Different HPV-11E6 Fusion Proteins Trap P53 in the Cytoplasm and Induce Apoptosis. Cancer Biol. Ther. 2008, 7, 1909–1915. [Google Scholar] [CrossRef] [Green Version]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 Oncoprotein Encoded by Human Papillomavirus Types 16 and 18 Promotes the Degradation of P53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef]
- Leverrier, S.; Bergamaschi, D.; Ghali, L.; Ola, A.; Warnes, G.; Akgül, B.; Blight, K.; García-Escudero, R.; Penna, A.; Eddaoudi, A.; et al. Role of HPV E6 Proteins in Preventing UVB-Induced Release of pro-Apoptotic Factors from the Mitochondria. Apoptosis 2007, 12, 549–560. [Google Scholar] [CrossRef]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.C. Mitochondria and Cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gregorio, A.; Martínez-Ramírez, I.; Pedraza-Chaverri, J.; Lizano, M. Reprogramming of Energy Metabolism in Response to Radiotherapy in Head and Neck Squamous Cell Carcinoma. Cancers 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct Evidence That Sulfhydryl Groups of Keap1 Are the Sensors Regulating Induction of Phase 2 Enzymes That Protect against Carcinogens and Oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [Green Version]
- Putker, M.; Vos, H.R.; van Dorenmalen, K.; de Ruiter, H.; Duran, A.G.; Snel, B.; Burgering, B.M.T.; Vermeulen, M.; Dansen, T.B. Evolutionary Acquisition of Cysteines Determines FOXO Paralog-Specific Redox Signaling. Antioxid. Redox Signal. 2015, 22, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Klotz, L.-O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox Regulation of FoxO Transcription Factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef] [Green Version]
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Cadenas, E. Basic Mechanisms of Antioxidant Activity. Biofactors 1997, 6, 391–397. [Google Scholar] [CrossRef]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of Oxidative Stress and DNA Damage in Human Carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Cruz-Gregorio, A.; Manzo-Merino, J.; Lizano, M. Cellular Redox, Cancer and Human Papillomavirus. Virus Res. 2018, 246, 35–45. [Google Scholar] [CrossRef]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Liou, G.-Y.; Storz, P. Reactive Oxygen Species in Cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [Green Version]
- Craig, A.; Scott, M.; Burch, L.; Smith, G.; Ball, K.; Hupp, T. Allosteric Effects Mediate CHK2 Phosphorylation of the P53 Transactivation Domain. EMBO Rep. 2003, 4, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Zilfou, J.T.; Lowe, S.W. Tumor Suppressive Functions of P53. Cold Spring Harb. Perspect. Biol. 2009, 1, a001883. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-Related Inflammation, the Seventh Hallmark of Cancer: Links to Genetic Instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Willis, S.N.; Adams, J.M. Life in the Balance: How BH3-Only Proteins Induce Apoptosis. Curr. Opin. Cell Biol. 2005, 17, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Wang, X. Cytochrome C-Mediated Apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef]
- Yoshida, H.; Kong, Y.Y.; Yoshida, R.; Elia, A.J.; Hakem, A.; Hakem, R.; Penninger, J.M.; Mak, T.W. Apaf1 Is Required for Mitochondrial Pathways of Apoptosis and Brain Development. Cell 1998, 94, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Wang, X. Cytochrome c Promotes Caspase-9 Activation by Inducing Nucleotide Binding to Apaf-1. J. Biol. Chem. 2000, 275, 31199–31203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, S.; Nagase, H.; Kawane, K.; Mukae, N.; Fukuyama, H. Degradation of Chromosomal DNA during Apoptosis. Cell Death Differ. 2003, 10, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratton, D.L.; Fadok, V.A.; Richter, D.A.; Kailey, J.M.; Guthrie, L.A.; Henson, P.M. Appearance of Phosphatidylserine on Apoptotic Cells Requires Calcium-Mediated Nonspecific Flip-Flop and Is Enhanced by Loss of the Aminophospholipid Translocase. J. Biol. Chem. 1997, 272, 26159–26165. [Google Scholar] [CrossRef] [Green Version]
- McBride, A.A. The Papillomavirus E2 Proteins. Virology 2013, 445, 57–79. [Google Scholar] [CrossRef] [Green Version]
- Lai, D.; Tan, C.L.; Gunaratne, J.; Quek, L.S.; Nei, W.; Thierry, F.; Bellanger, S. Localization of HPV-18 E2 at Mitochondrial Membranes Induces ROS Release and Modulates Host Cell Metabolism. PLoS ONE 2013, 8, e75625. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Gregorio, A.; Manzo-Merino, J.; Gonzaléz-García, M.C.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Valverde, M.; Rojas, E.; Rodríguez-Sastre, M.A.; García-Cuellar, C.M.; Lizano, M. Human Papillomavirus Types 16 and 18 Early-Expressed Proteins Differentially Modulate the Cellular Redox State and DNA Damage. Int. J. Biol. Sci. 2018, 14, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Gu, P.; Zhao, W.; Ding, W.; Zhao, X.; Guo, S.; Zhong, T. The Role of Globular Heads of the C1q Receptor in HPV 16 E2-Induced Human Cervical Squamous Carcinoma Cell Apoptosis Is Associated with P38 MAPK/JNK Activation. J. Transl. Med. 2013, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Su, Y.; Zhang, H.; Gu, P.; Gao, L. The Role of the Globular Heads of the C1q Receptor in HPV-16 E2-Induced Human Cervical Squamous Carcinoma Cell Apoptosis via a Mitochondria-Dependent Pathway. J. Transl. Med. 2014, 12, 286. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Wang, P.; Yu, M.; Zhu, Y.; Teng, L.; Su, Y. The Role of the Hematopoietic Cell-Specific Protein 1-Associated Protein X-1 in Human Papillomavirus 16 E2-Induced Human Cervical Squamous Carcinoma Cell Apoptosis via a Mitochondria-Dependent Pathway. Gynecol. Obs. Investig. 2021, 86, 273–282. [Google Scholar] [CrossRef]
- Doorbar, J. The E4 Protein; Structure, Function and Patterns of Expression. Virology 2013, 445, 80–98. [Google Scholar] [CrossRef] [Green Version]
- Raj, K.; Berguerand, S.; Southern, S.; Doorbar, J.; Beard, P. E1 Empty Set E4 Protein of Human Papillomavirus Type 16 Associates with Mitochondria. J. Virol. 2004, 78, 7199–7207. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Aparicio-Trejo, O.E.; Coronado-Martínez, I.; Pedraza-Chaverri, J.; Lizano, M. E6 Oncoproteins from High-Risk Human Papillomavirus Induce Mitochondrial Metabolism in a Head and Neck Squamous Cell Carcinoma Model. Biomolecules 2019, 9, 351. [Google Scholar] [CrossRef] [Green Version]
- Pim, D.; Massimi, P.; Banks, L. Alternatively Spliced HPV-18 E6* Protein Inhibits E6 Mediated Degradation of P53 and Suppresses Transformed Cell Growth. Oncogene 1997, 15, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.; Filippova, M.; Filippov, V.; Bashkirova, S.; Zhang, G.; Reeves, M.E.; Duerksen-Hughes, P. Overexpression of HPV16 E6* Alters β-Integrin and Mitochondrial Dysfunction Pathways in Cervical Cancer Cells. Cancer Genom. Proteom. 2016, 13, 259–273. [Google Scholar]
- Tamura, R.E.; de Vasconcellos, J.F.; Sarkar, D.; Libermann, T.A.; Fisher, P.B.; Zerbini, L.F. GADD45 Proteins: Central Players in Tumorigenesis. Curr. Mol. Med. 2012, 12, 634–651. [Google Scholar] [CrossRef]
- Cho, C.W.; Poo, H.; Cho, Y.S.; Cho, M.C.; Lee, K.A.; Lee, S.J.; Park, S.N.; Kim, I.K.; Jung, Y.K.; Choe, Y.K.; et al. HPV E6 Antisense Induces Apoptosis in CaSki Cells via Suppression of E6 Splicing. Exp. Mol. Med. 2002, 34, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Vogt, M.; Butz, K.; Dymalla, S.; Semzow, J.; Hoppe-Seyler, F. Inhibition of Bax Activity Is Crucial for the Antiapoptotic Function of the Human Papillomavirus E6 Oncoprotein. Oncogene 2006, 25, 4009–4015. [Google Scholar] [CrossRef] [Green Version]
- Cherry, J.J.; Rietz, A.; Malinkevich, A.; Liu, Y.; Xie, M.; Bartolowits, M.; Davisson, V.J.; Baleja, J.D.; Androphy, E.J. Structure Based Identification and Characterization of Flavonoids That Disrupt Human Papillomavirus-16 E6 Function. PLoS ONE 2013, 8, e84506. [Google Scholar] [CrossRef] [Green Version]
- Clemente-Soto, A.F.; Salas-Vidal, E.; Milan-Pacheco, C.; Sánchez-Carranza, J.N.; Peralta-Zaragoza, O.; González-Maya, L. Quercetin Induces G2 Phase Arrest and Apoptosis with the Activation of P53 in an E6 Expression-Independent Manner in HPV-Positive Human Cervical Cancer-Derived Cells. Mol. Med. Rep. 2019, 19, 2097–2106. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wei, Y.; Zhu, J.; Wang, Q.; Bao, L.; Ma, Y.; Chen, Y.; Feng, D.; Zhang, A.; Sun, J.; et al. GRIM-19 Disrupts E6/E6AP Complex to Rescue P53 and Induce Apoptosis in Cervical Cancers. PLoS ONE 2011, 6, e22065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Singh, N. Induction of Apoptosis by Hydrogen Peroxide in HPV 16 Positive Human Cervical Cancer Cells: Involvement of Mitochondrial Pathway. Mol. Cell Biochem. 2008, 310, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.N.; Wazer, D.E.; Band, V. E7 Protein of Human Papilloma Virus-16 Induces Degradation of Retinoblastoma Protein through the Ubiquitin-Proteasome Pathway. Cancer Res. 1996, 56, 4620–4624. [Google Scholar] [PubMed]
- Shim, J.-H.; Kim, K.-H.; Cho, Y.-S.; Choi, H.-S.; Song, E.Y.; Myung, P.-K.; Kang, J.S.; Suh, S.-K.; Park, S.N.; Yoon, D.-Y. Protective Effect of Oxidative Stress in HaCaT Keratinocytes Expressing E7 Oncogene. Amino Acids 2008, 34, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Finzer, P.; Krueger, A.; Stöhr, M.; Brenner, D.; Soto, U.; Kuntzen, C.; Krammer, P.H.; Rösl, F. HDAC Inhibitors Trigger Apoptosis in HPV-Positive Cells by Inducing the E2F-P73 Pathway. Oncogene 2004, 23, 4807–4817. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, H.; Kang, J.-W.; Kim, J.-H.; Lee, D.H.; Kim, M.-S.; Yang, Y.; Woo, E.-R.; Kim, Y.M.; Hong, J.; et al. The Biflavonoid Amentoflavone Induces Apoptosis via Suppressing E7 Expression, Cell Cycle Arrest at Sub-G₁ Phase, and Mitochondria-Emanated Intrinsic Pathways in Human Cervical Cancer Cells. J. Med. Food 2011, 14, 808–816. [Google Scholar] [CrossRef]
- Gao, L.-J.; Gu, P.-Q.; Fan, W.-M.; Liu, Z.; Qiu, F.; Peng, Y.-Z.; Guo, X.-R. The Role of GC1qR in Regulating Survival of Human Papillomavirus 16 Oncogene-Transfected Cervical Cancer Cells. Int. J. Oncol. 2011, 39, 1265–1272. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Chung, W.-Y.; Kim, J.; Park, H.-J.; Kim, E.-C.; Park, K.-K. Buddlejasaponin IV Induces Cell Cycle Arrest at G2/M Phase and Apoptosis in Immortalized Human Oral Keratinocytes. Phytother Res. 2011, 25, 1503–1510. [Google Scholar] [CrossRef]
- Jing, K.; Shin, S.; Jeong, S.; Kim, S.; Song, K.-S.; Park, J.-H.; Heo, J.-Y.; Seo, K.-S.; Park, S.-K.; Kweon, G.-R.; et al. Docosahexaenoic Acid Induces the Degradation of HPV E6/E7 Oncoproteins by Activating the Ubiquitin-Proteasome System. Cell Death Dis. 2014, 5, e1524. [Google Scholar] [CrossRef] [Green Version]
- Kabsch, K.; Alonso, A. The Human Papillomavirus Type 16 (HPV-16) E5 Protein Sensitizes Human Keratinocytes to Apoptosis Induced by Osmotic Stress. Oncogene 2002, 21, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.; Higo, H.; McKalip, A.; Herman, B. Human Papillomavirus (HPV) 16 E6 Sensitizes Cells to Atractyloside-Induced Apoptosis: Role of P53, ICE-like Proteases and the Mitochondrial Permeability Transition. J. Cell. Biochem. 1997, 66, 245–255. [Google Scholar] [CrossRef]
- Vikhanskaya, F.; Falugi, C.; Valente, P.; Russo, P. Human Papillomavirus Type 16 E6-Enhanced Susceptibility to Apoptosis Induced by TNF in A2780 Human Ovarian Cancer Cell Line. Int. J. Cancer 2002, 97, 732–739. [Google Scholar] [CrossRef]
- Thomas, R.J.; Oleinik, N.; Panneer Selvam, S.; Vaena, S.G.; Dany, M.; Nganga, R.N.; Depalma, R.; Baron, K.D.; Kim, J.; Szulc, Z.M.; et al. HPV/E7 Induces Chemotherapy-Mediated Tumor Suppression by Ceramide-Dependent Mitophagy. EMBO Mol. Med. 2017, 9, 1030–1051. [Google Scholar] [CrossRef]
- Sun, W.; Qin, X.; Zhou, J.; Xu, M.; Lyu, Z.; Li, X.; Zhang, K.; Dai, M.; Li, N.; Hang, D. Mitochondrial DNA copy number in cervical exfoliated cells and risk of cervical cancer among HPV-positive women. BMC Womens Health. 2020, 20, 139. [Google Scholar] [CrossRef]
- Guo, Y.; Meng, X.; Ma, J.; Zheng, Y.; Wang, Q.; Wang, Y.; Shang, H. Human Papillomavirus 16 E6 Contributes HIF-1α Induced Warburg Effect by Attenuating the VHL-HIF-1α Interaction. Int. J. Mol. Sci. 2014, 15, 7974–7986. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.; Hou, W.-J.; Zhao, Y.-J.; Liu, S.-L.; Qiu, X.-S.; Wang, E.-H.; Wu, G.-P. Overexpression of HPV16 E6/E7 Mediated HIF-1α Upregulation of GLUT1 Expression in Lung Cancer Cells. Tumour Biol. 2016, 37, 4655–4663. [Google Scholar] [CrossRef]
- Zhai, K.; Chang, L.; Zhang, Q.; Liu, B.; Wu, Y. Mitochondrial C150T Polymorphism Increases the Risk of Cervical Cancer and HPV Infection. Mitochondrion 2011, 11, 559–563. [Google Scholar] [CrossRef]
- Agarwal, N.R.; Maurya, N.; Pawar, J.S.; Ghosh, I. A Combined Approach against Tumorigenesis Using Glucose Deprivation and Mitochondrial Complex 1 Inhibition by Rotenone. Cell Biol. Int. 2016, 40, 821–831. [Google Scholar] [CrossRef]
- Höti, N.; Ma, J.; Tabassum, S.; Wang, Y.; Wu, M. Triphenyl Tin Benzimidazolethiol, a Novel Antitumor Agent, Induces Mitochondrial-Mediated Apoptosis in Human Cervical Cancer Cells via Suppression of HPV-18 Encoded E6. J. Biochem. 2003, 134, 521–528. [Google Scholar] [CrossRef]
- Khan, S.; Chib, R.; Shah, B.A.; Wani, Z.A.; Dhar, N.; Mondhe, D.M.; Lattoo, S.; Jain, S.K.; Taneja, S.C.; Singh, J. A Cyano Analogue of Boswellic Acid Induces Crosstalk between P53/PUMA/Bax and Telomerase That Stages the Human Papillomavirus Type 18 Positive HeLa Cells to Apoptotic Death. Eur. J. Pharmacol. 2011, 660, 241–248. [Google Scholar] [CrossRef]
- Marasco, D.; Vicidomini, C.; Krupa, P.; Cioffi, F.; Huy, P.D.Q.; Li, M.S.; Florio, D.; Broersen, K.; De Pandis, M.F.; Roviello, G.N. Plant Isoquinoline Alkaloids as Potential Neurodrugs: A Comparative Study of the Effects of Benzo[c]Phenanthridine and Berberine-Based Compounds on β-Amyloid Aggregation. Chem. Biol. Interact. 2021, 334, 109300. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.; Bharti, A.C.; Shukla, S.; Tyagi, A.; Husain, S.A.; Das, B.C. Berberine Modulates AP-1 Activity to Suppress HPV Transcription and Downstream Signaling to Induce Growth Arrest and Apoptosis in Cervical Cancer Cells. Mol. Cancer 2011, 10, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Singh, R.; Bhui, K.; Tyagi, S.; Mahmood, Z.; Shukla, Y. Tea Polyphenols Induce Apoptosis Through Mitochondrial Pathway and by Inhibiting Nuclear Factor-ΚB and Akt Activation in Human Cervical Cancer Cells. Oncol. Res. 2011, 19, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hu, S.-Y.; Luo, D.-Q.; Zhu, S.-Y.; Zhou, C.-Q. Potential Antitumor Agent from the Endophytic Fungus Pestalotiopsis Photiniae Induces Apoptosis via the Mitochondrial Pathway in HeLa Cells. Oncol. Rep. 2013, 30, 1773–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Singh, N. Molecular Mechanism of Curcumin Induced Cytotoxicity in Human Cervical Carcinoma Cells. Mol. Cell Biochem. 2009, 325, 107–119. [Google Scholar] [CrossRef]
- Tian, S.; Chen, H.; Tan, W. Targeting Mitochondrial Respiration as a Therapeutic Strategy for Cervical Cancer. Biochem. Biophys. Res. Commun. 2018, 499, 1019–1024. [Google Scholar] [CrossRef]
- Bernard, B.; Prétet, J.-L.; Charlot, J.-F.; Mougin, C. Human Papillomaviruses Type 16+ and 18+ Cervical Carcinoma Cells Are Sensitive to Staurosporine-Mediated Apoptosis. Biol. Cell 2003, 95, 17–26. [Google Scholar] [CrossRef]
- Zhao, X.; Song, X.; Zhao, J.; Zhu, W.; Hou, J.; Wang, Y.; Zhang, W. Juglone Inhibits Proliferation of HPV-Positive Cervical Cancer Cells Specifically. Biol. Pharm. Bull. 2019, 42, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Riyasdeen, A.; Periasamy, V.S.; Paul, P.; Alshatwi, A.A.; Akbarsha, M.A. Chloroform Extract of Rasagenthi Mezhugu, a Siddha Formulation, as an Evidence-Based Complementary and Alternative Medicine for HPV-Positive Cervical Cancers. Evid. Based Complement. Altern. Med. 2012, 2012, 136527. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Patra, D.; Kundu, R. Lignan Enriched Fraction (LRF) of Phyllanthus Amarus Promotes Apoptotic Cell Death in Human Cervical Cancer Cells in Vitro. Sci. Rep. 2019, 9, 14950. [Google Scholar] [CrossRef] [Green Version]
- Li, G.-L.; Jiang, W.; Xia, Q.; Chen, S.-H.; Ge, X.-R.; Gui, S.-Q.; Xu, C.-J. HPV E6 Down-Regulation and Apoptosis Induction of Human Cervical Cancer Cells by a Novel Lipid-Soluble Extract (PE) from Pinellia Pedatisecta Schott in Vitro. J. Ethnopharmacol. 2010, 132, 56–64. [Google Scholar] [CrossRef]
- Britt, E.L.; Raman, S.; Leek, K.; Sheehy, C.H.; Kim, S.W.; Harada, H. Combination of Fenretinide and ABT-263 Induces Apoptosis through NOXA for Head and Neck Squamous Cell Carcinoma Treatment. PLoS ONE 2019, 14, e0219398. [Google Scholar] [CrossRef]
- Bruno, S.; Tenca, C.; Saverino, D.; Ciccone, E.; Grossi, C.E. Apoptosis of Squamous Cells at Different Stages of Carcinogenesis Following 4-HPR Treatment. Carcinogenesis 2002, 23, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-C.; Chang, H.-S.; Wu, Y.-C.; Cheng, W.-L.; Lin, T.-T.; Chang, H.-J.; Kuo, S.-J.; Chen, S.-T.; Liu, C.-S. Mitochondrial Transplantation Regulates Antitumour Activity, Chemoresistance and Mitochondrial Dynamics in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 30. [Google Scholar] [CrossRef] [Green Version]


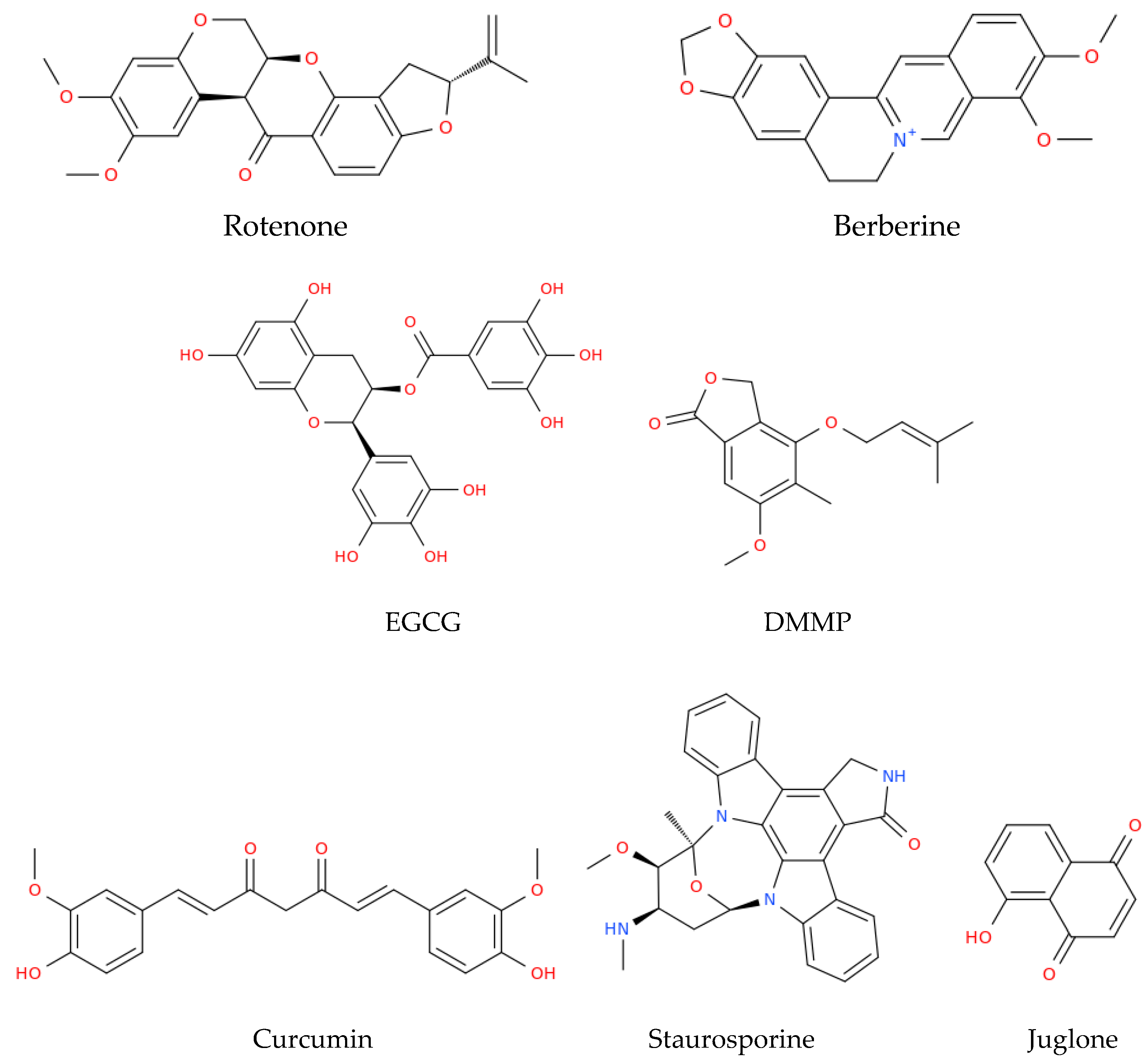
| Mitochondria Targeting Treatment | Mitochondrial Proteins Involved | Regulation of HPV Oncoproteins | Effects | Ref. | |
|---|---|---|---|---|---|
| HeLa cells | Rotenone | ↑Bax | Not studied | ↑ROS, ↓ATP, cell cycle arrest, ↑caspase-3, resulting in mitochondrial apoptosis | [79] |
| HeLa cells | TPT-benzimidazolethiol | ↑Bax mRNA Cyt c and Smac/DIABLO release | ↓E6 oncoprotein | ↑p53, ↑caspase-3 and -9, G0/G1 cell cycle arrest, resulting in mitochondrial apoptosis | [80] |
| HeLa cells | Cyano derivative of 11-keto-β-boswellic acid, BCDD | ↑Drp1 ↑Bax ↓Bcl2 ↑Cyt c release | ↓E6 mRNA | ↑p53, resulting in mitochondrial apoptosis | [81] |
| SiHa and HeLa cells | Berberine | ↓ ΔΨm | ↓E6 and E7 oncoproteins | ↑Caspase-3, resulting in mitochondrial apoptosis and ↓cell viability | [82] |
| HeLa cells | EGCG and TF | Cyt c release | Not studied | ↑ROS, ↑caspase-3 and -9, resulting in mitochondrial apoptosis | [84] |
| HeLa cells | DMMP | ↑PUMA, ↑NOXA, ↑Bax, ↑Bad, ↑Bim, ↓ ΔΨm | Not studied | Cell cycle arrest in G1, mitochondrial apoptosis | [85] |
| HeLa, SiHa cells and a xenograft mouse model | Curcumin | ↑Caspase-3 and -9 ↑Bax Cyt c release | Not studied | Mitochondrial apoptosis | [86] |
| SiHa cells and a xenograft mouse model | Atovaquone | Inhibits Complex III, inhibits mitochondrial respiration | Not studied | ↓Cell viability | [87] |
| Caski and HeLa cells | Staurosporine | ↑ Cyt c release | ↓E6 and E7 oncoproteins | ↑p53, ↑caspase-3 and -9, resulting in mitochondrial apoptosis | [88] |
| CaSki cell lines | Juglone | ↑Bax ↓Bcl2 ↑ Cyt c release | Not studied | Cell cycle arrest in G2/M, ↑caspase-3, resulting in mitochondrial apoptosis | [89] |
| ME-180 and SiHa cell lines | Chloroform extract of Rasagenthi mezhugu | ↓ ΔΨm | Not studied | Mitochondrial apoptosis | [90] |
| HeLa, and CaSki cell lines | LEF of Phyllanthus amarus | ↑Bax ↓Bcl2 ↓ ΔΨm | Not studied | ↑ROS, ↓E6, ↑p53, resulting in mitochondrial apoptosis | [91] |
| HeLa and CaSki cells | Lipid derived from Pinellia pedatisecta | ↑ Bax | ↓E6 mRNA | ↑p53, ↑caspase-3, resulting in mitochondrial apoptosis | [92] |
| HNSCC HPV(+) cells | Fenretinide | ↑NOXA | Not studied | Mitochondrial apoptosis | [93] |
| HNSCC HPV(+) cells | 4-HPR | ↑Caspase-3 | Not studied | ↑ROS, MTP, resulting in mitochondrial apoptosis | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Roviello, G.N.; Pedraza-Chaverri, J. Targeting Mitochondrial Therapy in the Regulation of HPV Infection and HPV-Related Cancers. Pathogens 2023, 12, 402. https://doi.org/10.3390/pathogens12030402
Cruz-Gregorio A, Aranda-Rivera AK, Roviello GN, Pedraza-Chaverri J. Targeting Mitochondrial Therapy in the Regulation of HPV Infection and HPV-Related Cancers. Pathogens. 2023; 12(3):402. https://doi.org/10.3390/pathogens12030402
Chicago/Turabian StyleCruz-Gregorio, Alfredo, Ana Karina Aranda-Rivera, Giovanni N. Roviello, and José Pedraza-Chaverri. 2023. "Targeting Mitochondrial Therapy in the Regulation of HPV Infection and HPV-Related Cancers" Pathogens 12, no. 3: 402. https://doi.org/10.3390/pathogens12030402







