Newly Designed Poxviral Promoters to Improve Immunogenicity and Efficacy of MVA-NP Candidate Vaccines against Lethal Influenza Virus Infection in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
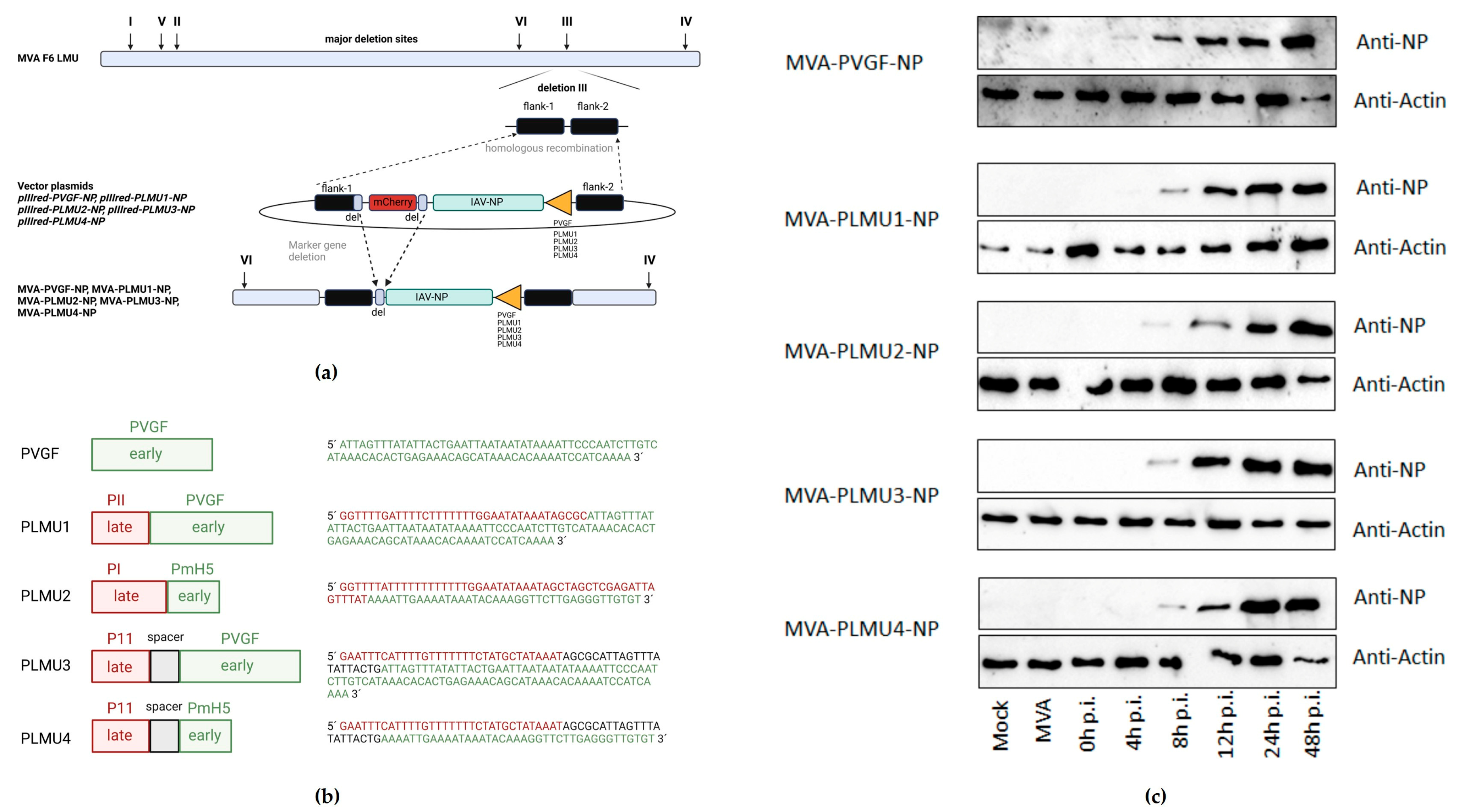
2.2. Plasmid Construction
2.3. Generation of Recombinant MVA Vector Viruses
2.4. Western Blot Analysis of Recombinant Proteins
2.5. Mouse Immunization and Infection Experiments
2.6. Depletion of Specific Subsets of Immune Cells
2.7. Necropsy, Histology and Immunohistochemistry
2.8. Quantitative Stereologic Examinations
2.9. Determination of IAV Loads in Mouse Lung Lobes
2.10. Quantification of NP-Specifc IgG Antibodies by Enzyme-Linked Immunosorbant Assay (ELISA)
2.11. CD8+ T-Cell Analysis by Enzyme-Linked Immunospot Assay (ELISpot)
2.12. T Cell Analysis in Blood Using FACS Analysis
2.13. Data Analysis
3. Results
3.1. Design and In Vitro Testing of Recombinant MVA Vaccines Delivering NP by Chimeric Promoters
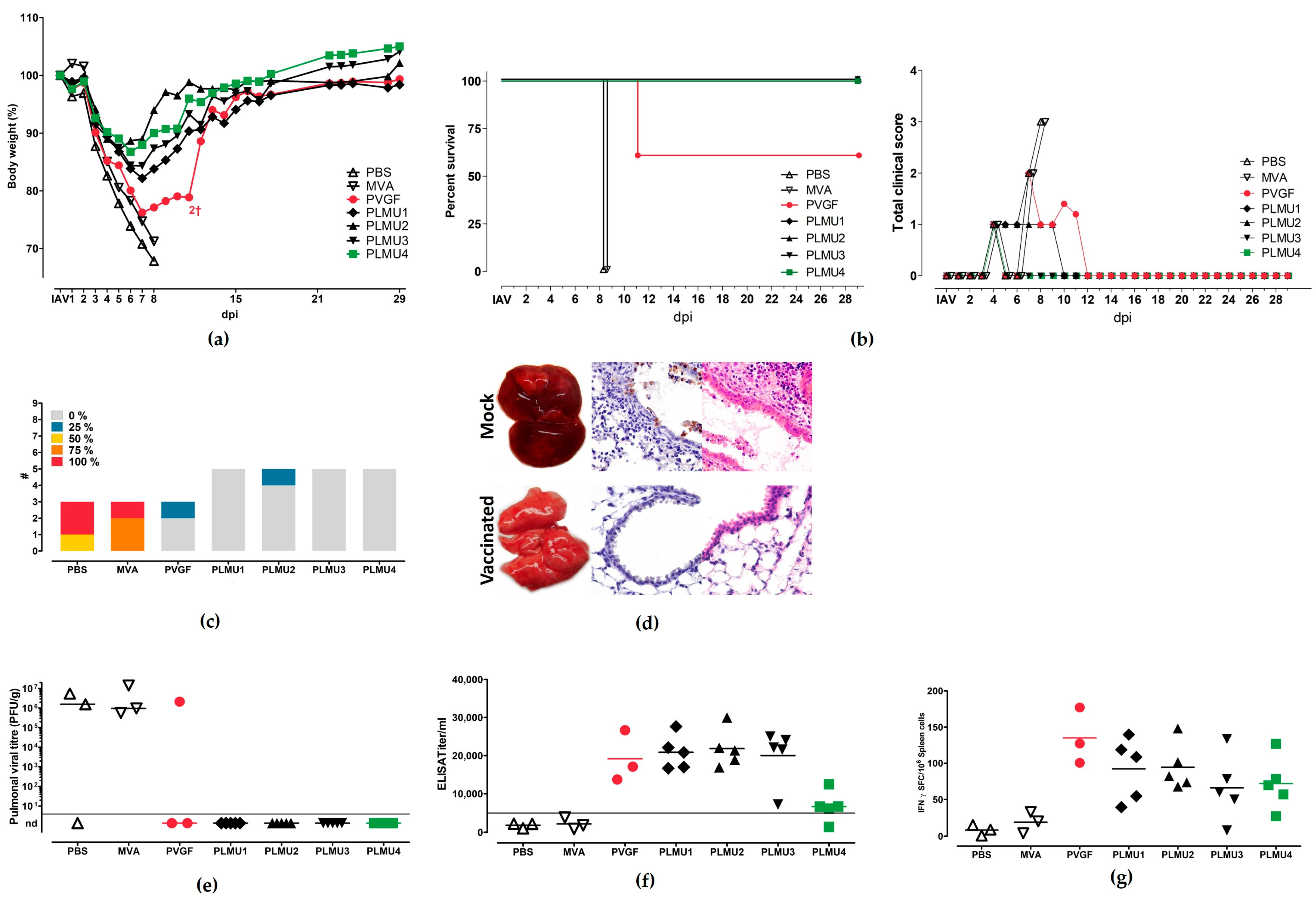
3.2. Immunogenicity and Protective Efficacy of MVA-NPcandidate Vaccine after a Prime-Boost Vaccination Regimen
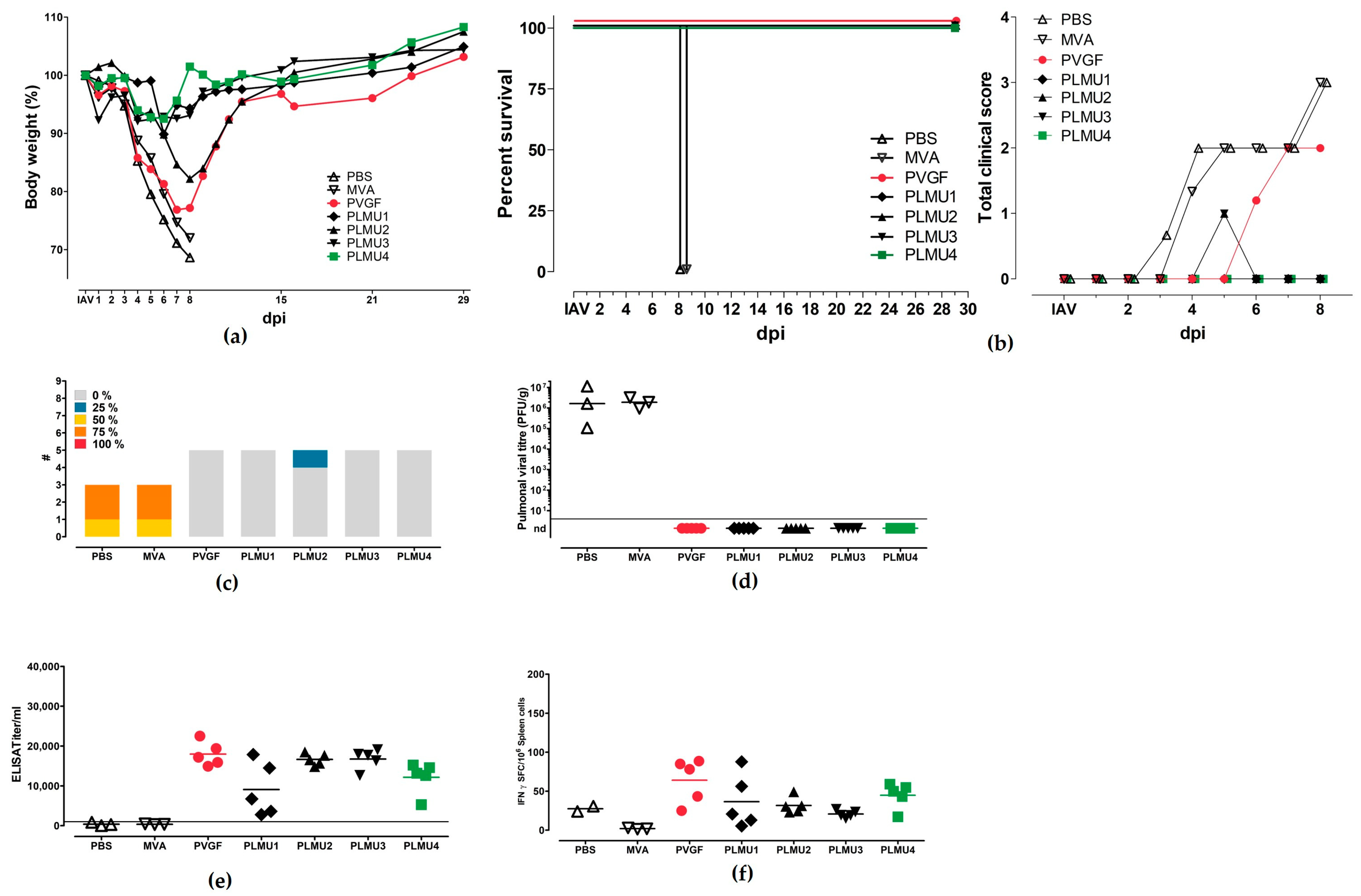
3.3. Immunogenicity and Protective Efficacy of MVA-NP Candidate Vaccines Using a Prime Vaccination Regimen
3.4. Immunogenicity Is Already Established at d8 p.i. in Selected Vaccines after Prime Vaccination
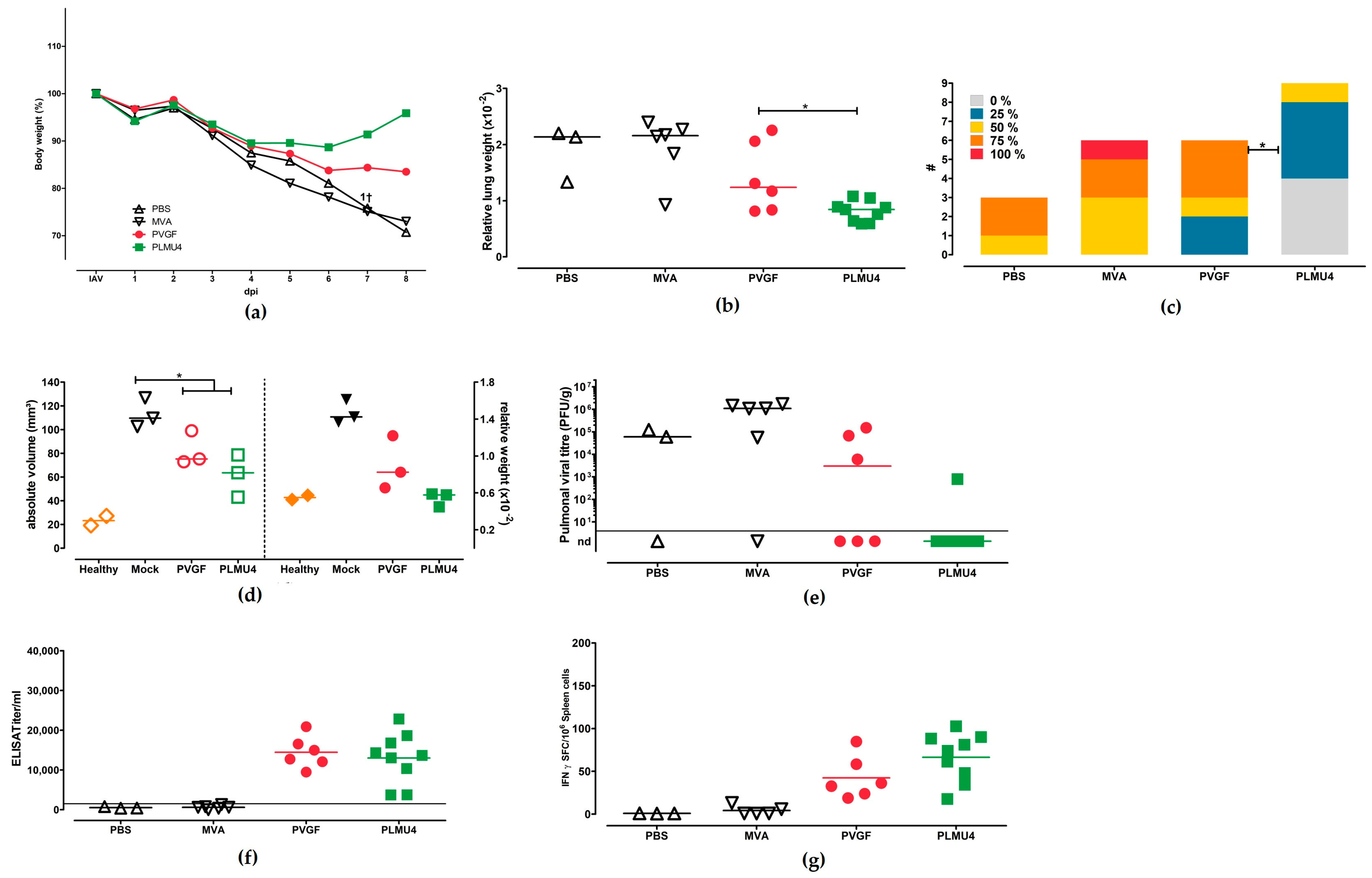
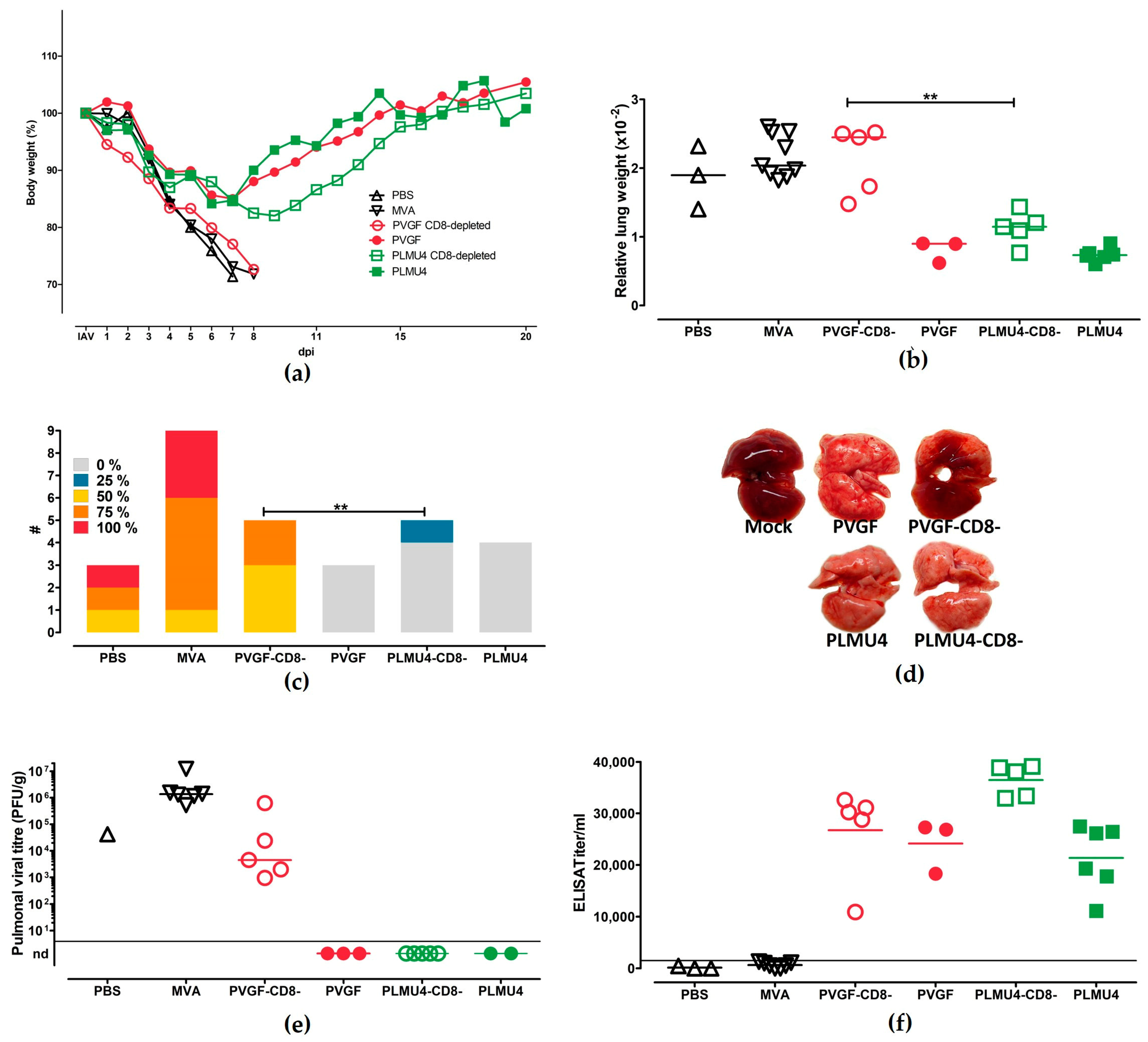
3.5. Role of CD8+ T Cells for the Protective Capacity of MVA-PVGF-NP and MVA-PLMU4-NP Induced Protection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mameli, C.; Cocchi, I.; Fumagalli, M.; Zuccotti, G. Influenza Vaccination: Effectiveness, Indications and Limits in the Pediatric Population. Front. Pediatr. 2019, 7, 317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, D.M.; Elliot, A.J. The impact of influenza on the health and health care utilisation of elderly people. Vaccine 2005, 23 (Suppl. 1), S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Sandbulte, M.R.; Westgeest, K.B.; Gao, J.; Xu, X.; Klimov, A.I.; Russell, C.A.; Burke, D.F.; Smith, D.J.; Fouchier, R.A.M.; Eichelberger, M.C. Discordant antigenic drift of neuraminidase and hemagglutinin in H1N1 and H3N2 influenza viruses. Proc. Natl. Acad. Sci. USA 2011, 108, 20748–20753. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018, 31, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Lapedes, A.S.; de Jong, J.C.; Bestebroer, T.M.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Mapping the antigenic and genetic evolution of influenza virus. Science 2004, 305, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Palese, P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 2015, 14, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Gouma, S.; Anderson, E.M.; Hensley, S.E. Challenges of Making Effective Influenza Vaccines. Annu. Rev. Virol. 2020, 7, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-J.; Crank, M.C.; Shiver, J.; Graham, B.S.; Mascola, J.R.; Nabel, G.J. Next-generation influenza vaccines: Opportunities and challenges. Nat. Rev. Drug Discov. 2020, 19, 239–252. [Google Scholar] [CrossRef]
- Doud, M.B.; Ashenberg, O.; Bloom, J.D. Site-Specific Amino Acid Preferences Are Mostly Conserved in Two Closely Related Protein Homologs. Mol. Biol. Evol. 2015, 32, 2944–2960. [Google Scholar] [CrossRef]
- Van Reeth, K.; Brown, I.; Essen, S.; Pensaert, M. Genetic relationships, serological cross-reaction and cross-protection between H1N2 and other influenza A virus subtypes endemic in European pigs. Virus Res. 2004, 103, 115–124. [Google Scholar] [CrossRef]
- Altmüller, A.; Fitch, W.M.; Scholtissek, C. Biological and genetic evolution of the nucleoprotein gene of human influenza A viruses. J. Gen. Virol. 1989, 70 Pt 8, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Antón, L.C.; Bennink, J.R.; Yewdell, J.W. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 2000, 12, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Hessel, A.; Savidis-Dacho, H.; Coulibaly, S.; Portsmouth, D.; Kreil, T.R.; Crowe, B.A.; Schwendinger, M.G.; Pilz, A.; Barrett, P.N.; Falkner, F.G.; et al. MVA vectors expressing conserved influenza proteins protect mice against lethal challenge with H5N1, H9N2 and H7N1 viruses. PLoS ONE 2014, 9, e88340. [Google Scholar] [CrossRef] [PubMed]
- Hillaire, M.L.B.; Osterhaus, A.D.M.E.; Rimmelzwaan, G.F. Induction of virus-specific cytotoxic T lymphocytes as a basis for the development of broadly protective influenza vaccines. J. Biomed. Biotechnol. 2011, 2011, 939860. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Krammer, F. Universal influenza virus vaccines and therapeutic antibodies. Clin. Microbiol. Infect. 2017, 23, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Fux, R.; Provacia, L.B.; Volz, A.; Eickmann, M.; Becker, S.; Osterhaus, A.D.M.E.; Haagmans, B.L.; Sutter, G. Middle East Respiratory Syndrome Coronavirus Spike Protein Delivered by Modified Vaccinia Virus Ankara Efficiently Induces Virus-Neutralizing Antibodies. J. Virol. 2013, 87, 11950–11954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer zu Natrup, C.; Tscherne, A.; Dahlke, C.; Ciurkiewicz, M.; Shin, D.-L.; Fathi, A.; Rohde, C.; Kalodimou, G.; Halwe, S.; Limpinsel, L.; et al. Stabilized recombinant SARS-CoV-2 spike antigen enhances vaccine immunogenicity and protective capacity. J. Clin. Investig. 2022, 132, e159895. [Google Scholar] [CrossRef]
- Tscherne, A.; Schwarz, J.H.; Rohde, C.; Kupke, A.; Kalodimou, G.; Limpinsel, L.; Okba, N.M.A.; Bošnjak, B.; Sandrock, I.; Odak, I.; et al. Immunogenicity and efficacy of the COVID-19 candidate vector vaccine MVA-SARS-2-S in preclinical vaccination. Proc. Natl. Acad. Sci. USA 2021, 118, e2026207118. [Google Scholar] [CrossRef] [PubMed]
- Kupke, A.; Volz, A.; Dietzel, E.; Freudenstein, A.; Schmidt, J.; Shams-Eldin, H.; Jany, S.; Sauerhering, L.; Krähling, V.; Gellhorn Serra, M.; et al. Protective CD8+ T Cell Response Induced by Modified Vaccinia Virus Ankara Delivering Ebola Virus Nucleoprotein. Vaccines 2022, 10, 533. [Google Scholar] [CrossRef]
- Koch, T.; Dahlke, C.; Fathi, A.; Kupke, A.; Krähling, V.; Okba, N.M.A.; Halwe, S.; Rohde, C.; Eickmann, M.; Volz, A.; et al. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: An open-label, phase 1 trial. Lancet Infect. Dis. 2020, 20, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Nyombayire, J.; Ingabire, R.; Magod, B.; Mazzei, A.; Mazarati, J.-B.; Noben, J.; Katwere, M.; Parker, R.; Nsanzimana, S.; Wall, K.M.; et al. Monitoring of Adverse Events in Recipients of the 2-Dose Ebola Vaccine Regimen of Ad26.ZEBOV Followed by MVA-BN-Filo in the UMURINZI Ebola Vaccination Campaign. J. Infect. Dis. 2022, 227, 268–277. [Google Scholar] [CrossRef]
- Volkmann, A.; Williamson, A.-L.; Weidenthaler, H.; Meyer, T.P.; Robertson, J.S.; Excler, J.-L.; Condit, R.C.; Evans, E.; Smith, E.R.; Kim, D.; et al. The Brighton Collaboration standardized template for collection of key information for risk/benefit assessment of a Modified Vaccinia Ankara (MVA) vaccine platform. Vaccine 2020, 39, 3067–3080. [Google Scholar] [CrossRef]
- Gilbert, S.C. Clinical development of Modified Vaccinia virus Ankara vaccines. Vaccine 2013, 31, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Martinez, J.; Zhou, W.; La Rosa, C.; Srivastava, T.; Dasgupta, A.; Rawal, R.; Li, Z.; Britt, W.J.; Diamond, D. Modified H5 promoter improves stability of insert genes while maintaining immunogenicity during extended passage of genetically engineered MVA vaccines. Vaccine 2009, 28, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Volz, A.; Sutter, G. Modified Vaccinia Virus Ankara: History, Value in Basic Research, and Current Perspectives for Vaccine Development. Adv. Virus Res. 2017, 97, 187–243. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K. Poxviral promoters for improving the immunogenicity of MVA delivered vaccines. Hum. Vaccin. Immunother. 2018, 15, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Moss, B. Genetically engineered poxviruses for recombinant gene expression, vaccination and safety. Proc. Natl. Acad. Sci. USA 1996, 93, 11341–11348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Maruri-Avidal, L.; Sisler, J.; Stuart, C.A.; Moss, B. Cascade regulation of vaccinia virus gene expression is modulated by multistage promoters. Virology 2013, 447, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Moss, B. Poxvirus DNA Replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a010199. [Google Scholar] [CrossRef] [Green Version]
- McFadden, G. Poxvirus tropism. Nat. Rev. Microbiol. 2005, 3, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Brechling, K.; Moss, B. Vaccinia virus expression vector: Coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 1985, 5, 3403–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutter, G.; Moss, B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 1992, 89, 10847–10851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oseroff, C.; Peters, B.; Pasquetto, V.; Moutaftsi, M.; Sidney, J.; Panchanathan, V.; Tscharke, D.C.; Maillere, B.; Grey, H.; Sette, A. Dissociation between epitope hierarchy and immunoprevalence in CD8 responses to vaccinia virus western reserve. J. Immunol. 2008, 180, 7193–7202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tscharke, D.C.; Karupiah, G.; Zhou, J.; Palmore, T.; Irvine, K.R.; Haeryfar, S.M.M.; Williams, S.; Sidney, J.; Sette, A.; Bennink, J.R.; et al. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J. Exp. Med. 2005, 201, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baur, K.; Brinkmann, K.; Schweneker, M.; Pätzold, J.; Meisinger-Henschel, C.; Hermann, J.; Steigerwald, R.; Chaplin, P.; Suter, M.; Hausmann, J. Immediate-early expression of a recombinant antigen by modified vaccinia virus ankara breaks the immunodominance of strong vector-specific B8R antigen in acute and memory CD8 T-cell responses. J. Virol. 2010, 84, 8743–8752. [Google Scholar] [CrossRef] [Green Version]
- Orubu, T.; Alharbi, N.K.; Lambe, T.; Gilbert, S.C.; Cottingham, M.G. Expression and cellular immunogenicity of a transgenic antigen driven by endogenous poxviral early promoters at their authentic loci in MVA. PLoS ONE 2012, 7, e40167. [Google Scholar] [CrossRef]
- Marr, L.; Lülf, A.-T.; Freudenstein, A.; Sutter, G.; Volz, A. Myristoylation increases the CD8+ T-cell response to a GFP prototype antigen delivered by modified vaccinia virus Ankara. J. Gen. Virol. 2016, 97, 934–940. [Google Scholar] [CrossRef]
- Kremer, M.; Volz, A.; Kreijtz, J.H.C.M.; Fux, R.; Lehmann, M.H.; Sutter, G. Easy and efficient protocols for working with recombinant vaccinia virus MVA. Methods Mol. Biol. 2012, 890, 59–92. [Google Scholar] [CrossRef]
- Volz, A.; Langenmayer, M.; Jany, S.; Kalinke, U.; Sutter, G. Rapid Expansion of CD8+ T Cells in Wild-Type and Type I Interferon Receptor-Deficient Mice Correlates with Protection after Low-Dose Emergency Immunization with Modified Vaccinia Virus Ankara. J. Virol. 2014, 88, 10946–10957. [Google Scholar] [CrossRef] [Green Version]
- Kittel, B.; Ruehl-Fehlert, C.; Morawietz, G.; Klapwijk, J.; Elwell, M.R.; Lenz, B.; O’Sullivan, M.G.; Roth, D.R.; Wadsworth, P.F. Revised guides for organ sampling and trimming in rats and mice—Part 2: A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 2004, 55, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Morawietz, G.; Ruehl-Fehlert, C.; Kittel, B.; Bube, A.; Keane, K.; Halm, S.; Heuser, A.; Hellmann, J. Revised guides for organ sampling and trimming in rats and mice—Part 3: A joint publication of the RITA and NACAD groups. Exp. Toxicol. Pathol. 2004, 55, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U. Revised guides for organ sampling and trimming in rats and mice—Part 1. Exp. Toxicol. Pathol. 2003, 55, 91–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodpaster, T.; Randolph-Habecker, J. A flexible mouse-on-mouse immunohistochemical staining technique adaptable to biotin-free reagents, immunofluorescence and multiple antibody staining. J. Histochem. Cytochem. 2014, 62, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Langenmayer, M.C.; Lülf-Averhoff, A.-T.; Adam-Neumair, S.; Fux, R.; Sutter, G.; Volz, A. Distribution and absence of generalized lesions in mice following single dose intramuscular inoculation of the vaccine candidate MVA-MERS-S. Biologicals 2018, 54, 58–62. [Google Scholar] [CrossRef]
- Knust, J.; Ochs, M.; Gundersen, H.J.G.; Nyengaard, J.R. Stereological estimates of alveolar number and size and capillary length and surface area in mice lungs. Anat. Rec. 2009, 292, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Weibel, E.R.; Kistler, G.S.; Scherle, W.F. Practical stereological methods for morphometric cytology. J. Cell Biol. 1966, 30, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Howard, V.; Reed, M. Unbiased Stereology; Garland Science: New York, NY, USA, 2004; ISBN 9781135331689. [Google Scholar]
- Kalodimou, G.; Veit, S.; Jany, S.; Kalinke, U.; Broder, C.C.; Sutter, G.; Volz, A. A Soluble Version of Nipah Virus Glycoprotein G Delivered by Vaccinia Virus MVA Activates Specific CD8 and CD4 T Cells in Mice. Viruses 2019, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Fux, R.; Wolf, G. Transient elimination of circulating bovine viral diarrhoea virus by colostral antibodies in persistently infected calves: A pitfall for BVDV-eradication programs? Vet. Microbiol. 2012, 161, 13–19. [Google Scholar] [CrossRef]
- Veit, S.; Jany, S.; Fux, R.; Sutter, G.; Volz, A. CD8+ T Cells Responding to the Middle East Respiratory Syndrome Coronavirus Nucleocapsid Protein Delivered by Vaccinia Virus MVA in Mice. Viruses 2018, 10, 718. [Google Scholar] [CrossRef] [Green Version]
- Townsend, A.R.; Rothbard, J.; Gotch, F.M.; Bahadur, G.; Wraith, D.; McMichael, A.J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 1986, 44, 959–968. [Google Scholar] [CrossRef]
- Vitiello, A.; Yuan, L.; Chesnut, R.W.; Sidney, J.; Southwood, S.; Farness, P.; Jackson, M.R.; Peterson, P.A.; Sette, A. Immunodominance analysis of CTL responses to influenza PR8 virus reveals two new dominant and subdominant Kb-restricted epitopes. J. Immunol. 1996, 157, 5555–5562. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.; Suezer, Y.; Volz, A.; Frenz, T.; Majzoub, M.; Hanschmann, K.-M.; Lehmann, M.H.; Kalinke, U.; Sutter, G. Critical Role of Perforin-dependent CD8+ T Cell Immunity for Rapid Protective Vaccination in a Murine Model for Human Smallpox. PLoS Pathog. 2012, 8, e1002557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, A.F.; Magnusson, S.E.; Bosman, F.; Stertman, L.; de Vries, R.D.; Rimmelzwaan, G.F. Protein and modified vaccinia virus Ankara-based influenza virus nucleoprotein vaccines are differentially immunogenic in BALB/c mice. Clin. Exp. Immunol. 2017, 190, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamere, M.W.; Lam, H.-T.; Moquin, A.; Haynes, L.; Lund, F.E.; Randall, T.D.; Kaminski, D.A. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J. Immunol. 2011, 186, 4331–4339. [Google Scholar] [CrossRef] [Green Version]
- Lamere, M.W.; Moquin, A.; Lee, F.E.-H.; Misra, R.S.; Blair, P.J.; Haynes, L.; Randall, T.D.; Lund, F.E.; Kaminski, D.A. Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance. J. Virol. 2011, 85, 5027–5035. [Google Scholar] [CrossRef] [Green Version]
- Kastenmuller, W.; Gasteiger, G.; Gronau, J.H.; Baier, R.; Ljapoci, R.; Busch, D.H.; Drexler, I. Cross-competition of CD8+ T cells shapes the immunodominance hierarchy during boost vaccination. J. Exp. Med. 2007, 204, 2187–2198. [Google Scholar] [CrossRef] [Green Version]
- Florek, K.R.; Weinfurter, J.T.; Jegaskanda, S.; Brewoo, J.N.; Powell, T.D.; Young, G.R.; Das, S.C.; Hatta, M.; Broman, K.W.; Hungnes, O.; et al. Modified vaccinia virus Ankara encoding influenza virus hemagglutinin induces heterosubtypic immunity in macaques. J. Virol. 2014, 88, 13418–13428. [Google Scholar] [CrossRef] [Green Version]
- Becker, P.D.; Nörder, M.; Weissmann, S.; Ljapoci, R.; Erfle, V.; Drexler, I.; Guzmán, C.A. Gene Expression Driven by a Strong Viral Promoter in MVA Increases Vaccination Efficiency by Enhancing Antibody Responses and Unmasking CD8⁺ T Cell Epitopes. Vaccines 2014, 2, 581–600. [Google Scholar] [CrossRef] [Green Version]
- Lanthier, P.A.; Huston, G.E.; Moquin, A.; Eaton, S.M.; Szaba, F.M.; Kummer, L.W.; Tighe, M.P.; Kohlmeier, J.E.; Blair, P.J.; Broderick, M.; et al. Live attenuated influenza vaccine (LAIV) impacts innate and adaptive immune responses. Vaccine 2011, 29, 7849–7856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasteiger, G.; Kastenmuller, W.; Ljapoci, R.; Sutter, G.; Drexler, I. Cross-Priming of Cytotoxic T Cells Dictates Antigen Requisites for Modified Vaccinia Virus Ankara Vector Vaccines. J. Virol. 2007, 81, 11925–11936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akondy, R.S.; Johnson, P.L.F.; Nakaya, H.I.; Edupuganti, S.; Mulligan, M.J.; Lawson, B.; Miller, J.D.; Pulendran, B.; Antia, R.; Ahmed, R. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc. Natl. Acad. Sci. USA 2015, 112, 3050–3055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruder, D.; Srikiatkhachorn, A.; Enelow, R.I. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 2006, 19, 147–155. [Google Scholar] [CrossRef]
- Heinen, P.P.; de Boer-Luijtze, E.A.; Bianchi, A.T.J. Respiratory and systemic humoral and cellular immune responses of pigs to a heterosubtypic influenza A virus infection. J. Gen. Virol. 2001, 82, 2697–2707. [Google Scholar] [CrossRef]
- Hayward, A.C.; Wang, L.; Goonetilleke, N.; Fragaszy, E.B.; Bermingham, A.; Copas, A.; Dukes, O.; Millett, E.R.C.; Nazareth, I.; Nguyen-Van-Tam, J.S.; et al. Natural T Cell-mediated Protection against Seasonal and Pandemic Influenza: Results of the Flu Watch Cohort Study. Am. J. Respir. Crit. Care Med. 2015, 191, 1422–1431. [Google Scholar] [CrossRef] [Green Version]
- Grant, E.J.; Josephs, T.M.; Loh, L.; Clemens, E.B.; Sant, S.; Bharadwaj, M.; Chen, W.; Rossjohn, J.; Gras, S.; Kedzierska, K. Broad CD8+ T cell cross-recognition of distinct influenza A strains in humans. Nat. Commun. 2018, 9, 5427. [Google Scholar] [CrossRef] [Green Version]
- Berthoud, T.K.; Hamill, M.; Lillie, P.J.; Hwenda, L.; Collins, K.A.; Ewer, K.J.; Milicic, A.; Poyntz, H.C.; Lambe, T.; Fletcher, H.A.; et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin. Infect. Dis. 2011, 52, 1–7. [Google Scholar] [CrossRef]
- Lillie, P.J.; Berthoud, T.K.; Powell, T.J.; Lambe, T.; Mullarkey, C.; Spencer, A.J.; Hamill, M.; Peng, Y.; Blais, M.-E.; Duncan, C.J.A.; et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin. Infect. Dis. 2012, 55, 19–25. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langenmayer, M.C.; Luelf-Averhoff, A.-T.; Marr, L.; Jany, S.; Freudenstein, A.; Adam-Neumair, S.; Tscherne, A.; Fux, R.; Rojas, J.J.; Blutke, A.; et al. Newly Designed Poxviral Promoters to Improve Immunogenicity and Efficacy of MVA-NP Candidate Vaccines against Lethal Influenza Virus Infection in Mice. Pathogens 2023, 12, 867. https://doi.org/10.3390/pathogens12070867
Langenmayer MC, Luelf-Averhoff A-T, Marr L, Jany S, Freudenstein A, Adam-Neumair S, Tscherne A, Fux R, Rojas JJ, Blutke A, et al. Newly Designed Poxviral Promoters to Improve Immunogenicity and Efficacy of MVA-NP Candidate Vaccines against Lethal Influenza Virus Infection in Mice. Pathogens. 2023; 12(7):867. https://doi.org/10.3390/pathogens12070867
Chicago/Turabian StyleLangenmayer, Martin C., Anna-Theresa Luelf-Averhoff, Lisa Marr, Sylvia Jany, Astrid Freudenstein, Silvia Adam-Neumair, Alina Tscherne, Robert Fux, Juan J. Rojas, Andreas Blutke, and et al. 2023. "Newly Designed Poxviral Promoters to Improve Immunogenicity and Efficacy of MVA-NP Candidate Vaccines against Lethal Influenza Virus Infection in Mice" Pathogens 12, no. 7: 867. https://doi.org/10.3390/pathogens12070867





