Major Emerging Fungal Diseases of Reptiles and Amphibians
Abstract
:1. Introduction
2. Overview of Fungal Pathogens
2.1. Reptiles
2.1.1. Nannizziopsis spp.
2.1.2. Ophidiomyces ophidiicola
2.2. Amphibians
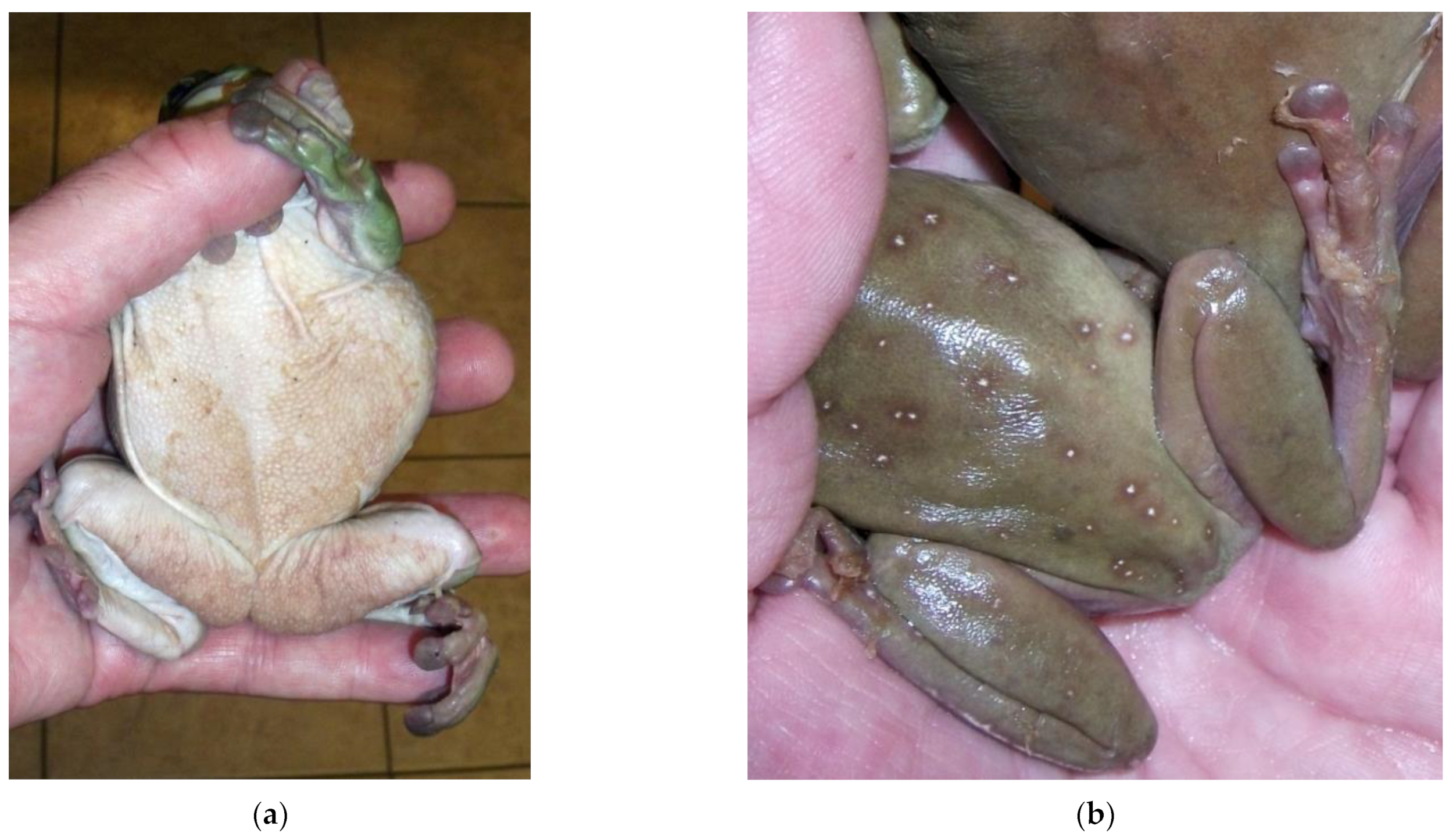
2.2.1. Chytridiomycosis
Batrachochytrium dendrobatidis
Batrachochytrium salamandrivorans
3. Host–Pathogen Relationship
3.1. Host-Related Factors
3.2. Pathogen-Related Factors
3.3. Environment-Related Factors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McArthur, D.B. Emerging Infectious Diseases. Nurs. Clin. North Am. 2019, 54, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Weldon, C. Chytridiomycosis, an Emerging Infectious Disease of Amphibians in South Africa. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2005. [Google Scholar]
- Gibbons, P.; Steffes, Z. Emerging Infectious Diseases of Chelonians. Vet. Clin. North Am. Exot. Anim. Pract. 2013, 16, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.; Knowles, S.; Lankton, J.; Michell, K.; Edwards, J.; Kapfer, J.; Staffen, R.; Wild, E.; Schmidt, K.; Ballmann, A.; et al. Snake Fungal Disease: An Emerging Threat to Wild Snakes. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latney, L.V.; Wellehan, J. Selected Emerging Infectious Diseases of Squamata. Vet. Clin. North Am. Exot. Anim. Pract. 2013, 16, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.; Beckmann, K.; Perkins, M.; Fitzpatrick, L.; Cromie, R.; Redbond, J.; O’Brien, M.; Ghosh, P.; Shelton, J.; Fisher, M. Emerging Disease in UK Amphibians. Vet. Rec. 2015, 176, 468. [Google Scholar] [CrossRef]
- Xie, G.Y.; Olson, D.H.; Blaustein, A.R. Projecting the Global Distribution of the Emerging Amphibian Fungal Pathogen, Batrachochytrium Dendrobatidis, Based on IPCC Climate Futures. PLoS ONE 2016, 11, e0160746. [Google Scholar] [CrossRef] [Green Version]
- Blaustein, A.R.; Urbina, J.; Snyder, P.W.; Reynolds, E.; Dang, T.; Hoverman, J.T.; Han, B.; Olson, D.H.; Searle, C.; Hambalek, N.M. Effects of Emerging Infectious Diseases on Amphibians: A Review of Experimental Studies. Diversity 2018, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Latney, L.V.; Klaphake, E. Selected Emerging Infectious Diseases of Amphibians. Vet. Clin. North Am. Exot. Anim. Pract. 2020, 23, 397–412. [Google Scholar] [CrossRef]
- Adamovicz, L.; Allender, M.C.; Gibbons, P.M. Emerging Infectious Diseases of Chelonians: An Update. Vet. Clin. North Am.-Exot. Anim. Pract. 2020, 23, 263–283. [Google Scholar] [CrossRef]
- Ladner, J.T.; Palmer, J.M.; Ettinger, C.L.; Stajich, J.E.; Farrell, T.M.; Glorioso, B.M.; Lawson, B.; Price, S.J.; Stengle, A.G.; Grear, D.A.; et al. The Population Genetics of the Causative Agent of Snake Fungal Disease Indicate Recent Introductions to the USA. PLoS Biol. 2022, 20, e3001676. [Google Scholar] [CrossRef]
- Spitzen-van der Sluijs, A.; Martel, A.; Asselberghs, J.; Bales, E.; Beukema, W.; Bletz, M.; Dalbeck, L.; Goverse, E.; Kerres, A.; Kinet, T.; et al. Expanding Distribution of Lethal Amphibian Fungus Batrachochytrium salamandrivorans in Europe. Emerg. Infect. Dis. 2016, 22, 1286–1288. [Google Scholar] [CrossRef] [Green Version]
- Woodburn, D.; Miller, A.; Allender, M.; Maddox, C.; Terio, K. Emydomyces testavorans, a New Genus and Species of Onygenalean Fungus Isolated from Shell Lesions of Freshwater Aquatic Turtles. J. Clin. Microbiol. 2019, 57, e00628-18. [Google Scholar] [CrossRef] [Green Version]
- Parrish, K.; Kirkland, P.D.; Skerratt, L.F.; Ariel, E. Nidoviruses in Reptiles: A Review. Front. Vet. Sci. 2021, 8, 733404. [Google Scholar] [CrossRef]
- Baker, S.; Kessler, E.; Darville-Bowleg, L.; Merchant, M. Different Mechanisms of Serum Complement Activation in the Plasma of Common (Chelydra serpentina) and Alligator (Macrochelys temminckii) Snapping Turtles. PLoS ONE 2019, 14, e0217626. [Google Scholar]
- Haynes, E.; Allender, M. History, Epidemiology, and Pathogenesis of Ophidiomycosis: A Review. Herpetol. Rev. 2021, 52, 521–536. [Google Scholar]
- Cabañes, F. Chytridiomycosis in Amphibians. Rev. Iberoam. Micol. 2019, 36, 171–172. [Google Scholar] [CrossRef]
- Latney, L.V.; Wellehan, J.F. Selected Emerging Infectious Diseases of Squamata: An Update. Vet. Clin. North Am.-Exot. Anim. Pract. 2020, 23, 353–371. [Google Scholar] [CrossRef]
- Stchigel, A.M.; Sutton, D.; Cano, J.; Cabañes, F.; Abarca, L.; Tintelnot, K.; Wickes, B.; García, D.; Guarro, J. Phylogeny of Chrysosporia Infecting Reptiles: Proposal of the New Family Nannizziopsiaceae and Five New Species. Persoonia 2013, 31, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Paré, J.; Sigler, L. An Overview of Reptile Fungal Pathogens in the Genera nannizziopsis, paranannizziopsis, and ophidiomyces. J. Herpetol. Med. Surg. 2016, 26, 46–53. [Google Scholar] [CrossRef]
- Sayers, E.W.; Cavanaugh, M.; Clark, K.; Ostell, J.; Pruitt, K.D.; Karsch-Mizrachi, I. GenBank. Nucleic Acids Res. 2020, 48, D84–D86. [Google Scholar] [CrossRef] [Green Version]
- Paré, J.; Wellehan, J.; Perry, S.; Scheelings, T.; Keller, K.; Boyer, T. Onygenalean Dermatomycoses (Formerly Yellow Fungus Disease, Snake Fungal Disease) in Reptiles. J. Herpetol. Med. Surg. 2021, 30, 198–209. [Google Scholar] [CrossRef]
- Allain, S.; Duffus, A.; Marschang, R. Editorial: Emerging Infections and Diseases of Herpetofauna. Front. Vet. Sci 2022, 9, 9616. [Google Scholar] [CrossRef] [PubMed]
- Paré, J.A.; Sigler, L.; Hunter, D.; Summerbell, R.; Smith, D.; Machin, K. Cutaneous Mycoses in Chameleons Caused by the Chrysosporium Anamorph of Nannizziopsis vriesii (Apinis) Currah. J. Zoo Wildl. Med. 1997, 28, 443–453. [Google Scholar] [PubMed]
- Bowman, M.; Paré, J.; Sigler, L.; Naeser, J.; Sladky, K.; Hanley, C.; Helmer, P.; Phillips, L.; Brower, A.; Porter, R. Deep Fungal Dermatitis in Three Inland Bearded Dragons (Pogona vitticeps) Caused by the Chrysosporium Anamorph of Nannizziopsis vriesii. Med. Mycol. 2007, 45, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.A.; Walden, M.R. Chrysosporium Anamorph Nannizziopsis vriesii: An Emerging Fungal Pathogen of Captive and Wild Reptiles. Vet. Clin. North Am. Exot. Anim. Pract. 2013, 16, 659–668. [Google Scholar] [CrossRef]
- Sigler, L.; Hambleton, S.; Paré, J. Molecular Characterization of Reptile Pathogens Currently Known as Members of the Chrysosporium Anamorph of Nannizziopsis vriesii Complex and Relationship with Some Human-Associated Isolates. J. Clin. Microbiol. 2013, 51, 3338–3357. [Google Scholar] [CrossRef] [Green Version]
- Peterson, N.; Rose, K.; Shaw, S.; Hyndman, T.; Sigler, L.; Kurtböke, D.; Llinas, J.; Littleford Colquhoun, B.; Cristescu, R.; Frere, C. Cross-Continental Emergence of Nannizziopsis Barbatae Disease May Threaten Wild Australian Lizards. Sci. Rep. 2020, 10, 20976. [Google Scholar] [CrossRef]
- Johnson, R.; Sangster, C.; Sigler, L.; Hambleton, S.; Paré, J.A. Deep Fungal Dermatitis Caused by the Chrysosporium Anamorph of Nannizziopsis vriesii in Captive Coastal Bearded Dragons (Pogona barbata). Aust. Vet. J. 2011, 89, 515–519. [Google Scholar] [CrossRef]
- Gentry, S.L.; Lorch, J.M.; Lankton, J.S.; Pringle, A. Koch’s Postulates: Confirming Nannizziopsis guarroi as the Cause of Yellow Fungal Disease in Pogona Vitticeps. Mycologia 2021, 113, 1253–1263. [Google Scholar] [CrossRef]
- Wellehan, J.; Divers, S. Mycology. In Mader’s Reptile and Amphibian Medicine and Surgery; Divers, S., Stahl, S., Eds.; Saunders: Philadelphia, PA, USA, 2019; pp. 270–280. [Google Scholar]
- Hill, A.; Sandy, J.; Begg, A. Mycotic Dermatitis in Juvenile Freshwater Crocodiles (Crocodylus johnstoni) Caused by Nannizziopsis crocodili. J. Zoo Wildl. Med. 2019, 50, 225–230. [Google Scholar] [CrossRef]
- Paré, J.A.; Coyle, K.A.; Sigler, L.; Maas, A.K., III; Mitchell, R.L. Pathogenicity of the Chrysosporium Anamorph of Nannizziopsis vriesii for Veiled Chameleons (Chamaeleo calyptratus). Med. Mycol. 2006, 44, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Hellebuyck, T.; Pasmans, F.; Haesebrouck, F.; Martel, A. Dermatological Diseases in Lizards. Vet. J. 2012, 193, 38–45. [Google Scholar] [CrossRef]
- Hellebuyck, T.; Scheelings, T. Dermatology—Skin. In Mader’s Reptile and Amphibian Medicine and Surgery; Divers, S., Stahl, S., Eds.; Saunders: Philadelphia, PA, USA, 2019; pp. 699–711. [Google Scholar]
- Schneider, J.; Heydel, T.; Klasen, L.; Pees, M.; Schrödl, W.; Schmidt, V. Characterization of Nannizziopsis guarroi with Genomic and Proteomic Analysis in Three Lizard Species. Med. Mycol. 2018, 56, 610–620. [Google Scholar] [CrossRef]
- Foltin, E.T.; Keller, K.A. Successful Treatment of Nannizziopsis guarroi Infection Using Systemic Terbinafine in a Central Bearded Dragon (Pogona vitticeps). J. Herpetol. Med. Surg. 2022, 32, 20–25. [Google Scholar] [CrossRef]
- Van Waeyenberghe, L.; Baert, K.; Pasmans, F.; Van Rooij, P.; Hellebuyck, T.; Beernaert, L.; Backer, P.; Haesebrouck, F.; Martel, A. Voriconazole, a Safe Alternative for Treating Infections Caused by the Chrysosporium Anamorph of Nannizziopsis vriesii in Bearded Dragons (Pogona vitticeps). Med. Mycol. 2010, 48, 880–885. [Google Scholar] [CrossRef] [Green Version]
- McEntire, M.S.; Reinhart, J.M.; Cox, S.K.; Keller, K.A. Single-Dose Pharmacokinetics of Orally Administered Terbinafine in Bearded Dragons (Pogona vitticeps) and the Antifungal Susceptibility Patterns of Nannizziopsis guarroi. Am. J. Vet. Res. 2022, 83, 256–263. [Google Scholar] [CrossRef]
- Jourdan, B.; Hemby, C.; Allender, M.C.; Levy, I.; Foltin, E.; Keller, K.A. Effectiveness of Common Disinfecting Agents Against Isolates of Nannizziopsis guarroi. J. Herpetol. Med. Surg. 2022. [Google Scholar] [CrossRef]
- Gray, M.; Duffus, A.; Haman, K.; Harris, R.; Allender, M.; Thompson, T.; Christman, M.; Sacredote-Velat, A.; Sprague, L.; Williams, J.; et al. Pathogen Surveillance in Herpetofaunal Populations: Guidance on Study Design, Sample Collection, Biosecurity, and Intervention Strategies. Herpetol. Rev. 2017, 48, 334. [Google Scholar]
- Rajeev, S.; Sutton, D.; Wickes, B.; Miller, D.; Giri, D.; Meter, M.; Thompson, E.; Rinaldi, M.; Romanelli, A.; Cano, J.; et al. Isolation and Characterization of a New Fungal Species, Chrysosporium ophiodiicola, from a Mycotic Granuloma of a Black Rat Snake (Elaphe obsoleta obsoleta). J. Clin. Microbiol. 2009, 47, 1264–1268. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.; Weyant, R.; Lamirande, E.; Sigler, L.; Mason, R. Fatal Mycotic Dermatitis in Captive Brown Tree Snakes (Boiga irregularis). J. Zoo Wildl. Med. 1999, 30, 111–118. [Google Scholar]
- Cheatwood, J.; Jacobson, E.; May, P.; Farrell, T.; Homer, B.; Samuelson, D.; Kimbrough, J. An Outbreak of Fungal Dermatitis and Stomatitis in a Free-Ranging Population of Pigmy Rattlesnakes (Sistrurus miliarius barbouri) in Florida. J. Wildl. Dis. 2003, 39, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.; Steeil, J.; Neiffer, D.; Evans, M.; Peters, A.; Allender, M.; Cartoceti, A. Retrospective Review of Ophidiomycosis (Ophidiomyces ophiodiicola) at the Smithsonian’s National Zoological Park (1983–2017). J. Zoo Wildl. Med. 2021, 52, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.; Price, S.; Lankton, J.; Drayer, A. Confirmed Cases of Ophidiomycosis in Museum Specimens from as Early as 1945, United States. Emerg. Infect. Dis. J. 2021, 27, 1986. [Google Scholar] [CrossRef] [PubMed]
- Origgi, F.; Pisano, S.; Glaizot, O.; Hertwig, S.; Schmitz, A.; Ursenbacher, S. Ophiodimyces Ophiodiicola, Etiologic Agent of Snake Fungal Disease, in Europe since Late 1950s. Emerg. Infect. Dis. 2022, 28, 2064–2068. [Google Scholar] [CrossRef]
- Di Nicola, M.R.; Coppari, L.; Notomista, T.; Marini, D. Ophidiomyces ophidiicola Detection and Infection: A Global Review on a Potential Threat to the World’s Snake Populations. Eur. J. Wildl. Res. 2022, 68, 64. [Google Scholar] [CrossRef]
- McBride, M.P.; Wojick, K.B.; Georoff, T.A.; Kimbro, J.; Garner, M.M.; Wang, X.; Childress, A.L.; Wellehan, J.F.X. Ophidiomyces ophiodiicola Dermatitis in Eight Free-Ranging Timber Rattlesnakes (Crotalus horridus) from Massachusetts. J. Zoo Wildl. Med. 2015, 46, 86–94. [Google Scholar] [CrossRef]
- Lindemann, D.M.; Allender, M.C.; Rzadkowska, M.; Archer, G.; Kane, L.; Baitchman, E.; Driskell, E.A.; Chu, C.T.; Singh, K.; Hsiao, S.-H.; et al. Pharmacokinetics, Efficacy, and Safety of Voriconazole and Itraconazole in Healthy Cottonmouths (Agkistrodon piscivorus) and Massasauga Rattlesnakes (Sistrurus catenatus) with Snake Fungal Disease. J. Zoo Wildl. Med. 2017, 48, 757–766. [Google Scholar] [CrossRef]
- Meier, G.; Notomista, T.; Marini, D.; Ferri, V. First Case of Snake Fungal Disease Affecting a Free-Ranging Natrix natrix (Linnaeus, 1758) in Ticino Canton, Switzerland. Herpetol. Notes 2018, 11, 885–891. [Google Scholar]
- Allender, M.C.; Raudabaugh, D.B.; Gleason, F.H.; Miller, A.N. The Natural History, Ecology, and Epidemiology of Ophidiomyces ophiodiicola and Its Potential Impact on Free-Ranging Snake Populations. Fungal Ecol. 2015, 17, 187–196. [Google Scholar] [CrossRef]
- McKenzie, C.; Oesterle, P.; Stevens, B.; Shirose, L.; Lillie, B.; Davy, C.; Jardine, C.; Nemeth, N. Pathology Associated with Ophidiomycosis in Wild Snakes in Ontario, Canada. Can. Vet. J. 2020, 2020, 957–962. [Google Scholar]
- Sun, P.; Yang, C.K.; Li, W.-T.; Lai, W.-Y.; Chen, F.; Huang, H.; Yu, P. Infection with Nannizziopsis guarroi and Ophidiomyces ophiodiicola in Reptiles in Taiwan. Transbound. Emerg. Dis. 2021, 69, 764–775. [Google Scholar] [CrossRef]
- Grioni, A.; To, K.; Crow, P.; Rose-Jeffreys, L.; Ching, K.; Chu, L.; Hill, F.; Chan, K.H.-K.; Cheung, K. Detection of Ophidiomyces Ophidiicola in a Wild Burmese Python (Python bivittatus) in Hong Kong SAR, China. J. Herpetol. Med. Surg. 2021, 31, 283–291. [Google Scholar] [CrossRef]
- Takami, Y.; Une, Y.; Mitsui, I.; Hemmi, C.; Takaki, Y.; Hosoya, T.; Nam, K.-O. First Report of Emerging Snake Fungal Disease Caused by Ophidiomyces ophiodiicola from Asia in Imported Captive Snakes in Japan. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lorch, J.; Lankton, J.; Werner, K.; Falendysz, E.; McCurley, K.; Blehert, D. Experimental Infection of Snakes with Ophidiomyces ophiodiicola Causes Pathological Changes That Typify Snake Fungal Disease. mBio 2015, 6, e01534-15. [Google Scholar] [CrossRef] [Green Version]
- Bohuski, E.; Lorch, J.M.; Griffin, K.M.; Blehert, D.S. TaqMan Real-Time Polymerase Chain Reaction for Detection of Ophidiomyces ophiodiicola, the Fungus Associated with Snake Fungal Disease. BMC Vet. Res. 2015, 11, 95. [Google Scholar] [CrossRef] [Green Version]
- Paré, J.; Sigler, L.; Rypien, K.; Gibas, C. Cutaneous Mycobiota of Captive Squamate Reptiles with Notes on the Scarcity of Chrysosporium Anamorph of Nannizziopsis vriesii. J. Herpetol. Med. Surg. 2003, 13, 10–15. [Google Scholar] [CrossRef]
- Stengle, A.G.; Farrell, T.M.; Freitas, K.S.; Lind, C.M.; Price, S.J.; Butler, B.O.; Tadevosyan, T.; Isidoro-Ayza, M.; Taylor, D.R.; Winzeler, M.; et al. Evidence of Vertical Transmission of the Snake Fungal Pathogen Ophidiomyces ophiodiicola. J. Wildl. Dis. 2019, 55, 961–964. [Google Scholar] [CrossRef]
- Kane, L.P.; Allender, M.C.; Archer, G.; Leister, K.; Rzadkowska, M.; Boers, K.; Souza, M.; Cox, S. Pharmacokinetics of Nebulized and Subcutaneously Implanted Terbinafine in Cottonmouths (Agkistrodon piscivorus). J. Vet. Pharmacol. Ther. 2017, 40, 575–579. [Google Scholar] [CrossRef]
- Rzadkowska, M.; Allender, M.C.; O’Dell, M.; Maddox, C. Evaluation of Common Disinfectants Effective against Ophidiomyces ophiodiicola, the Causative Agent of Snake Fungal Disease. J. Wildl. Dis. 2016, 52, 759–762. [Google Scholar] [CrossRef]
- Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; et al. Batrachochytrium salamandrivorans Sp. Nov. Causes Lethal Chytridiomycosis in Amphibians. Proc. Natl. Acad. Sci. USA 2013, 110, 15325–15329. [Google Scholar] [CrossRef] [Green Version]
- Van Rooij, P.; Martel, A.; Haesebrouck, F.; Pasmans, F. Amphibian Chytridiomycosis: A Review with Focus on Fungus-Host Interactions. Vet. Res. 2015, 46, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, N.; Whitaker, B. Amphibian Chytridiomycosis. In Mader’s Reptile and Amphibian Medicine and Surgery; Divers, S., Stahl, S., Eds.; Saunders: Philadelphia, PA, USA, 2019; pp. 1292–1293. [Google Scholar]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis Gen. et Sp. Nov., a Chytrid Pathogenic to Amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Sonn, J.; Berman, S.; Richards Zawacki, C. The Influence of Temperature on Chytridiomycosis In Vivo. Ecohealth 2017, 14, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Hagman, M.; Alford, R. Patterns of Batrachochytrium dendrobatidis Transmission between Tadpoles in a High-Elevation Rainforest Stream in Tropical Australia. Dis. Aquat. Organ. 2015, 115, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, J.S.; Annis, S.L.; Longcore, J.E. Physiology of Batrachochytrium dendrobatidis, a Chytrid Pathogen of Amphibians. Mycologia 2004, 96, 9–15. [Google Scholar] [CrossRef]
- Stegen, G.; Pasmans, F.; Schmidt, B.; Rouffaer, L.; Praet, S.; Schaub, M.; Canessa, S.; Laudelout, A.; Kinet, T.; Adriaensen, C.; et al. Drivers of Salamander Extirpation Mediated by Batrachochytrium salamandrivorans. Nature 2017, 544, 353. [Google Scholar] [CrossRef]
- Spitzen-van der Sluijs, A.; Spikmans, F.; Bosman, W.; Zeeuw, M.; Meij, T.; Goverse, E.; Kik, M.; Pasmans, F.; Martel, A. Rapid Enigmatic Decline Drives the Fire Salamander (Salamandra salamandra) to the Edge of Extinction in the Netherlands. Amphib.-Reptil. 2013, 34, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Berger, L.; Speare, R.; Skerratt, L. Distribution of Batrachochytrium dendrobatidis and Pathology in the Skin of Green Tree Frogs Litoria Caerulea with Severe Chytridiomycosis. Dis. Aquat. Organ. 2006, 68, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.; Pasmans, F.; Muñoz, J.F.; Shea, T.; Carranza, S.; Cuomo, C.; Martel, A. Diversity, Multifaceted Evolution, and Facultative Saprotrophism in the European Batrachochytrium salamandrivorans Epidemic. Nat. Commun. 2021, 12, 6688. [Google Scholar] [CrossRef]
- Hanlon, S.; Lynch, K.; Kerby, J. Batrachochytrium dendrobatidis Exposure Effects on Foraging Efficiencies and Body Size in Anuran Tadpoles. Dis. Aquat. Organ. 2015, 112, 237–242. [Google Scholar] [CrossRef]
- Borteiro, C.; Kolenc, F.; Verdes, J.; Martinez Debat, C.; Ubilla, M. Sensitivity of Histology for the Detection of the Amphibian Chytrid Fungus Batrachochytrium dendrobatidis. J. Vet. Diagn. Investig. 2019, 31, 246–249. [Google Scholar] [CrossRef] [Green Version]
- Kamoroff, C.; Goldberg, C.; Grasso, R. Rapid Detection of the Amphibian Chytrid Fungus (Batrachochytrium dendrobatidis) Using In-Situ DNA Extraction and a Handheld Mobile Thermocycler. Authorea 2020. [Google Scholar] [CrossRef]
- Kamoroff, C.; Goldberg, C. Using Environmental DNA for Early Detection of Amphibian Chytrid Fungus Batrachochytrium dendrobatidis Prior to a Ranid Die Off. Dis. Aquat. Organ. 2017, 127, 75–79. [Google Scholar] [CrossRef]
- Lastra González, D.; Baláž, V.; Vojar, J.; Chajma, P. Dual Detection of the Chytrid Fungi Batrachochytrium Spp. with an Enhanced Environmental DNA Approach. J. Fungi 2021, 7, 258. [Google Scholar] [CrossRef]
- Osman, O.A.; Andersson, J.; Martin-Sanchez, P.M.; Eiler, A. National EDNA-Based Monitoring of Batrachochytrium dendrobatidis and Amphibian Species in Norway. Metabarcoding Metagenom. 2022, 6, e85199. [Google Scholar] [CrossRef]
- Congram, M.; Torres Vilaça, S.; Wilson, C.C.; Kyle, C.J.; Lesbarrères, D.; Wikston, M.J.H.; Beaty, L.; Murray, D.L. Tracking the Prevalence of a Fungal Pathogen, Batrachochytrium dendrobatidis (Chytrid Fungus), Using Environmental DNA. Environ. DNA 2022, 4, 687–699. [Google Scholar] [CrossRef]
- Mutschmann, F. Chytridiomycosis in Amphibians. J. Exot. Pet Med. 2015, 24, 276–282. [Google Scholar] [CrossRef]
- Kueneman, J.G.; Woodhams, D.C.; Harris, R.; Archer, H.M.; Knight, R.; McKenzie, V.J. Probiotic Treatment Restores Protection against Lethal Fungal Infection Lost during Amphibian Captivity. Proc. Biol. Sci. 2016, 283, 20161553. [Google Scholar] [CrossRef] [Green Version]
- Bletz, M.; Loudon, A.; Becker, M.; Bell, S.; Woodhams, D.; Minbiole, K.; Harris, R. Mitigating Amphibian Chytridiomycosis with Bioaugmentation: Characteristics of Effective Probiotics and Strategies for Their Selection and Use. Ecol. Lett. 2013, 16, 807–820. [Google Scholar] [CrossRef]
- Harrison, X.A.; Sewell, T.; Fisher, M.; Antwis, R.E. Designing Probiotic Therapies With Broad-Spectrum Activity Against a Wildlife Pathogen. Front. Microbiol. 2020, 10, 3134. [Google Scholar] [CrossRef]
- Blooi, M.; Martel, A.; Haesebrouck, F.; Vercammen, F.; Bonte, D.; Pasmans, F. Treatment of Urodelans Based on Temperature Dependent Infection Dynamics of Batrachochytrium salamandrivorans. Sci. Rep. 2015, 5, 8037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voyles, J.; Johnson, L.; Briggs, C.; Cashins, S.; Alford, R.; Berger, L.; Skerratt, L.; Speare, R.; Rosenblum, E. Temperature Alters Reproductive Life History Patterns in Batrachochytrium dendrobatidis, a Lethal Pathogen Associated with the Global Loss of Amphibians. Ecol. Evol. 2012, 2, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, M.; Richards-Zawacki, C. Elevated Temperature as a Treatment for Batrachochytrium dendrobatidis Infection in Captive Frogs. Dis. Aquat. Organ. 2011, 94, 235–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, S.; Parker, J.; Briggs, C. Effect of Temperature on Host Response to Batrachochytrium dendrobatidis Infection in the Mountain Yellow-Legged Frog (Rana muscosa). J. Wildl. Dis. 2008, 44, 716–720. [Google Scholar] [CrossRef] [Green Version]
- Geiger, C.; Küpfer, E.; Schär, S.; Wolf, S.; Schmidt, B. Elevated Temperature Clears Chytrid Fungus Infections from Tadpoles of the Midwife Toad, Alytes Obstetricans. Amphib.-Reptil. 2011, 32, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.; Berger, L.; Philips, L.; Speare, R. Fungicidal Effects of Chemical Disinfectants, UV Light, Desiccation and Heat on the Amphibian Chytrid Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 2004, 57, 255–260. [Google Scholar] [CrossRef]
- Poole, V.; Grow, S. Amphibian Husbandry Resource Guide, 2nd ed.; Poole, V., Grow, S., Eds.; Association of Zoos and Aquariums: Silver Spring, MD, USA, 2012. [Google Scholar]
- Speare, R. Developing Management Strategies to Control Amphibian Diseases; School of Public Health and Tropical Medicine, James Cook University: Douglas, Australia, 2001; pp. 171–183. [Google Scholar]
- Garmyn, A.; Van Rooij, P.; Pasmans, F.; Hellebuyck, T.; Van Den Broeck, W.; Haesebrouck, F.; Martel, A. Waterfowl: Potential Environmental Reservoirs of the Chytrid Fungus Batrachochytrium dendrobatidis. PLoS ONE 2012, 7, e35038. [Google Scholar] [CrossRef]
- Hanlon, S.; Henson, J.; Kerby, J. Detection of Amphibian Chytrid Fungus on Waterfowl Integument in Natural Settings. Dis. Aquat. Organ. 2017, 126, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Infectious Disease and Amphibian Population Declines. Divers. Distrib. 2003, 9, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.L.; Speare, R. Possible Modes of Dissemination of the Amphibian Chytrid Batrachochytrium dendrobatidis in the Environment. Dis. Aquat. Organ. 2005, 65, 181–186. [Google Scholar] [CrossRef]
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis Causes Amphibian Mortality Associated with Population Declines in the Rain Forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef] [Green Version]
- O’Hanlon, S.; Rieux, A.; Farrer, R.; Rosa, G.; Waldman, B.; Bataille, A.; Kosch, T.; Murray, K.; Brankovics, B.; Fumagalli, M.; et al. Recent Asian Origin of Chytrid Fungi Causing Global Amphibian Declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Talley, B.; Muletz Wolz, C.; Vredenburg, V.; Fleischer, R.; Lips, K. A Century of Batrachochytrium dendrobatidis in Illinois Amphibians (1888–1989). Biol. Conserv. 2015, 182, 254–261. [Google Scholar] [CrossRef]
- Rodriguez, D.; Becker, C.G.; Pupin, N.C.; Haddad, C.F.B.; Zamudio, K.R. Long-term Endemism of Two Highly Divergent Lineages of the Amphibian-Killing Fungus in the Atlantic Forest of Brazil. Mol. Ecol. 2014, 23, 774–787. [Google Scholar] [CrossRef]
- Goka, K.; Yokoyama, J.; Une, Y.; Kuroki, T.; Suzuki, K.; Nakahara, M.; Kobayashi, A.; Inaba, S.; Mizutani, T.; Hyatt, A. Amphibian Chytridiomycosis in Japan: Distribution, Haplotypes and Possible Route of Entry into Japan. Mol. Ecol. 2009, 18, 4757–4774. [Google Scholar] [CrossRef]
- Soto-Azat, C.; Clarke, B.; Poynton, J.; Cunningham, A. Widespread Historical Presence of Batrachochytrium dendrobatidis in African Pipid Frogs. Divers. Distrib. 2010, 16, 126–131. [Google Scholar] [CrossRef]
- Ouellet, M.; Mikaelian, I.; Pauli, B.; Rodrigue, J.; Green, D. Historical Evidence of Widespread Chytrid Infection in North American Amphibian Populations. Conserv. Biol. 2005, 19, 1431–1440. [Google Scholar] [CrossRef]
- Garner, T.W.J.; Walker, S.; Bosch, J.; Hyatt, A.D.; Cunningham, A.A.; Fisher, M.C. Chytrid Fungus in Europe. Emerg. Infect. Dis. 2005, 11, 1639–1641. [Google Scholar] [CrossRef]
- Davidson, E.; Parris, M.; Collins, J.; Longcore, J.; Pessier, A.; Brunner, J.; Beaupre, S. Pathogenicity and Transmission of Chytridiomycosis in Tiger Salamanders (Ambystoma tigrinum). Copeia 2003, 2003, 601–607. [Google Scholar] [CrossRef]
- Olson, D.; Ronnenberg, K.; Glidden, C.; Christiansen, K.; Blaustein, A. Global Patterns of the Fungal Pathogen Batrachochytrium dendrobatidis Support Conservation Urgency. Front. Vet. Sci. 2021, 8, 685877. [Google Scholar] [CrossRef]
- Gower, D.; Doherty-Bone, T.; Loader, S.; Wilkinson, M.; Kouete, M.; Tapley, B.; Orton, F.; Daniel, O.; Wynne, F.; Flach, E.; et al. Batrachochytrium dendrobatidis Infection and Lethal Chytridiomycosis in Caecilian Amphibians (Gymnophiona). Ecohealth 2013, 10, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.; Vredenburg, V.; Martel, A.; Pasmans, F.; Bell, R.; Blackburn, D.; Bletz, M.; Bosch, J.; Briggs, C.; Brown, R.; et al. Cryptic Diversity of a Widespread Global Pathogen Reveals Expanded Threats to Amphibian Conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 20382–20387. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, R.; Cunningham, A.; Daszak, P.; Apolo, A.; Perdomo, E.; Speranza, G. Emerging Pathogen of Wild Amphibians in Frogs (Rana catesbeiana) Farmed for International Trade. Emerg. Infect. Dis. 2003, 9, 995–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloegel, L.; Picco, A.; Kilpatrick, A.; Davies, A.; Hyatt, A.; Daszak, P. Magnitude of the US Trade in Amphibians and Presence of Batrachochytrium dendrobatidis and Ranavirus Infection in Imported North American Bullfrogs (Rana catesbeiana). Biol. Conserv. 2009, 142, 1420–1426. [Google Scholar] [CrossRef]
- Bai, C.-M.; Garner, T.; Yiming, L. First Evidence of Batrachochytrium dendrobatidis in China: Discovery of Chytridiomycosis in Introduced American Bullfrogs and Native Amphibians in the Yunnan Province, China. EcoHealth 2010, 7, 127–134. [Google Scholar] [CrossRef]
- Weldon, C.; Preez, L.; Hyatt, A.; Muller, R.; Spears, R. Origin of the Amphibian Chytrid Fungus. Emerg. Infect. Dis. 2005, 10, 2100–2105. [Google Scholar] [CrossRef]
- Scheele, B.; Pasmans, F.; Skerratt, L.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.; Burrowes, P.; Carvalho, T.; Catenazzi, A.; et al. Amphibian Fungal Panzootic Causes Catastrophic and Ongoing Loss of Biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- González-Maya, J.; Belant, J.; Wyatt, S.; Schipper, J.; Cardenal, J.; Corrales, D.; Cruz-Lizano, I.; Hoepker, A.; Escobedo-Galván, A.; Castañeda, F.; et al. Renewing Hope: The Rediscovery of Atelopus Varius in Costa Rica. Amphib.-Reptil. 2013, 34, 573–578. [Google Scholar] [CrossRef]
- Belasen, A.M.; Russell, I.D.; Zamudio, K.R.; Bletz, M.C. Endemic Lineages of Batrachochytrium dendrobatidis Are Associated With Reduced Chytridiomycosis-Induced Mortality in Amphibians: Evidence From a Meta-Analysis of Experimental Infection Studies. Front. Vet. Sci. 2022, 9, 756686. [Google Scholar] [CrossRef]
- Schloegel, L.; Toledo, L.F.; Longcore, J.; Greenspan, S.; Vieira, C.; Lee, M.; Zhao, S.; Wangen, C.; Mosterio, C.; Hipolito, M.; et al. Novel, Panzootic and Hybrid Genotypes of Amphibian Chytridiomycosis Associated with the Bullfrog Trade. Mol. Ecol. 2012, 21, 5162–5177. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, T.S.; Betancourt Román, C.M.; Lambertini, C.; Valencia-Aguilar, A.; Rodriguez, D.; Nunes-de-Almeida, C.H.L.; Ruggeri, J.; Belasen, A.M.; da Silva Leite, D.; Zamudio, K.R.; et al. Amphibian-Killing Chytrid in Brazil Comprises Both Locally Endemic and Globally Expanding Populations. Mol. Ecol. 2016, 25, 2978–2996. [Google Scholar] [CrossRef]
- Martel, A.; Blooi, M.; Adriaensen, C.; Van Rooij, P.; Beukema, W.; Fisher, M.; Farrer, R.; Schmidt, B.; Tobler, U.; Goka, K.; et al. Recent Introduction of a Chytrid Fungus Endangers Western Palearctic Salamanders. Science 2014, 346, 630–631. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, L.; Pasmans, F.; Martel, A.; Cunningham, A. Epidemiological Tracing of Batrachochytrium salamandrivorans Identifies Widespread Infection and Associated Mortalities in Private Amphibian Collections. Sci. Rep. 2018, 8, 13845. [Google Scholar] [CrossRef] [Green Version]
- Sabino Pinto, J.; Bletz, M.; Hendrix, R.; Perl, R.; Martel, A.; Pasmans, F.; Lötters, S.; Mutschmann, F.; Schmeller, D.; Schmidt, B.; et al. First Detection of the Emerging Fungal Pathogen Batrachochytrium salamandrivorans in Germany. Amphib.-Reptil. 2015, 36, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Van Nguyen, T.; Ziegler, T.; Pasmans, F.; Martel, A. Trade in Wild Anurans Vectors the Urodelan Pathogen Batrachochytrium salamandrivorans into Europe. Amphib.-Reptil. 2017, 38, 554–556. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Martel, A.; Wu, J.; Praet, S.; Canessa, S.; Pasmans, F. Widespread Occurrence of an Emerging Fungal Pathogen in Heavily Traded Chinese Urodelan Species. Conserv. Lett. 2018, 11, e12436. [Google Scholar] [CrossRef] [Green Version]
- Laking, A.; Ngo, H.; Pasmans, F.; Martel, A.; Nguyen, T. Batrachochytrium salamandrivorans Is the Predominant Chytrid Fungus in Vietnamese Salamanders. Sci. Rep. 2017, 7, 44443. [Google Scholar] [CrossRef] [Green Version]
- North American Bsal Task Force. A North American Strategic Plan to Prevent and Control Invasions of the Lethal Salamander Pathogen Batrachochytrium salamandrivorans; North American Bsal Task Force: Fort Collins, CO, USA, 2022. [Google Scholar]
- Yap, T.; Nguyen, N.; Serr, M.; Shepack, A.; Vredenburg, V. Batrachochytrium salamandrivorans and the Risk of a Second Amphibian Pandemic. EcoHealth 2017, 14, 851–864. [Google Scholar] [CrossRef]
- Towe, A.; Gray, M.; Carter, E.; Wilber, M.; Ossiboff, R.; Ash, K.; Bohanon, M.; Bajo, B.; Miller, D. Batrachochytrium salamandrivorans Can Devour More than Salamanders. J. Wildl. Dis. 2021, 57, 942–948. [Google Scholar] [CrossRef]
- Bosch, J.; Martel, A.; Sopniewski, J.; Thumsová, B.; Ayres, C.; Scheele, B.; Velo-Antón, G.; Pasmans, F. Batrachochytrium salamandrivorans Threat to the Iberian Urodele Hotspot. J. Fungi 2021, 7, 644. [Google Scholar] [CrossRef]
- Can, Ö.E.; D’Cruze, N.; Macdonald, D.W. Dealing in Deadly Pathogens: Taking Stock of the Legal Trade in Live Wildlife and Potential Risks to Human Health. Glob. Ecol. Conserv. 2019, 17, e00515. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, D.; Barnhart, K.; Bletz, M.; Campos, A.; Ganem, S.; Hertz, A.; Labumbard, B.; Nanjappa, P.; Tokash-Peters, A. Batrachochytrium: Biology and Management of Amphibian Chytridiomycosis. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2018; pp. 1–18. ISBN 978-0-47001-617-6. [Google Scholar]
- Beukema, W.; Martel, A.; Nguyen, T.; Goka, K.; Schmeller, D.; Yuan, Z.; Laking, A.; Nguyen, T.; Lin, C.-F.; Shelton, J.; et al. Environmental Context and Differences between Native and Invasive Observed Niches of Batrachochytrium salamandrivorans Affect Invasion Risk Assessments in the Western Palaearctic. Divers. Distrib. 2018, 24, 1788–1801. [Google Scholar] [CrossRef] [Green Version]
- Brucker, R.M.; Harris, R.N.; Schwantes, C.; Gallaher, T.N.; Flaherty, D.C.; Lam, B.A.; Minbiole, K.P.C. Amphibian Chemical Defense: Antifungal Metabolites of the Microsymbiont Janthinobacterium lividum on the Salamander Plethodon cinereus. J. Chem. Ecol. 2008, 34, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.K.; Pasmans, F.; Dhaenens, M.; Deforce, D.; Bonte, D.; Verheyen, K.; Lens, L.; Martel, A. Skin Mucosome Activity as an Indicator of Batrachochytrium salamandrivorans Susceptibility in Salamanders. PLoS ONE 2018, 13, e0199295. [Google Scholar] [CrossRef]
- Jiménez, R.R.; Carfagno, A.; Linhoff, L.; Gratwicke, B.; Woodhams, D.C.; Chafran, L.S.; Bletz, M.C.; Bishop, B.; Muletz-Wolz, C.R. Inhibitory Bacterial Diversity and Mucosome Function Differentiate Susceptibility of Appalachian Salamanders to Chytrid Fungal Infection. Appl. Environ. Microbiol. 2022, 88, e01818-21. [Google Scholar] [CrossRef]
- Myers, J.M.; Ramsey, J.P.; Blackman, A.L.; Nichols, A.E.; Minbiole, K.P.C.; Harris, R.N. Synergistic Inhibition of the Lethal Fungal Pathogen Batrachochytrium dendrobatidis: The Combined Effect of Symbiotic Bacterial Metabolites and Antimicrobial Peptides of the Frog Rana Muscosa. J. Chem. Ecol. 2012, 38, 958–965. [Google Scholar] [CrossRef]
- Lam, B.; Walke, J.; Vredenburg, V.; Harris, R. Proportion of Individuals with Anti-Batrachochytrium dendrobatidis Skin Bacteria Is Associated with Population Persistence in the Frog Rana Muscosa. Biol. Conserv. 2010, 143, 529–531. [Google Scholar] [CrossRef]
- Lam, B.A.; Walton, D.B.; Harris, R.N. Motile Zoospores of Batrachochytrium dendrobatidis Move Away from Antifungal Metabolites Produced by Amphibian Skin Bacteria. EcoHealth 2011, 8, 36–45. [Google Scholar] [CrossRef]
- Ramsey, J.; Reinert, L.; Harper, L.; Woodhams, D.; Rollins-Smith, L. Immune Defenses against Batrachochytrium dendrobatidis, a Fungus Linked to Global Amphibian Declines, in the South African Clawed Frog, Xenopus laevis. Infect. Immun. 2010, 78, 3981–3992. [Google Scholar] [CrossRef] [Green Version]
- Rebollar, E.A.; Gutiérrez-Preciado, A.; Noecker, C.; Eng, A.; Hughey, M.C.; Medina, D.; Walke, J.B.; Borenstein, E.; Jensen, R.V.; Belden, L.K.; et al. The Skin Microbiome of the Neotropical Frog Craugastor fitzingeri: Inferring Potential Bacterial-Host-Pathogen Interactions from Metagenomic Data. Front. Microbiol. 2018, 9, 466. [Google Scholar] [CrossRef] [Green Version]
- Bosch, J.; Thumsová, B.; López-Rojo, N.; Pérez, J.; Alonso, A.; Fisher, M.; Boyero, L. Microplastics Increase Susceptibility of Amphibian Larvae to the Chytrid Fungus Batrachochytrium dendrobatidis. Sci. Rep. 2021, 11, 22438. [Google Scholar] [CrossRef]
- McKenzie, C.; Oesterle, P.; Stevens, B.; Shirose, L.; Mastromonaco, G.; Lillie, B.; Davy, C.; Jardine, C.; Nemeth, N. Ophidiomycosis in Red Cornsnakes (Pantherophis guttatus): Potential Roles of Brumation and Temperature on Pathogenesis and Transmission. Vet. Pathol. 2020, 57, 825–837. [Google Scholar] [CrossRef]
- Romer, A.; Grinath, J.; Moe, K.; Walker, D. Host Microbiome Responses to the Snake Fungal Disease Pathogen (Ophidiomyces ophidiicola) Are Driven by Changes in Microbial Richness. Sci. Rep. 2022, 12, 3078. [Google Scholar] [CrossRef]
- Origgi, F.; Tecilla, M. Immunology of Reptiles. In Infectious Diseases and Pathology of Reptiles Color Atlas and Text; Jacobson, E., Garner, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 215–266. [Google Scholar]
- Zapata, A.G.; Varas, A.; Torroba, M. Seasonal Variations in the Immune System of Lower Vertebrates. Immunol. Today 1992, 13, 142–147. [Google Scholar] [CrossRef]
- Wright, R.K.; Cooper, E. Temperature Effects on Ectotherm Immune Responses. Dev. Comp. Immunol. 1981, 5, 117–122. [Google Scholar] [CrossRef]
- Campbell, T. Peripheral Blood of Reptiles. In Exotic Animal Hematology and Cytology; John Wiley & Sons, I.: Hoboken, NJ, USA, 2015; pp. 67–87. [Google Scholar]
- Rakus, K.; Ronsmans, M.; Vanderplasschen, A. Behavioral Fever in Ectothermic Vertebrates. Dev. Comp. Immunol. 2016, 66, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Merchant, M.; Williams, S.; Trosclair, P.L.; Elsey, R.M.; Mills, K. Febrile Response to Infection in the American Alligator (Alligator mississippiensis). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 921–925. [Google Scholar] [CrossRef]
- Ramos, A.B.; Don, M.T.; Muchlinski, A.E. The Effect of Bacteria Infection on Mean Selected Body Temperature in the Common Agama, Agama Agama: A Dose-Response Study. Comp. Biochem. Physiol. Part A Physiol. 1993, 105, 479–484. [Google Scholar] [CrossRef]
- Muchlinski, A.E.; Estany, A.; Don, M.T. The Response of Anolis Equestris and Oplurus cyclurus (Reptilia: Iguanidae) to Bacterial Endotoxin. J. Therm. Biol. 1995, 20, 315–320. [Google Scholar] [CrossRef]
- Richards-Zawacki, C.L. Thermoregulatory Behaviour Affects Prevalence of Chytrid Fungal Infection in a Wild Population of Panamanian Golden Frogs. Proc. Biol. Sci. 2010, 277, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Agha, M.; Price, S.; Nowakowski, A.; Augustine, B.; Todd, B. Mass Mortality of Eastern Box Turtles with Upper Respiratory Disease Following Atypical Cold Weather. Dis. Aquat. Organ. 2017, 124, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.M.; Venesky, M.D.; Sauer, E.L.; Civitello, D.J.; McMahon, T.A.; Roznik, E.A.; Rohr, J.R. The Thermal Mismatch Hypothesis Explains Host Susceptibility to an Emerging Infectious Disease. Ecol. Lett. 2017, 20, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Venesky, M.D.; DeMarchi, J.; Hickerson, C.; Anthony, C.D. Does the Thermal Mismatch Hypothesis Predict Disease Outcomes in Different Morphs of a Terrestrial Salamander? J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022, 337, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Civitello, D.J.; Venesky, M.D.; McMahon, T.A.; Rohr, J.R. Thermal Mismatches Explain How Climate Change and Infectious Disease Drove Widespread Amphibian Extinctions. bioRxiv 2017. [Google Scholar] [CrossRef] [Green Version]
- Chandler, H.; Allender, M.; Stegenga, B.; Haynes, E.; Ospina, E.; Stevenson, D. Ophidiomycosis Prevalence in Georgia’s Eastern Indigo Snake (Drymarchon couperi) Populations. PLoS ONE 2019, 14, e0218351. [Google Scholar] [CrossRef]
- Lind, C.; McCoy, C.; Farrell, T. Tracking Outcomes of Snake Fungal Disease in Free-Ranging Pigmy Rattlesnakes (Sistrurus miliarius). J. Wildl. Dis. 2018, 54, 352–356. [Google Scholar] [CrossRef]
- McKenzie, J.; Price, S.; Fleckenstein, L.; Drayer, A.; Connette, G.; Bohuski, E.; Lorch, J. Field Diagnostics and Seasonality of Ophidiomyces ophiodiicola in Wild Snake Populations. EcoHealth 2018, 16, 141–150. [Google Scholar] [CrossRef]
- Narayan, E. Non-Invasive Reproductive and Stress Endocrinology in Amphibian Conservation Physiology. Conserv. Physiol. 2013, 1, cot011. [Google Scholar] [CrossRef]
- Gabor, C.; Fisher, M.; Bosch, J. A Non-Invasive Stress Assay Shows That Tadpole Populations Infected with Batrachochytrium dendrobatidis Have Elevated Corticosterone Levels. PLoS ONE 2013, 8, e56054. [Google Scholar] [CrossRef] [Green Version]
- Searle, C.; Belden, L.; Du, P.; Blaustein, A. Stress and Chytridiomycosis: Exogenous Exposure to Corticosterone Does Not Alter Amphibian Susceptibility to a Fungal Pathogen. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2014, 321, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Fonner, C.; Patel, S.; Boord, S.; Venesky, M.; Woodley, S. Effects of Corticosterone on Infection and Disease in Salamanders Exposed to the Amphibian Fungal Pathogen, Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 2016, 123, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Barnhart, K.; Bletz, M.; Labumbard, B.; Tokash-Peters, A.; Gabor, C.; Woodhams, D. Batrachochytrium salamandrivorans Elicits Acute Stress Response in Spotted Salamanders but Not Infection or Mortality. Anim. Conserv. 2020, 23, 533–546. [Google Scholar] [CrossRef]
- McCoy, C.; Lind, C.; Farrell, T. Environmental and Physiological Correlates of the Severity of Clinical Signs of Snake Fungal Disease in a Population of Pigmy Rattlesnakes, Sistrurus miliarius. Conserv. Physiol. 2017, 5, cow077. [Google Scholar] [CrossRef] [Green Version]
- Agugliaro, J.; Lind, C.M.; Lorch, J.M.; Farrell, T.M. An Emerging Fungal Pathogen Is Associated with Increased Resting Metabolic Rate and Total Evaporative Water Loss Rate in a Winter-Active Snake. Funct. Ecol. 2020, 34, 486–496. [Google Scholar] [CrossRef]
- Rosenblum, E.; Poorten, T.; Settles, M.; Murdoch, G. Only Skin Deep: Shared Genetic Response to the Deadly Chytrid Fungus in Susceptible Frog Species. Mol. Ecol. 2012, 21, 3110–3120. [Google Scholar] [CrossRef]
- Farrer, R.A.; Weinert, L.A.; Bielby, J.; Garner, T.W.J.; Balloux, F.; Clare, F.; Bosch, J.; Cunningham, A.A.; Weldon, C.; du Preez, L.H.; et al. Multiple Emergences of Genetically Diverse Amphibian-Infecting Chytrids Include a Globalized Hypervirulent Recombinant Lineage. Proc. Natl. Acad. Sci. USA 2011, 108, 18732–18736. [Google Scholar] [CrossRef] [Green Version]
- Rosenblum, E.; James, T.; Zamudio, K.; Poorten, T.; Ilut, D.; Rodriguez, D.; Eastman, J.; Richards-Hrdlicka, K.; Joneson, S.; Jenkinson, T.; et al. Complex History of the Amphibian-Killing Chytrid Fungus Revealed with Genome Resequencing Data. Proc. Natl. Acad. Sci. USA 2013, 110, 9385–9390. [Google Scholar] [CrossRef] [Green Version]
- Dang, T.; Searle, C.; Blaustein, A. Virulence Variation among Strains of the Emerging Infectious Fungus Batrachochytrium dendrobatidis in Multiple Amphibian Host Species. Dis. Aquat. Organ. 2017, 124, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Franklinos, L.H.V.; Lorch, J.M.; Bohuski, E.; Rodriguez-Ramos Fernandez, J.; Wright, O.N.; Fitzpatrick, L.; Petrovan, S.; Durrant, C.; Linton, C.; Baláž, V.; et al. Emerging Fungal Pathogen Ophidiomyces ophiodiicola in Wild European Snakes. Sci. Rep. 2017, 7, 3844. [Google Scholar] [CrossRef]
- Clark, R.; Marchand, M.; Clifford, B.; Stechert, R.; Stephens, S. Decline of an Isolated Timber Rattlesnake (Crotalus horridus) Population: Interactions between Climate Change, Disease, and Loss of Genetic Diversity. Biol. Conserv. 2011, 144, 886–891. [Google Scholar] [CrossRef]
- Campbell, L.; Burger, J.; Zappalorti, R.; Bunnell, J.; Winzeler, M.; Taylor, D.; Lorch, J. Soil Reservoir Dynamics of Ophidiomyces ophidiicola, the Causative Agent of Snake Fungal Disease. J. Fungi 2021, 7, 461. [Google Scholar] [CrossRef] [PubMed]
- Le Donne, V.; Crossland, N.; Brandão, J.; Sokolova, Y.; Fowlkes, N.; Nevarez, J.; Langohr, I.; Gaunt, S. Nannizziopsis guarroi Infection in 2 Inland Bearded Dragons (Pogona vitticeps): Clinical, Cytologic, Histologic, and Ultrastructural Aspects. Vet. Clin. Pathol. 2016, 45, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.D.; Sigler, L.; Peucker, S.K.J.; Norton, J.H.; Nielan, A. Chrysosporium Anamorph of Nannizziopsis vriesii Associated with Fatal Cutaneous Mycoses in the Salt-Water Crocodile (Crocodylus porosus). Med. Mycol. 2002, 40, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, M.; Sánchez, C.; Becker, D.; Byers, K.; Worsley-Tonks, K.; Craft, M. City Sicker? A Meta-analysis of Wildlife Health and Urbanization. Front. Ecol. Environ. 2019, 17, 575–583. [Google Scholar] [CrossRef]
- Woodhams, D.; Alford, R. Ecology of Chytridiomycosis in Rainforest Stream Frog Assemblages of Tropical Queensland. Conserv. Biol. 2005, 19, 1449–1459. [Google Scholar] [CrossRef]
- Cramp, R.; Ohmer, M.; Franklin, C. UV Exposure Causes Energy Trade-Offs Leading to Increased Chytrid Fungus Susceptibility in Green Tree Frog Larvae. Conserv. Physiol. 2022, 10, coac038. [Google Scholar] [CrossRef]
- Ortiz-Santaliestra, M.; Fisher, M.; Fernández-Beaskoetxea, S.; Fernández-Benéitez, M.; Bosch, J. Ambient Ultraviolet B Radiation and Prevalence of Infection by Batrachochytrium dendrobatidis in Two Amphibian Species. Conserv. Biol. 2011, 25, 975–982. [Google Scholar] [CrossRef]
- Doddington, B.J.; Bosch, J.; Oliver, J.A.; Grassly, N.C.; Garcia, G.; Schmidt, B.; Garner, T.; Fisher, M.C. Context-Dependent Amphibian Host Population Response to an Invading Pathogen. Ecology 2013, 98, 1795–1804. [Google Scholar] [CrossRef] [Green Version]
- Schmeller, D.; Blooi, M.; Martel, A.; Garner, T.; Fisher, M.; Azémar, F.; Clare, F.; Leclerc, C.; Jäger, L.; Guevara-Nieto, H.M.; et al. Microscopic Aquatic Predators Strongly Affect Infection Dynamics of a Globally Emerged Pathogen. Curr. Biol. 2013, 24, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Searle, C.L.; Mendelson, J.R.; Green, L.E.; Duffy, M.A. Daphnia Predation on the Amphibian Chytrid Fungus and Its Impacts on Disease Risk in Tadpoles. Ecol. Evol. 2013, 3, 4129–4138. [Google Scholar] [CrossRef] [Green Version]
- Battaglin, W.; Smalling, K.; Anderson, C.; Calhoun, D.; Chestnut, T.; Muths, E. Potential Interactions among Disease, Pesticides, Water Quality and Adjacent Land Cover in Amphibian Habitats in the United States. Sci. Total Environ. 2016, 566–567, 320–332. [Google Scholar] [CrossRef]
- Tompros, A.; Wilber, M.Q.; Fenton, A.; Carter, E.D.; Gray, M.J. Efficacy of Plant-Derived Fungicides at Inhibiting Batrachochytrium salamandrivorans Growth. J. Fungi 2022, 8, 1025. [Google Scholar] [CrossRef]
- Barbi, A.; Goessens, T.; Strubbe, D.; Deknock, A.; Van Leeuwenberg, R.; De Troyer, N.; Verbrugghe, E.; Greener, M.; De Baere, S.; Lens, L.; et al. Widespread Triazole Pesticide Use Affects Infection Dynamics of a Global Amphibian Pathogen. Ecol. Lett. 2023, 26, 313–322. [Google Scholar] [CrossRef]
- Romansic, J.; Johnson, J.; Wagner, R.; Hill, R.; Gaulke, C.; Vredenburg, V.; Blaustein, A. Complex Interactive Effects of Water Mold, Herbicide, and the Fungus Batrachochytrium dendrobatidis on Pacific Treefrog Hyliola Regilla Hosts. Dis. Aquat. Organ. 2016, 123, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, M.P.; Clulow, J.; Mahony, M.J. Evidence of a Salt Refuge: Chytrid Infection Loads Are Suppressed in Hosts Exposed to Salt. Oecologia 2015, 177, 901–910. [Google Scholar] [CrossRef]
- Thomas, V.; Wang, Y.; Van Rooij, P.; Verbrugghe, E.; Baláž, V.; Bosch, J.; Cunningham, A.; Fisher, M.; Garner, T.; Gilbert, M.; et al. Mitigating Batrachochytrium salamandrivorans in Europe. Amphib.-Reptil. 2019, 40, 265–290. [Google Scholar] [CrossRef] [Green Version]
- Woodburn, D.; Kinsel, M.; Poll, C.; Langan, J.; Haman, K.; Gamble, K.; Maddox, C.; Jeon, A.; Wellehan, J.; Ossiboff, R.; et al. Shell Lesions Associated With Emydomyces testavorans Infection in Freshwater Aquatic Turtles. Vet. Pathol. 2021, 58, 578–586. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schilliger, L.; Paillusseau, C.; François, C.; Bonwitt, J. Major Emerging Fungal Diseases of Reptiles and Amphibians. Pathogens 2023, 12, 429. https://doi.org/10.3390/pathogens12030429
Schilliger L, Paillusseau C, François C, Bonwitt J. Major Emerging Fungal Diseases of Reptiles and Amphibians. Pathogens. 2023; 12(3):429. https://doi.org/10.3390/pathogens12030429
Chicago/Turabian StyleSchilliger, Lionel, Clément Paillusseau, Camille François, and Jesse Bonwitt. 2023. "Major Emerging Fungal Diseases of Reptiles and Amphibians" Pathogens 12, no. 3: 429. https://doi.org/10.3390/pathogens12030429




