Unveiling the Relevance of the Oral Cavity as a Staphylococcus aureus Colonization Site and Potential Source of Antimicrobial Resistance
Abstract
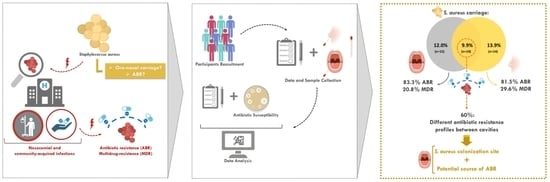
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Oral and Nasal Bacteria Isolation and Identification
2.3. Antibiotic Resistance Assessment
2.4. Data Analysis
3. Results
3.1. Population Characterization
3.2. Prevalence of S. aureus
3.3. S. aureus Carriage and Its Correlation with Clinical and Demographic Factors
3.4. Antibiotic Resistance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pickering, A.C.; Yebra, G.; Gong, X.; Goncheva, M.I.; Wee, B.A.; MacFadyen, A.C.; Muehlbauer, L.F.; Alves, J.; Cartwright, R.A.; Paterson, G.K.; et al. Evolutionary and Functional Analysis of Coagulase Positivity among the Staphylococci. mSphere 2021, 6, e00381-21. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Priority Pathogens List for R&D of New Antibiotics. 2017. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 April 2023).
- Borg, M.A.; Camilleri, L. What Is Driving the Epidemiology of Methicillin-Resistant Staphylococcus aureus Infections in Europe? Microb. Drug Resist. 2021, 27, 889–894. [Google Scholar] [CrossRef] [PubMed]
- John, J. The treatment of resistant staphylococcal infections. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef]
- World Health Organization. Ten Threats to Global Health in 2019. 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 1 April 2023).
- Sakr, A.; Brégeon, F.; Mège, J.L.; Rolain, J.M.; Blin, O. Staphylococcus aureus Nasal Colonization: An Update on Mechanisms, Epidemiology, Risk Factors, and Subsequent Infections. Front Microbiol. 2018, 9. Available online: https://pubmed.ncbi.nlm.nih.gov/30349525/ (accessed on 1 April 2023). [CrossRef]
- Kluytmans, J.; van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Krismer, B.; Weidenmaier, C.; Zipperer, A.; Peschel, A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol. 2017, 15, 675–687. [Google Scholar] [CrossRef]
- Zanger, P.; Nurjadi, D.; Vath, B.; Kremsner, P.G. Persistent Nasal Carriage of Staphylococcus aureus Is Associated with Deficient Induction of Human β-Defensin 3 after Sterile Wounding of Healthy Skin Vivo Infect. Immun. 2011, 79, 2658. [Google Scholar] [CrossRef]
- Sørensen, M.; Wickman, M.; Sollid, J.U.E.; Furberg, A.S.; Klingenberg, C. Allergic disease and Staphylococcus aureus carriage in adolescents in the Arctic region of Norway. Pediatr. Allergy Immunol. 2016, 27, 728–735. [Google Scholar] [CrossRef]
- Laux, C.; Peschel, A.; Krismer, B. Staphylococcus aureus Colonization of the Human Nose and Interaction with Other Microbiome Members. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Nowicka, D.; Grywalska, E. Staphylococcus aureus and Host Immunity in Recurrent Furunculosis. Dermatology 2019, 235, 295–305. [Google Scholar] [CrossRef]
- Kozak, M.; Pawlik, A. The Role of the Oral Microbiome in the Development of Diseases. Int. J. Mol. Sci. 2023, 24, 5231. [Google Scholar] [CrossRef] [PubMed]
- Stamatova, I.; Meurman, J.H. Probiotics: Health benefits in the mouth. Am. J. Dent. 2009, 22, 329–338. [Google Scholar] [PubMed]
- McCormack, M.G.; Smith, A.J.; Akram, A.N.; Jackson, M.; Robertson, D.; Edwards, G. Staphylococcus aureus and the oral cavity: An overlooked source of carriage and infection? Am. J. Infect. Control. 2015, 43, 35–37. [Google Scholar] [CrossRef]
- Lima, B.P.; Hu, L.I.; Vreeman, G.W.; Weibel, D.B.; Lux, R. The Oral Bacterium Fusobacterium nucleatum Binds Staphylococcus aureus and Alters Expression of the Staphylococcal Accessory Regulator sarA. Microb. Ecol. 2019, 78, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.L.; Mcgrath, C.; Bandara, H.; Li, L.S.W.; Samaranayake, L.P. Oral health promotion interventions on oral reservoirs of staphylococcus aureus: A systematic review. Oral Dis. 2012, 18, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Passariello, C.; Puttini, M.; Iebba, V.; Pera, P.; Gigola, P. Influence of oral conditions on colonization by highly toxigenic Staphylococcus aureus strains. Oral Dis. 2012, 18, 402–409. [Google Scholar] [CrossRef]
- Simões-Silva, L.; Ferreira, S.; Santos-Araujo, C.; Tabaio, M.; Pestana, M.; Soares-Silva, I.; Sampaio-Maia, B. Oral Colonization of Staphylococcus Species in a Peritoneal Dialysis Population: A Possible Reservoir for PD-Related Infections? Can. J. Infect. Dis. Med. 2018, 2018, 5789094. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef]
- Mergenhagen, K.A.; Starr, K.E.; Wattengel, B.A.; Lesse, A.J.; Sumon, Z.; Sellick, J.A. Determining the Utility of Methicillin-Resistant Staphylococcus aureus Nares Screening in Antimicrobial Stewardship. Clin. Infect Dis. 2020, 71, 1142–1148. [Google Scholar] [CrossRef]
- Carr, A.L.; Daley, M.J.; Givens Merkel, K.; Rose, D.T. Clinical Utility of Methicillin-Resistant Staphylococcus aureus Nasal Screening for Antimicrobial Stewardship: A Review of Current Literature. Pharmacotherapy 2018, 38, 1216–1228. [Google Scholar] [CrossRef]
- Scholten, R.; Hannink, G.; Willemsen, K.; Mascini, E.M.; Somford, M.P.; Schreurs, B.W.; van Susante, J.L.C. Preoperative Staphylococcus aureus screening and eradication. Bone Jt. J. 2020, 102-B, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.; Brégeon, F.; Rolain, J.M.; Blin, O. Staphylococcus aureus nasal decolonization strategies: A review. Expert Rev. Anti-Infect. Ther. 2019, 17, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, K.; Wierzbowska, M.; Kwapisz, E.; Kosecka-Strojek, M.; Bronk, M.; Saki, M.; Międzobrodzki, J. Distribution and antibiotic-resistance of different Staphylococcus species identified by matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) isolated from the oral cavity. J. Oral Microbiol. 2021, 13, 1983322. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S.; Kotey, F.C. Methicillin-Resistant Staphylococcus aureus in the Oral Cavity: Implications for Antibiotic Prophylaxis and Surveillance. Infect. Dis. 2020, 13, 117863372097658. [Google Scholar] [CrossRef]
- Garbacz, K.; Kwapisz, E.; Piechowicz, L.; Wierzbowska, M. Staphylococcus aureus Isolated from the Oral Cavity: Phage Susceptibility in Relation to Antibiotic Resistance. Antibiotics 2021, 10, 1329. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables and Dosages, Version 12.0. 2022. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Expert_Rules/2020/Intrinsic_Resistance_and_Unusual_Phenotypes_Tables_v3.2_20200225.pdf (accessed on 16 July 2022).
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 32nd Edition. 2022. Available online: https://file.qums.ac.ir/repository/mmrc/clsi%202017.pdf (accessed on 1 April 2023).
- Reyes, N.; Montes, O.; Figueroa, S.; Tiwari, R.; Sollecito, C.C.; Emmerich, R.; Usyk, M.; Geliebter, J.; Burk, R.D. Staphylococcus aureus nasal carriage and microbiome composition among medical students from Colombia: A cross-sectional study. F1000Research 2020, 9, 78. [Google Scholar] [CrossRef]
- Price, J.R.; Cole, K.; Bexley, A.; Kostiou, V.; Eyre, D.W.; Golubchik, T.; Wilson, D.J.; Crook, D.W.; Walker, A.S.; EA Peto, T.; et al. Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: A longitudinal cohort study based on whole-genome sequencing. Lancet Infect Dis. 2017, 17, 207–214. [Google Scholar] [CrossRef]
- Chen, B.; Dai, X.; He, B.; Pan, K.; Li, H.; Liu, X.; Bao, Y.; Lao, W.; Wu, X.; Yao, Y.; et al. Differences in Staphylococcus aureus nasal carriage and molecular characteristics among community residents and healthcare workers at Sun Yat-Sen University, Guangzhou, Southern China. BMC Infect. Dis. 2015, 15, 303. [Google Scholar] [CrossRef]
- Saadatian-Elahi, M.; Tristan, A.; Laurent, F.; Rasigade, J.-P.; Bouchiat, C.; Ranc, A.-G.; Lina, G.; Dauwalder, O.; Etienne, J.; Bes, M.; et al. Basic Rules of Hygiene Protect Health Care and Lab Workers from Nasal Colonization by Staphylococcus aureus: An International Cross-Sectional Study. PLoS ONE 2013, 8, 82851. [Google Scholar] [CrossRef]
- Elie-Turenne, M.-C.; Fernandes, H.; Mediavilla, J.R.; Rosenthal, M.; Mathema, B.; Singh, A.; Cohen, T.R.; Pawar, K.A.; Shahidi, H.; Kreiswirth, B.N.; et al. Prevalence and characteristics of Staphylococcus aureus colonization among healthcare professionals in an urban teaching hospital. Infect Control Hosp Epidemiol. 2010, 31, 574–580. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Ohara-Nemoto, Y.; Haraga, H.; Kimura, S.; Nemoto, T.K. Occurrence of staphylococci in the oral cavities of healthy adults and nasal oral trafficking of the bacteria. J. Med. Microbiol. 2008, 57 Pt 1, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Kearney, A.; Kinnevey, P.; Shore, A.; Earls, M.; Poovelikunnel, T.; Brennan, G.; Humphreys, H.; Coleman, D. The oral cavity revealed as a significant reservoir of Staphylococcus aureus in an acute hospital by extensive patient, healthcare worker and environmental sampling. J. Hosp. Infect. 2020, 105, 389–396. [Google Scholar] [CrossRef] [PubMed]


| Clinical and Demographic Factors | |
|---|---|
| Sex (female; male) | 82.1%; 17.9% |
| Age (years) | 21.81 ± 3.53 |
| Smoking habits | 2.29% |
| Hormonal contraception a | 64.19% |
| Obesity | 5.66% |
| Asthma and allergies | 7.02% |
| Atopic dermatitis | 2.67% |
| Fixed prothesis | 3.54% |
| Gingivitis | 4.35% |
| DMFT | 2.60 ± 2.62 |
| Clinical and Demographic Factors | Oral Carriers | p-Value b | Nasal Carriers | p-Value b | Oral and Nasal Carriers | p-Value b | |
|---|---|---|---|---|---|---|---|
| Gender | Male | 17.6% | >0.999 | 23.5% | >0.999 | 11.8% | 0.661 |
| Female | 21.8% | 23.1% | 9.0% | ||||
| Hormonal contraception a | Taking | 20.0% | 0.820 | 20.0% | 0.561 | 6.0% | 0.243 |
| Not taking | 25.0% | 28.6% | 14.3% | ||||
| DMFT | =0 teeth | 19.2% | >0.999 | 19.2% | 0.776 | 3.8% | 0.449 |
| >0 teeth | 21.7% | 24.6% | 11.6% | ||||
| Total | Oral | Nasal | |
|---|---|---|---|
| (51) | (24) | (27) | |
| R | 82.4% (42) | 83.3% (20) | 81.5% (22) |
| MDR | 25.5% (13) | 20.8% (5) | 29.6% (8) |
| AML | 43.1% (22) | 45.8% (11) | 40.7% (11) |
| FOX | 0% (0) | 0% (0) | 0% (0) |
| CIP | 0% (0) | 0% (0) | 0% (0) |
| C | 0% (0) | 0% (0) | 0% (0) |
| CN | 66.7% (34) | 58.3% (14) | 74.1% (20) |
| TE | 3.9% (2) | 4.2% (1) | 3.7% (1) |
| SXT | 0% (0) | 0% (0) | 0% (0) |
| E | 31.4% (16) | 29.2% (7) | 33.3% (9) |
| CD | 31.4 % (16) | 29.2% (7) | 33.3% (9) |
| QDA | 0% (0) | 0% (0) | 0% (0) |
| Phenotypic Resistance Profiles | Total | Oral | Nasal |
|---|---|---|---|
| (51) | (24) | (27) | |
| - | 17.6% (9) | 16.7% (4) | 18.5% (5) |
| AML | 7.8% (4) | 12.5% (3) | 3.7% (1) |
| CN | 17.6% (9) | 12.5% (3) | 22.2% (6) |
| AML-CN | 23.5% (12) | 25% (6) | 22.2% (6) |
| CD-E | 5.9% (3) | 8.3% (2) | 3.7% (1) |
| CN-TE | 2.0% (1) | 4.2% (1) | 0% (0) |
| AML-CD-E | 2.0% (1) | 4.2% (1) | 0% (0) |
| CD-CN-E | 13.7% (7) | 12.5% (3) | 14.8% (4) |
| AML-CD-CN-E | 7.8% (4) | 4.2% (1) | 11.1% (3) |
| AML-CD-CN-E-TE | 2.0% (1) | 0% (0) | 3.7% (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.; Pires, M.F.; Sousa, M.; Campos, C.; da Costa, C.F.F.A.; Sampaio-Maia, B. Unveiling the Relevance of the Oral Cavity as a Staphylococcus aureus Colonization Site and Potential Source of Antimicrobial Resistance. Pathogens 2023, 12, 765. https://doi.org/10.3390/pathogens12060765
Campos J, Pires MF, Sousa M, Campos C, da Costa CFFA, Sampaio-Maia B. Unveiling the Relevance of the Oral Cavity as a Staphylococcus aureus Colonization Site and Potential Source of Antimicrobial Resistance. Pathogens. 2023; 12(6):765. https://doi.org/10.3390/pathogens12060765
Chicago/Turabian StyleCampos, Joana, Mariana Faria Pires, Marta Sousa, Carla Campos, Carolina Fernandes Ferreira Alves da Costa, and Benedita Sampaio-Maia. 2023. "Unveiling the Relevance of the Oral Cavity as a Staphylococcus aureus Colonization Site and Potential Source of Antimicrobial Resistance" Pathogens 12, no. 6: 765. https://doi.org/10.3390/pathogens12060765