Novel Metallo-β-Lactamase blaCVI-1 Isolated from a Chromobaterium violaceum Clinical Strain Resistant to Colistin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification, Susceptibility Testing, and Microbiological Tests and PCRs
2.2. Whole Genome Sequencing (WGS) and Analysis
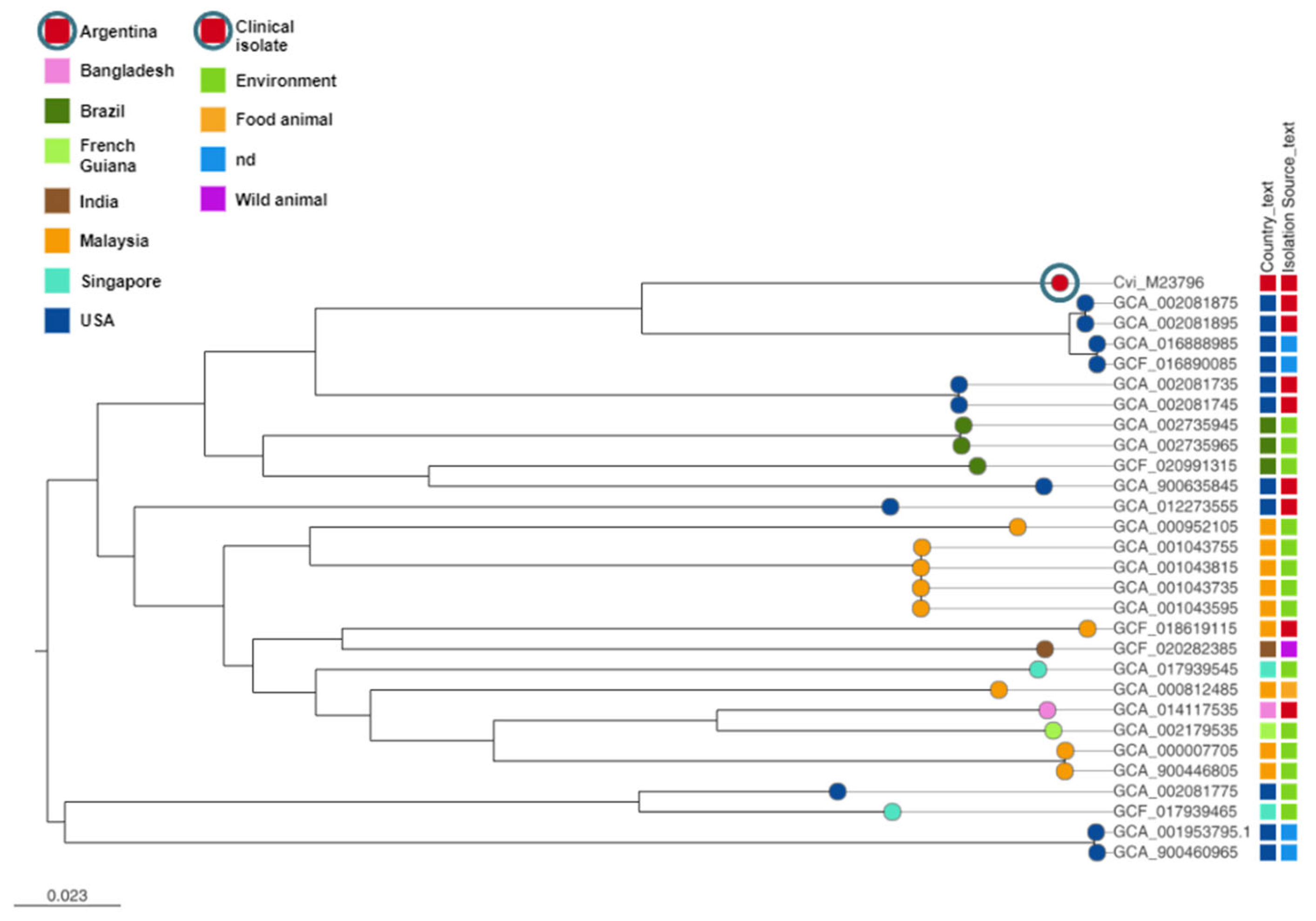
2.3. Phylogenetic Analysis of C. violaceum M23796
2.4. Data Availability
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Justo, G.Z.; Duran, N. Action and function of Chromobacterium violaceum in health and disease: Violacein as a promising metabolite to counteract gastroenterological diseases. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Araripe, J.R.; Rondinelli, E.; Urmenyi, T.P. Gene expression in Chromobacterium violaceum. Genet. Mol. Res. 2004, 3, 64–75. [Google Scholar] [PubMed]
- Brazilian National Genome Project Consortium. The complete genome sequence of Chromobacterium violaceum reveals remarkable and exploitable bacterial adaptability. Proc. Natl. Acad. Sci. USA 2003, 100, 11660–11665. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, K.E.; Valainis, G.T.; Sanders, C.V. Comparison of the in vitro activity of ciprofloxacin and 24 other antimicrobial agents against clinical strains of Chromobacterium violaceum. Diagn. Microbiol. Infect. Dis. 1988, 10, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Fantinatti-Garboggini, F.; Almeida, R.; Portillo Vdo, A.; Barbosa, T.A.; Trevilato, P.B.; Neto, C.E.; Coelho, R.D.; Silva, D.W.; Bartoleti, L.A.; Hanna, E.S.; et al. Drug resistance in Chromobacterium violaceum. Genet. Mol. Res. 2004, 3, 134–147. [Google Scholar] [PubMed]
- Farrar, W.E., Jr.; O’Dell, N.M. beta-Lactamase activity in Chromobacterium violaceum. J. Infect. Dis. 1976, 134, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Krco, S.; Davis, S.J.; Joshi, P.; Wilson, L.A.; Monteiro Pedroso, M.; Douw, A.; Schofield, C.J.; Hugenholtz, P.; Schenk, G.; Morris, M.T. Structure, function, and evolution of metallo-beta-lactamases from the B3 subgroup-emerging targets to combat antibiotic resistance. Front. Chem. 2023, 11, 1196073. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S.C.; Ceraso, D.; Schugurensky, A. First case report from Argentina of fatal septicemia caused by Chromobacterium violaceum. J. Clin. Microbiol. 1986, 23, 956–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2019. [Google Scholar]
- Pasteran, F.; Veliz, O.; Ceriana, P.; Lucero, C.; Rapoport, M.; Albornoz, E.; Gomez, S.; Corso, A.; Re, L.N.G. Evaluation of the Blue-Carba test for rapid detection of carbapenemases in gram-negative bacilli. J. Clin. Microbiol. 2015, 53, 1996–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M.; et al. The PATRIC Bioinformatics Resource Center: Expanding data and analysis capabilities. Nucleic Acids Res. 2020, 48, D606–D612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UniProt, C. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Tonkin-Hill, G.; Lees, J.A.; Bentley, S.D.; Frost, S.D.W.; Corander, J. Fast hierarchical Bayesian analysis of population structure. Nucleic Acids Res. 2019, 47, 5539–5549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argimon, S.; Abudahab, K.; Goater, R.J.E.; Fedosejev, A.; Bhai, J.; Glasner, C.; Feil, E.J.; Holden, M.T.G.; Yeats, C.A.; Grundmann, H.; et al. Microreact: Visualizing and sharing data for genomic epidemiology and phylogeography. Microb. Genom. 2016, 2, e000093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Corallo, T.; Aguirre, C.; Lamberti, D.; Colman, S.; Alvarez, M.; Sucin, G.; Albornoz, M.; Sotelo, O.; Euliarte, C. Septicemia por Chromobacterium violaceum en pediatría. Med. Infant. 2019, XXVI, 276–284. [Google Scholar]
- Hall, B.G.; Salipante, S.J.; Barlow, M. Independent origins of subgroup Bl + B2 and subgroup B3 metallo-beta-lactamases. J. Mol. Evol. 2004, 59, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Breazeale, S.D.; Ribeiro, A.A.; Raetz, C.R. Oxidative decarboxylation of UDP-glucuronic acid in extracts of polymyxin-resistant Escherichia coli. Origin of lipid a species modified with 4-amino-4-deoxy-L-arabinose. J. Biol. Chem. 2002, 277, 2886–2896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trent, M.S.; Ribeiro, A.A.; Lin, S.; Cotter, R.J.; Raetz, C.R. An inner membrane enzyme in Salmonella and Escherichia coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: Induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J. Biol. Chem. 2001, 276, 43122–43131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, J.H.; da Silva Neto, J.F. Chromobacterium violaceum Pathogenicity: Updates and Insights from Genome Sequencing of Novel Chromobacterium Species. Front. Microbiol. 2017, 8, 2213. [Google Scholar] [CrossRef] [Green Version]

| Disc Diffusion | MICs | |||
|---|---|---|---|---|
| Antimicrobial Agent | mm | Int. | µg/mL | Int. |
| Imipenem | 23 | S | 4 * | R |
| Meropenem | 32 | S | ≤1 | S |
| Ertapenem | 29 | S | 0.5 | S |
| Ampicillin | 6 | R | 32 | R |
| Ampicillin/Sulbactam | 11 | R | >16 | R |
| Amoxicillin/Clavulanic Acid | 6 | R | >16 | R |
| Ceftazidime | 22 | S | >16 | R |
| Ceftazidime/Clavulanic Acid | 20 | na | >2 | na |
| Ceftazidme/Avibactam | 30 | S | nd | na |
| Ceftolozano/Tazobactam | 30 | S | nd | na |
| Cefepime | 28 | S | 16 | R |
| Cefotaxime | 21 | R | >16 | R |
| Cefotaxime/Clavulanic acid | 19 | na | >4 | na |
| Cefoxitin | 21 | S | >16 | R |
| Aztreonam | 21 | S | >8 | I |
| Piperacillin/Tazobactam | 29 | S | ≤16 | S |
| Trimethoprime/Sulfamethoxazole | 30 | S | ≤2 | S |
| Amikacin | 20 | S | ≤16 | S |
| Gentamicin | 21 | S | ≤4 | S |
| Ciprofloxacin | 40 | S | ≤1 | S |
| Fosfomycin | 16 | na | ≤64 | na |
| Minocycline | 30 | S | nd | na |
| Tigecycline | 29 | na | ≤1 | na |
| Colistin | 6 | na | >4 | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, S.A.; Sanz, M.B.; Rapoport, M.; Sucin, G.; Corallo, T.A.; Poklepovich, T.; Campos, J.; Ceriana, P.; de Mendieta, J.M.; Prieto, M.; et al. Novel Metallo-β-Lactamase blaCVI-1 Isolated from a Chromobaterium violaceum Clinical Strain Resistant to Colistin. Pathogens 2023, 12, 961. https://doi.org/10.3390/pathogens12070961
Gomez SA, Sanz MB, Rapoport M, Sucin G, Corallo TA, Poklepovich T, Campos J, Ceriana P, de Mendieta JM, Prieto M, et al. Novel Metallo-β-Lactamase blaCVI-1 Isolated from a Chromobaterium violaceum Clinical Strain Resistant to Colistin. Pathogens. 2023; 12(7):961. https://doi.org/10.3390/pathogens12070961
Chicago/Turabian StyleGomez, Sonia A., María Belén Sanz, Melina Rapoport, Graciela Sucin, Teresa A. Corallo, Tomás Poklepovich, Josefina Campos, Paola Ceriana, Juan Manuel de Mendieta, Mónica Prieto, and et al. 2023. "Novel Metallo-β-Lactamase blaCVI-1 Isolated from a Chromobaterium violaceum Clinical Strain Resistant to Colistin" Pathogens 12, no. 7: 961. https://doi.org/10.3390/pathogens12070961





