Additive Effects of Cyclic Peptide [R4W4] When Added Alongside Azithromycin and Rifampicin against Mycobacterium avium Infection
Abstract
:1. Introduction
2. Results
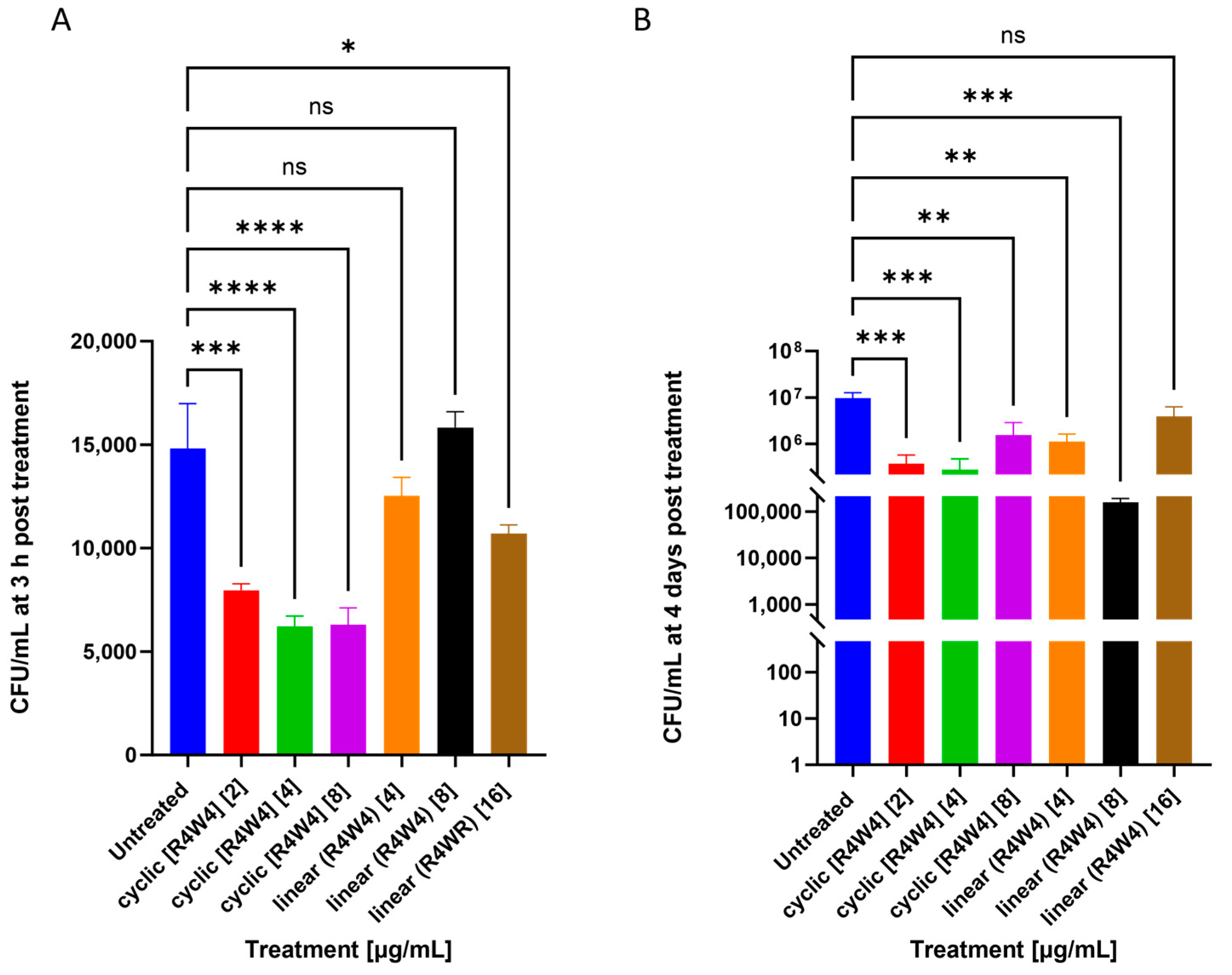
2.1. M. avium Treated with Cyclic Peptide [R4W4] and Linear Peptide (R4W4)
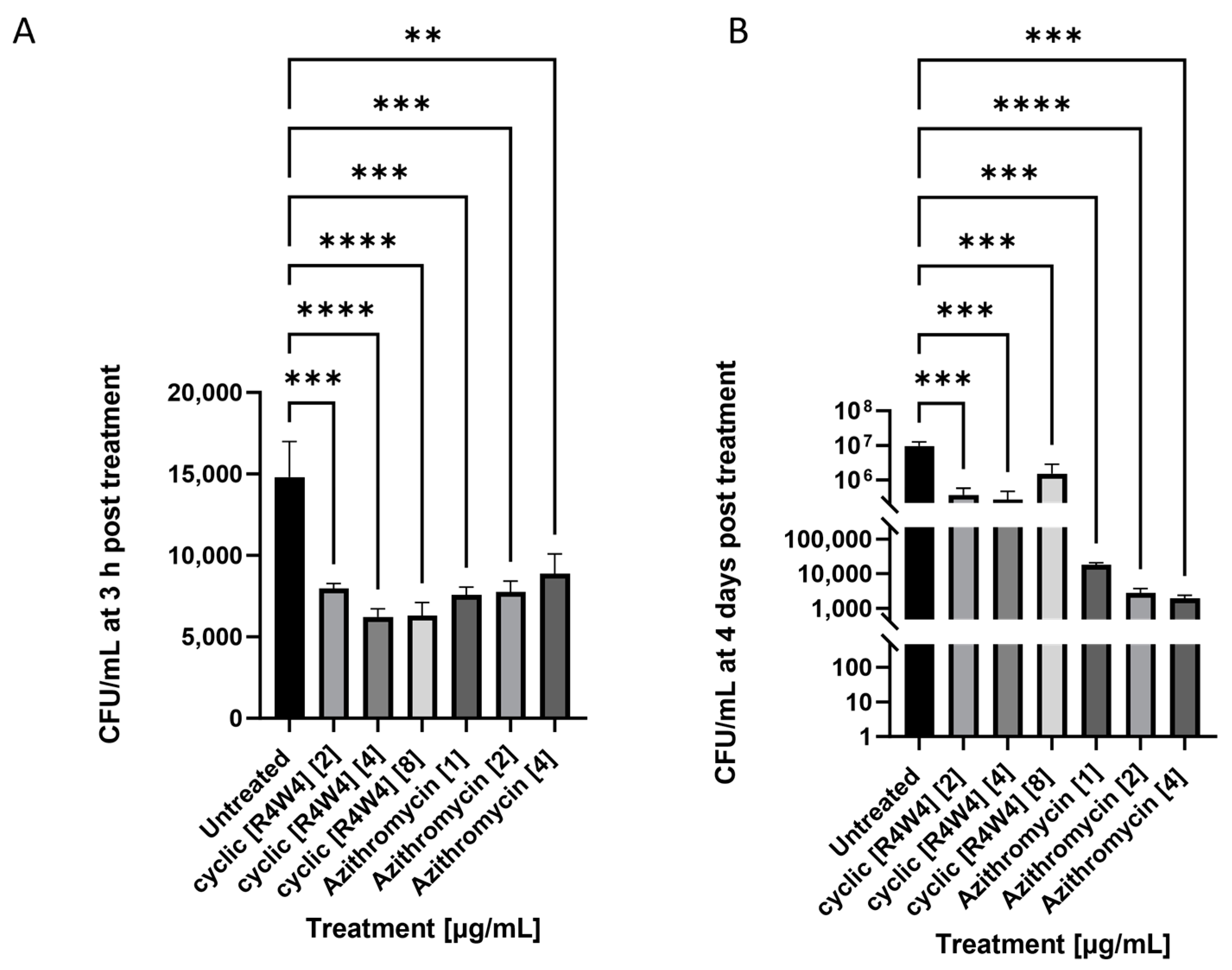
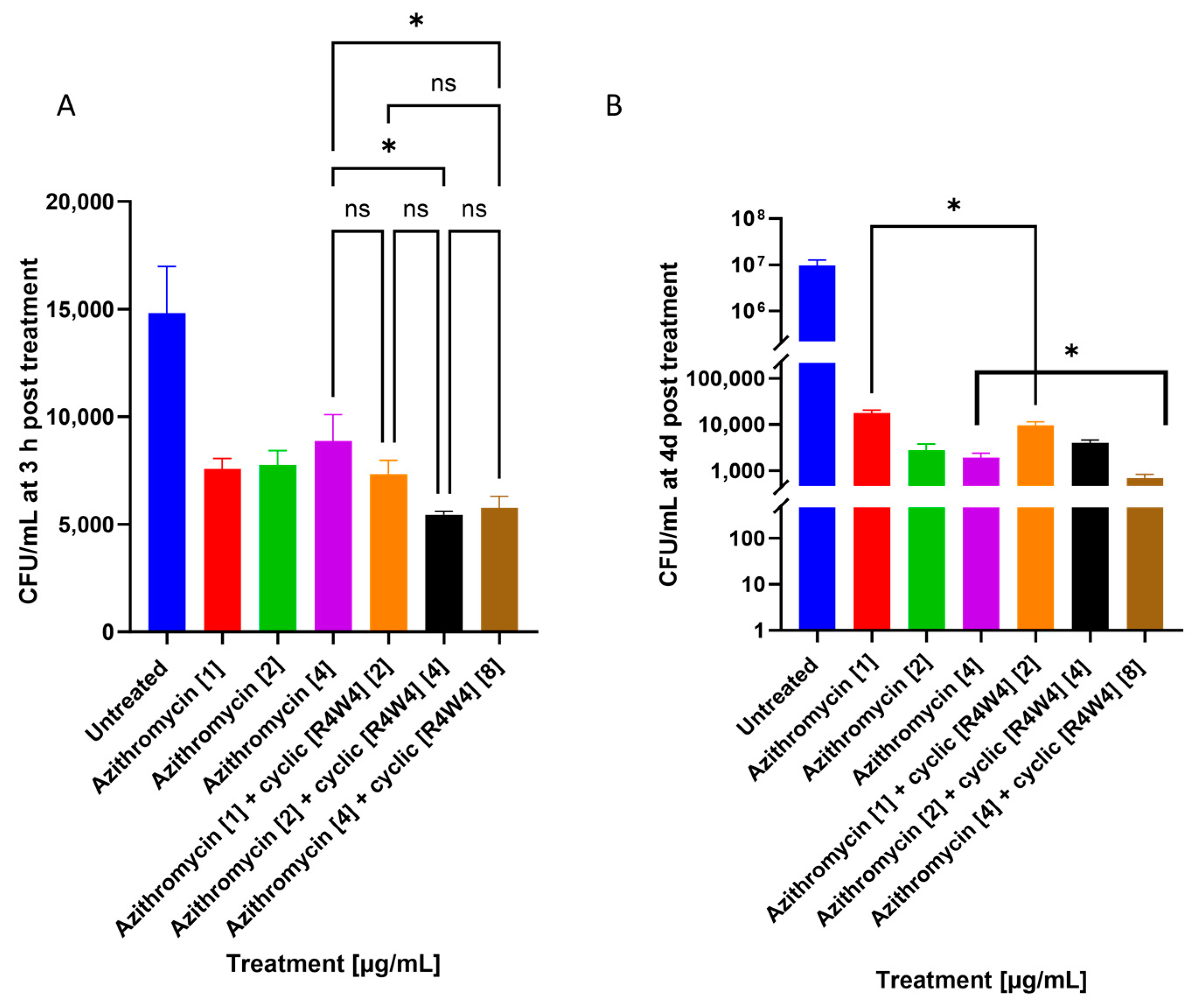
2.2. M. avium Treated with Azithromycin and Cyclic Peptide [R4W4]
2.3. M. avium Treated with Rifampicin and Cyclic Peptide [R4W4]
3. Discussion
4. Materials and Methods
4.1. Bacterial Processing and Preparation
4.2. Bacterial Cell Culture, Antibiotic Treatment, and CFU Counts
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Brode, S.K.; Daley, C.L.; Marras, T.K. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: A systematic review. Int. J. Tuberc. Lung Dis. 2014, 18, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L.; Winthrop, K.L. Mycobacterium avium Complex: Addressing Gaps in Diagnosis and Management. J. Infect. Dis. 2020, 222, S199–S211. [Google Scholar] [CrossRef] [PubMed]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium avium and Mycobacterium abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef] [PubMed]
- Thorel, M.F.; Huchzermeyer, H.F.; Michel, A.L. Mycobacterium avium and Mycobacterium intracellulare infection in mammals. Rev. Sci. Tech. 2001, 20, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Hosseiniporgham, S.; Cubeddu, T.; Rocca, S.; Sechi, L.A. Identification of Mycobacterium avium subsp. paratuberculosis (MAP) in Sheep Milk, a Zoonotic Problem. Microorganisms 2020, 8, 1264. [Google Scholar] [CrossRef]
- Kelley, V.A.; Schorey, J.S. Mycobacterium’s arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Mol. Biol. Cell. 2003, 14, 3366–3377. [Google Scholar] [CrossRef]
- Oh, Y.K.; Straubinger, R.M. Intracellular fate of Mycobacterium avium: Use of dual-label spectrofluorometry to investigate the influence of bacterial viability and opsonization on phagosomal pH and phagosome-lysosome interaction. Infect. Immun. 1996, 64, 319–325. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Young, L.S. Natural killer cell-dependent mycobacteriostatic and mycobactericidal activity in human macrophages. J. Immunol. 1991, 146, 265–270. [Google Scholar] [CrossRef]
- Saunders, B.M.; Cheers, C. Inflammatory response following intranasal infection with Mycobacterium avium complex: Role of T-cell subsets and gamma interferon. Infect. Immun. 1995, 63, 2282–2287. [Google Scholar] [CrossRef]
- Wagner, D.; Sangari, F.J.; Kim, S.; Petrofsky, M.; Bermudez, L.E. Mycobacterium avium infection of macrophages results in progressive suppression of interleukin-12 production in vitro and in vivo. J. Leukoc. Biol. 2002, 71, 80–88. [Google Scholar] [CrossRef]
- Fiogbe, A.A.; Liistro, G.; Hoton, D.; Pieters, T. Mycobacterium avium tumoral infection mimicking a lung adenocarcinoma: A potential diagnostic pitfall. Rev. Pneumol. Clin. 2016, 72, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Matesanz Lopez, C.; Loras Gallego, C.; Cacho Calvo, J.; Thuissard Vasallo, I.J.; Rio Ramirez, M.T. Patients with non-tuberculous mycobacteria in respiratory samples: A 5-year epidemiological study. Rev. Esp. Quimioter. 2021, 34, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Auster, L.; Sutton, M.; Gwin, M.C.; Nitkin, C.; Bonfield, T.L. Optimization of In Vitro Mycobacterium avium and Mycobacterium intracellulare Growth Assays for Therapeutic Development. Microorganisms 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef]
- Munjal, S.; Munjal, S.; Gao, J.; Venketaraman, V. Exploring Potential COPD Immunosuppression Pathways Causing Increased Susceptibility for MAC Infections among COPD Patients. Clin. Pract. 2021, 11, 619–630. [Google Scholar] [CrossRef]
- Akram, S.M.; Attia, F.N. Mycobacterium avium Complex. PubMed. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431110/ (accessed on 27 June 2023).
- Swenson, C.; Zerbe, C.S.; Fennelly, K. Host Variability in NTM Disease: Implications for Research Needs. Front. Microbiol. 2018, 9, 2901. [Google Scholar] [CrossRef]
- Dronamraju, V.; Singh, N.; Poon, J.; Shah, S.; Gorga, J.; Ojeda-Martinez, H.; McFarlane, S. Assessment of Bronchiectasis in HIV Patients among an Urban Population. Case Rep. Pulmonol. 2020, 2020, 8903809. [Google Scholar] [CrossRef]
- Buchacz, K.; Baker, R.K.; Palella, F.J.; Chmiel, J.S.; Lichtenstein, K.A.; Novak, R.M.; Wood, K.C.; Brooks, J.T.; HOPS Investigators. AIDS-defining opportunistic illnesses in US patients, 1994–2007: A cohort study. Aids 2010, 24, 1549–1559. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Koh, W.J.; Daley, C.L. Treatment of Mycobacterium avium Complex Pulmonary Disease. Tuberc. Respir. Dis. 2019, 82, 15–26. [Google Scholar] [CrossRef]
- Sharma, S.K.; Upadhyay, V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J. Med. Res. 2020, 152, 185–226. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline: Executive summary. Clin. Infect. Dis. 2020, 71, e1–e36. [Google Scholar] [CrossRef] [PubMed]
- Buziashvili, M.; Mirtskhulava, V.; Kipiani, M.; Blumberg, H.M.; Baliashvili, D.; Magee, M.J.; Furin, J.J.; Tukvadze, N.; Kempker, R.R. Rates and risk factors for nephrotoxicity and ototoxicity among tuberculosis patients in Tbilisi, Georgia. Int. J. Tuberc. Lung Dis. 2019, 23, 18–1011. [Google Scholar] [CrossRef]
- Kaplan, J.E.; Masur, H.; Holmes, K.K.; USPHS; Infectious Disease Society of America Guidelines for Preventing Opportunistic Infections among HIV-Infected Persons–2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2002, 51, 1–52. [Google Scholar]
- Corti, M.; Palmero, D. Mycobacterium avium complex infection in HIV/AIDS patients. Expert Rev. Anti Infect. Ther. 2008, 6, 351–363. [Google Scholar] [CrossRef]
- Wallace, R.J., Jr.; Brown-Elliott, B.A.; McNulty, S.; Philley, J.V.; Killingley, J.; Wilson, R.W.; York, D.S.; Shepherd, S.; Griffith, D.E. Macrolide/Azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest 2014, 146, 276–282. [Google Scholar] [CrossRef]
- Griffith, D.E.; Brown-Elliott, B.A.; Shepherd, S.; McLarty, J.; Griffith, L.; Wallace, R.J., Jr. Ethambutol ocular toxicity in treatment regimens for Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2005, 72, 250–253. [Google Scholar] [CrossRef]
- Jeong, B.H.; Jeon, K.; Park, H.Y.; Kim, S.Y.; Lee, K.S.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2015, 191, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Pasipanodya, J.G.; Ogbonna, D.; Deshpande, D.; Srivastava, S.; Gumbo, T. Meta-analyses and the evidence base for microbial outcomes in the treatment of pulmonary Mycobacterium avium-intracellulare complex disease. J. Antimicrob. Chemother. 2017, 72, i3–i19. [Google Scholar] [CrossRef]
- Riahifard, N.; Tavakoli, K.; Yamaki, J.; Parang, K.; Tiwari, R. Synthesis and Evaluation of Antimicrobial Activity of [R4W4K]-Levofloxacin and [R4W4K]-Levofloxacin-Q Conjugates. Molecules 2017, 22, 957. [Google Scholar] [CrossRef]
- Joo, S.H. Cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther. 2012, 20, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Findlay, B.; Zhanel, G.G.; Schweizer, F. Cationic amphiphiles, a new generation of antimicrobials inspired by the natural antimicrobial peptide scaffold. Antimicrob. Agents Chemother. 2010, 54, 4049–4058. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Sun, J.; Nasrolahi Shirazi, A.; LaPlante, K.L.; Rowley, D.C.; Parang, K. Antibacterial activities of amphiphilic cyclic cell-penetrating peptides against multidrug-resistant pathogens. Mol. Pharm. 2014, 11, 3528–3536. [Google Scholar] [CrossRef]
- Sajid, M.I.; Lohan, S.; Kato, S.; Tiwari, R.K. Combination of Amphiphilic Cyclic Peptide [R4W4] and Levofloxacin against Multidrug-Resistant Bacteria. Antibiotics 2022, 11, 416. [Google Scholar] [CrossRef]
- Hernandez, J.; Ashley, D.; Cao, R.; Abrahem, R.; Nguyen, T.; To, K.; Yegiazaryan, A.; Akinwale David, A.; Kumar Tiwari, R.; Venketaraman, V. Cyclic Peptide [R4W4] in Improving the Ability of First-Line Antibiotics to Inhibit Mycobacterium tuberculosis Inside in vitro Human Granulomas. Front. Immunol. 2020, 11, 1677. [Google Scholar] [CrossRef]
- Jin, Y.Y.; Kim, J.Y.; Yang, S.H.; Lee, H.; Suh, J.W. Improvement of the productivity of ecumicin, a novel anti-tuberculosis agent, from new Nonomuraea sp. MJM5123. J. Antibiot. 2016, 69, 362–367. [Google Scholar] [CrossRef]
- Gao, W.; Kim, J.Y.; Anderson, J.R.; Akopian, T.; Hong, S.; Jin, Y.Y.; Kandror, O.; Kim, J.W.; Lee, I.A.; Lee, S.Y.; et al. The cyclic peptide ecumicin targeting ClpC1 is active against Mycobacterium tuberculosis in vivo. Antimicrob. Agents Chemother. 2015, 59, 880–889. [Google Scholar] [CrossRef]
- Maurer, M.; Linder, D.; Franke, K.B.; Jäger, J.; Taylor, G.; Gloge, F.; Gremer, S.; Le Breton, L.; Mayer, M.P.; Weber-Ban, E.; et al. Toxic Activation of an AAA+ Protease by the Antibacterial Drug Cyclomarin A. Cell Chem. Biol. 2019, 26, 1169–1179.e4. [Google Scholar] [CrossRef]
- Zhu, S.; Su, Y.; Shams, S.; Feng, Y.; Tong, Y.; Zheng, G. Lassomycin and lariatin lasso peptides as suitable antibiotics for combating mycobacterial infections: Current state of biosynthesis and perspectives for production. Appl. Microbiol. Biotechnol. 2019, 103, 3931–3940. [Google Scholar] [CrossRef]
- Daley, C.L. Mycobacterium avium Complex Disease. Microbiol. Spectr. 2017, 5, 663–701. [Google Scholar] [CrossRef] [PubMed]
- Firth, A.; Prathapan, P. Azithromycin: The First Broad-spectrum Therapeutic. Eur. J. Med. Chem. 2020, 207, 112739. [Google Scholar] [CrossRef] [PubMed]
- Abulfathi, A.A.; Decloedt, E.H.; Svensson, E.M.; Diacon, A.H.; Donald, P.; Reuter, H. Clinical Pharmacokinetics and Pharmacodynamics of Rifampicin in Human Tuberculosis. Clin. Pharmacokinet. 2019, 58, 1103–1129. [Google Scholar] [CrossRef]
- Riahifard, N.; Mozaffari, S.; Aldakhil, T.; Nunez, F.; Alshammari, Q.; Alshammari, S.; Yamaki, J.; Parang, K.; Tiwari, R.K. Design, Synthesis, and Evaluation of Amphiphilic Cyclic and Linear Peptides Composed of Hydrophobic and Positively-Charged Amino Acids as Antibacterial Agents. Molecules 2018, 23, 2722. [Google Scholar] [CrossRef]
- Kwon, B.S.; Kim, M.N.; Sung, H.; Koh, Y.; Kim, W.S.; Song, J.W.; Oh, Y.M.; Lee, S.D.; Lee, S.W.; Lee, J.S.; et al. In Vitro MIC Values of Rifampin and Ethambutol and Treatment Outcome in Mycobacterium avium Complex Lung Disease. Antimicrob. Agents Chemother. 2018, 62, e00491-18. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.E.; Shin, S.J.; Won, C.J.; Min, K.N.; Oh, T.; Hahn, M.Y.; Lee, K.; Lee, S.H.; Daley, C.L.; Kim, S.; et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am. J. Respir. Crit. Care Med. 2012, 186, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Lohan, S.; Moreno, J.; Zoghebi, K.; Tiwari, R.K.; Parang, K. Cyclic and Linear Peptides Containing Alternate WW and RR Residues as Molecular Cargo Delivery Tools. Mol. Pharm. 2023, 20, 341–356. [Google Scholar] [CrossRef]
- Schmid, A.; Wolfensberger, A.; Nemeth, J.; Schreiber, P.W.; Sax, H.; Kuster, S.P. Monotherapy versus Combination Therapy for Multidrug-Resistant Gram-Negative Infections: Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 15290. [Google Scholar] [CrossRef]
- Sattar, A.; Zakaria, Z.; Abu, J.; Aziz, S.A.; Rojas-Ponce, G. Isolation of Mycobacterium avium and other nontuberculous mycobacteria in chickens and captive birds in peninsular Malaysia. BMC Vet. Res. 2021, 17, 13. [Google Scholar] [CrossRef]
- Shin, M.K.; Shin, S.J. Genetic Involvement of Mycobacterium avium Complex in the Regulation and Manipulation of Innate Immune Functions of Host Cells. Int. J. Mol. Sci. 2021, 22, 3011. [Google Scholar] [CrossRef]
- Mendoza-Coronel, E.; Castañón-Arreola, M. Comparative evaluation of in vitro human macrophage models for mycobacterial infection study. Pathog. Dis. 2016, 74, ftw052. [Google Scholar] [CrossRef] [PubMed]
- Early, J.; Fischer, K.; Bermudez, L.E. Mycobacterium Avium Uses Apoptotic Macrophages as Tools for Spreading. Microb. Pathog. 2011, 50, 132–139. [Google Scholar] [CrossRef]
- Torraca, V.; Masud, S.; Spaink, H.P.; Meijer, A.H. Macrophage-Pathogen Interactions in Infectious Diseases: New Therapeutic Insights from the Zebrafish Host Model. Dis. Model. Mech. 2014, 7, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Stapleton, M.; Gadwa, J.; Vongtongsalee, K.; Schenkel, A.R.; Chan, E.D.; Ordway, D. Mycobacterium avium Infection in a C3HeB/FeJ Mouse Model. Front. Microbiol. 2019, 10, 693. [Google Scholar] [CrossRef]
- Henao-Tamayo, M.; Obregón-Henao, A.; Creissen, E.; Shanley, C.; Orme, I.; Ordway, D.J. Differential Mycobacterium bovis BCG vaccine-derived efficacy in C3Heb/FeJ and C3H/HeOuJ mice exposed to a clinical strain of Mycobacterium tuberculosis. Clin. Vaccine Immunol. 2015, 22, 91–98. [Google Scholar] [CrossRef]
- Elguezabal, N.; Bastida, F.; Sevilla, I.A.; González, N.; Molina, E.; Garrido, J.M.; Juste, R.A. Estimation of Mycobacterium avium subsp. paratuberculosis growth parameters: Strain characterization and comparison of methods. Appl. Environ. Microbiol. 2011, 77, 8615–8624. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelley, M.; Sasaninia, K.; Abnousian, A.; Badaoui, A.; Owens, J.; Beever, A.; Kachour, N.; Tiwari, R.K.; Venketaraman, V. Additive Effects of Cyclic Peptide [R4W4] When Added Alongside Azithromycin and Rifampicin against Mycobacterium avium Infection. Pathogens 2023, 12, 1057. https://doi.org/10.3390/pathogens12081057
Kelley M, Sasaninia K, Abnousian A, Badaoui A, Owens J, Beever A, Kachour N, Tiwari RK, Venketaraman V. Additive Effects of Cyclic Peptide [R4W4] When Added Alongside Azithromycin and Rifampicin against Mycobacterium avium Infection. Pathogens. 2023; 12(8):1057. https://doi.org/10.3390/pathogens12081057
Chicago/Turabian StyleKelley, Melissa, Kayvan Sasaninia, Arbi Abnousian, Ali Badaoui, James Owens, Abrianna Beever, Nala Kachour, Rakesh Kumar Tiwari, and Vishwanath Venketaraman. 2023. "Additive Effects of Cyclic Peptide [R4W4] When Added Alongside Azithromycin and Rifampicin against Mycobacterium avium Infection" Pathogens 12, no. 8: 1057. https://doi.org/10.3390/pathogens12081057






