Characterization of Bovine Intraepithelial T Lymphocytes in the Gut
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cattle
2.2. Isolation of Cells from Lymph Nodes and Blood
2.3. T-IEL Isolation
2.4. Antibodies and Reagents
2.5. FACS
2.6. Statistical Analysis
3. Results
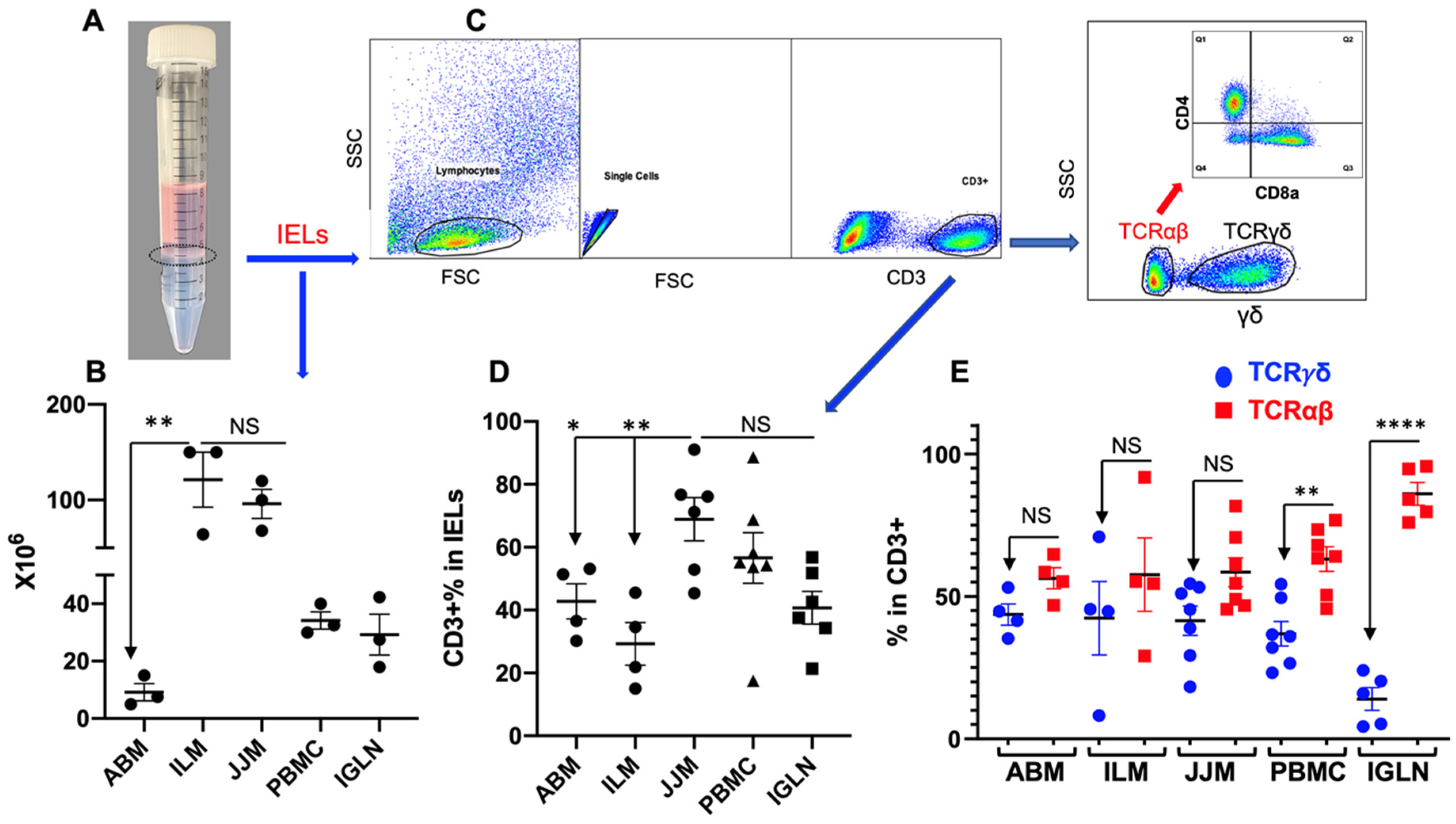
3.1. Similar Levels of TCRγδ+ and TCRαβ+ T Cells in Bovine T-IELs
3.2. TCRγδ+ T-IELs Are Dominantly CD8-Negative
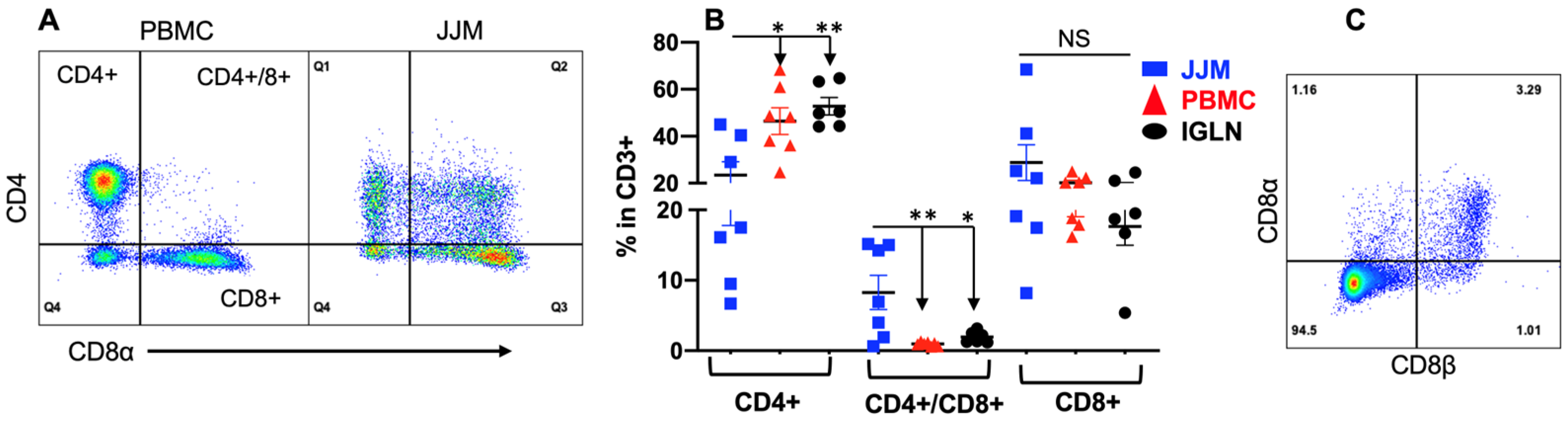
3.3. TCRαβ+CD4+CD8αβ+ T Cells Are Substantial in T-IELs but Not in the Blood and Lymph Nodes
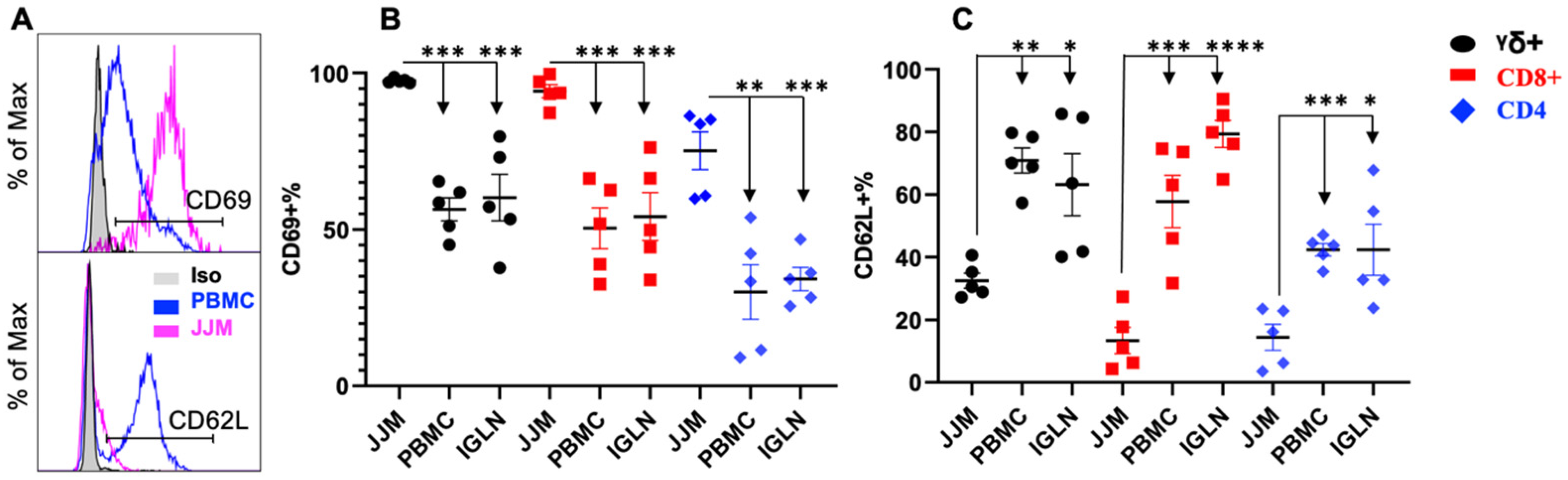
3.4. CD69 Is Highly Expressed in T-IELs, While CD62L Is Expressed at a Lower Level Compared to Their Counterparts in the Blood or Lymph Nodes
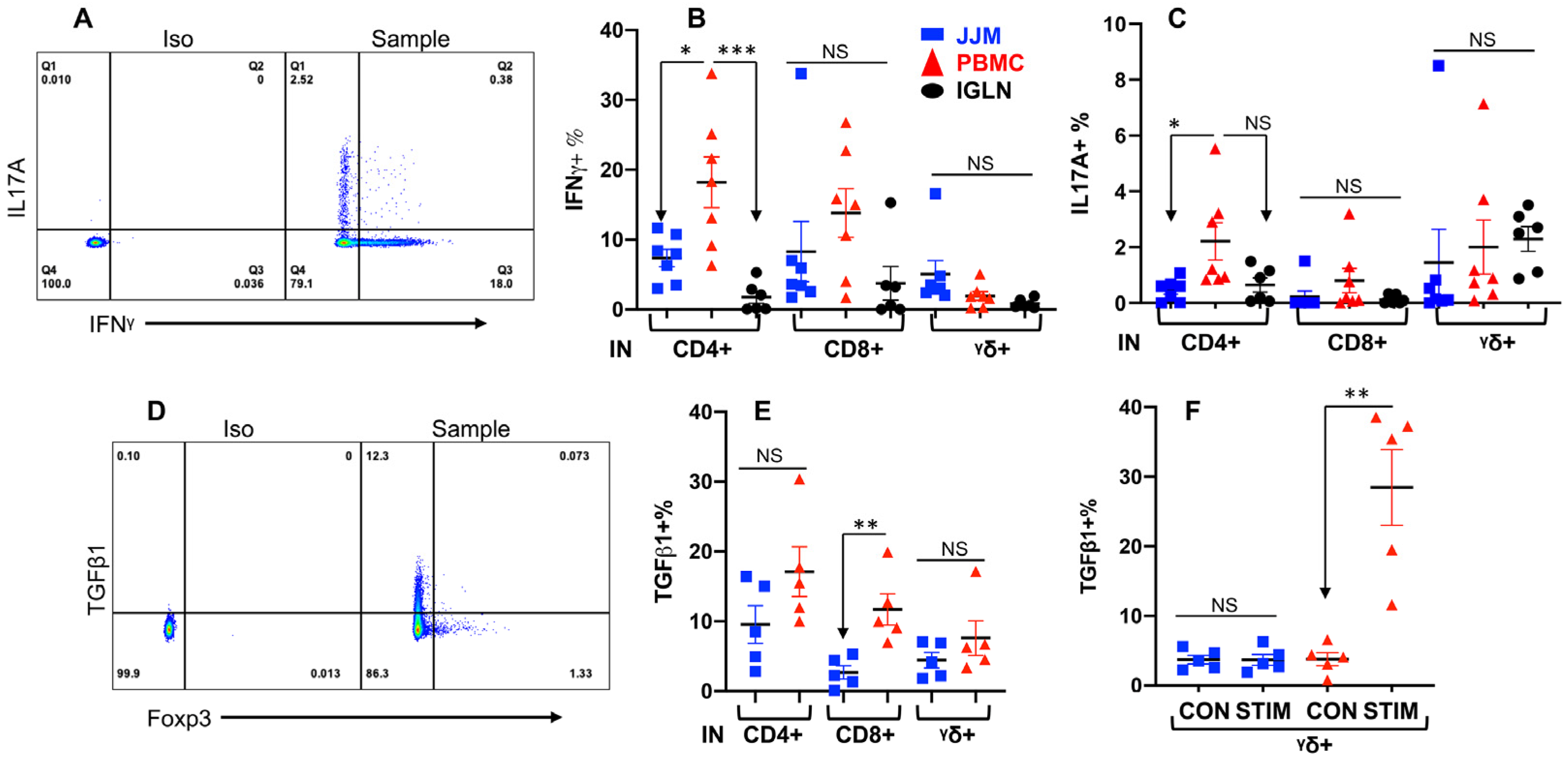
3.5. T-IELs Are Able to Produce Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olivares-Villagómez, D.; Van Kaer, L. Intestinal intraepithelial lymphocytes: Sentinels of the mucosal barrier. Trends Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qiu, Y.; Yang, H. Intestinal intraepithelial lymphocytes: Maintainers of intestinal immune tolerance and regulators of intestinal immunity. J. Leukoc. Biol. 2021, 109, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Edelblum, K.L.; Singh, G.; Odenwald, M.A.; Lingaraju, A.; El Bissati, K.; Mcleod, R.; Sperling, A.I.; Turner, J.R. γδ intraepithelial lymphocyte migration limits transepithelial pathogen invasion and systemic disease in mice. Gastroenterology 2015, 148, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.S.; Severson, K.M.; Vaishnava, S.; Behrendt, C.L.; Yu, X.; Benjamin, J.L.; Ruhn, K.A.; Hou, B.; Defranco, A.L.; Yarovinsky, F.; et al. γδ intraepithelial lymphocytes are essential mediators of host–microbial homeostasis at the intestinal mucosal surface. Proc. Natl. Acad. Sci. USA 2011, 108, 8743–8748. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.S.; Behrendt, C.L.; Hooper, L.V. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J. Immunol. 2009, 182, 3047–3054. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.E.; Cruickshank, S.M.; Egan, C.E.; Mears, R.; Newton, D.J.; Andrew, E.M.; Lawrence, B.; Howell, G.; Else, K.J.; Gubbels, M.J.; et al. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology 2006, 131, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Boismenu, R.; Havran, W.L. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science 1994, 266, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, M.S.; Lancto, C.A.; Walcheck, B.; Layton, W.; Jutila, M.A. Localization of α/β and γ/δ T lymphocytes in cryptosporidium parvum- infected tissues in naive and immune calves. Infect. Immun. 1997, 65, 2428–2433. [Google Scholar] [CrossRef]
- Fayer, R.; Gasbarre, L.; Pasquali, P.; Canals, A.; Almeria, S.; Zarlenga, D. Cryptosporidium parvum infection in bovine neonates: Dynamic clinical, parasitic and immunologic patterns. Int. J. Parasitol. 1998, 28, 49–56. [Google Scholar] [CrossRef]
- Menge, C.; Blessenohl, M.; Eisenberg, T.; Stamm, I.; Baljer, G. Bovine ileal intraepithelial lymphocytes represent target cells for shiga toxin 1 from escherichia coli. Infect. Immun. 2004, 72, 1896–1905. [Google Scholar] [CrossRef]
- Moussay, E.; Stamm, I.; Taubert, A.; Baljer, G.; Menge, C. Escherichia coli shiga toxin 1 enhances il-4 transcripts in bovine ileal intraepithelial lymphocytes. Vet. Immunol. Immunopathol. 2006, 113, 367–382. [Google Scholar] [CrossRef]
- Menge, C.; Stamm, I.; Van Diemen, P.M.; Sopp, P.; Baljer, G.; Wallis, T.S.; Stevens, M.P. Phenotypic and functional characterization of intraepithelial lymphocytes in a bovine ligated intestinal loop model of enterohaemorrhagic escherichia coli infection. J. Med. Microbiol. 2004, 53, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, L.; Egan, R.; Baquero, M.; Mansz, A.; Plattner, B.L. WC1(+) and WC1(neg) γδ T lymphocytes in intestinal mucosa of healthy and mycobacterium avium subspecies paratuberculosis-infected calves. Vet. Immunol. Immunopathol. 2019, 216, 109919. [Google Scholar] [CrossRef] [PubMed]
- Godson, D.L.; Campos, M.; Babiuk, L.A. Non-major histocompatibility complex-restricted cytotoxicity of bovine coronavirus-infected target cells mediated by bovine intestinal intraepithelial leukocytes. J. Gen. Virol. 1991, 72, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Clough, E.R.; Dean, H.J. Isolation and characterization of lymphocytes from bovine intestinal epithelium and lamina propria. Vet. Immunol. Immunopathol. 1988, 19, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, T.; Suzuki, Y.; Mori, K. Intraepithelial γδ T cells are closely associated with apoptotic enterocytes in the bovine intestine. Arch. Histol. Cytol. 1997, 60, 319–328. [Google Scholar] [CrossRef]
- Nagi, A.M.; Babiuk, L.A. Bovine gut-associated lymphoid tissue–morphologic and functional studies. I. Isolation and characterization of leukocytes from the epithelium and lamina propria of bovine small intestine. J. Immunol. Methods 1987, 105, 23–37. [Google Scholar] [CrossRef]
- Ray Waters, W.; Harp, J.A.; Nonnecke, B.J. Phenotypic analysis of peripheral blood lymphocytes and intestinal intra-epithelial lymphocytes in calves. Vet. Immunol. Immunopathol. 1995, 48, 249–259. [Google Scholar] [CrossRef]
- Waters, W.R.; Harp, J.A.; Nonnecke, B.J. In vitro blastogenic responses and interferon-γ production by intestinal intraepithelial lymphocytes of calves. Res. Vet. Sci. 1996, 61, 45–48. [Google Scholar] [CrossRef]
- Wyatt, C.R.; Barrett, W.J.; Brackett, E.J.; Davis, W.C.; Besser, T.E. Phenotypic comparison of ileal intraepithelial lymphocyte populations of suckling and weaned calves. Vet. Immunol. Immunopathol. 1999, 67, 213–222. [Google Scholar] [CrossRef]
- Almería, S.; Canals, A.; Zarlenga, D.S.; Gasbarre, L.C. Isolation and phenotypic characterization of abomasal mucosal lymphocytes in the course of a primary ostertagia ostertagi infection in calves. Vet. Immunol. Immunopathol. 1997, 57, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L.; Olivares-Villagómez, D. Development, homeostasis, and functions of intestinal intraepithelial lymphocytes. J. Immunol. 2018, 200, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Denucci, C.C.; Mitchell, J.S.; Shimizu, Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: Getting there and staying there. Crit. Rev.™ Immunol. 2009, 29, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Edelblum, K.L.; Shen, L.; Weber, C.R.; Marchiando, A.M.; Clay, B.S.; Wang, Y.; Prinz, I.; Malissen, B.; Sperling, A.I.; Turner, J.R. Dynamic migration of γδ intraepithelial lymphocytes requires occludin. Proc. Natl. Acad. Sci. USA 2012, 109, 7097–7102. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.D.; Ethridge, A.D.; Lipstein, R.; Kumar, S.; Wang, Y.; Jabri, B.; Turner, J.R.; Edelblum, K.L. Epithelial IL-15 Is a critical regulator of γδ intraepithelial lymphocyte motility within the intestinal mucosa. J. Immunol. 2018, 201, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Lebrero-Fernández, C.; Bergström, J.H.; Pelaseyed, T.; Bas-Forsberg, A. Murine butyrophilin-like 1 and Btnl6 form heteromeric complexes in small intestinal epithelial cells and promote proliferation of local T lymphocytes. Front. Immunol. 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Acero, L.F.; Zal, T.; Schluns, K.S. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J. Immunol. 2009, 183, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.D.; Golovchenko, N.B.; Burns, G.L.; Nair, P.M.; Kelly, T.J., IV; Agos, J.; Irani, M.Z.; Soh, W.S.; Zeglinski, M.R.; Lemenze, A.; et al. γδ intraepithelial lymphocytes facilitate pathological epithelial cell shedding via CD103-mediated granzyme release. Gastroenterology 2022, 162, 877–889.e7. [Google Scholar] [CrossRef]
- Vantourout, P.; Laing, A.; Woodward, M.J.; Zlatareva, I.; Apolonia, L.; Jones, A.W.; Snijders, A.P.; Malim, M.H.; Hayday, A.C. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology. Proc. Natl. Acad. Sci. USA 2018, 115, 1039–1044. [Google Scholar] [CrossRef]
- Lodolce, J.P.; Boone, D.L.; Chai, S.; Swain, R.E.; Dassopoulos, T.; Trettin, S.; Ma, A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 1998, 9, 669–676. [Google Scholar] [CrossRef]
- Ebert, E.C. Interleukin 15 is a potent stimulant of intraepithelial lymphocytes. Gastroenterology 1998, 115, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Kandel, A.; Li, L. Synergistic activation of bovine CD4+ T cells by neutrophils and IL-12. Pathogens 2021, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Mescher, M.F.; Curtsinger, J.M.; Agarwal, P.; Casey, K.A.; Gerner, M.; Hammerbeck, C.D.; Popescu, F.; Xiao, Z. Signals required for programming effector and memory development by CD8+T cells. Immunol. Rev. 2006, 211, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Masopust, D.; Vezys, V.; Wherry, E.J.; Barber, D.L.; Ahmed, R. Cutting edge: Gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 2006, 176, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, S.; Zhan, Y.; Harrison, L.C. Homeostatic proliferation of intestinal intraepithelial lymphocytes precedes their migration to extra-intestinal sites. Eur. J. Immunol. 2007, 37, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
- Billiet, L.; Goetgeluk, G.; Bonte, S.; De Munter, S.; De Cock, L.; Pille, M.; Ingels, J.; Jansen, H.; Weening, K.; Van Nieuwerburgh, F.; et al. Human thymic CD10+ PD-1+ intraepithelial lymphocyte precursors acquire interleukin-15 responsiveness at the CD1a– CD95+ CD28– CCR7– developmental stage. Int. J. Mol. Sci. 2020, 21, 8785. [Google Scholar] [CrossRef] [PubMed]
- Gorfu, G.; Rivera-Nieves, J.; Ley, K. Role of β7 integrins in intestinal lymphocyte homing and retention. Curr. Mol. Med. 2009, 9, 836–850. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, E. Beta-7 integrin controls enterocyte migration in the small intestine. World J. Gastroenterol. 2015, 21, 1759. [Google Scholar] [CrossRef]
- Svensson, M.; Marsal, J.; Ericsson, A.; Carramolino, L.; Brodén, T.; Márquez, G.; Agace, W.W. CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa. J. Clin. Investig. 2002, 110, 1113–1121. [Google Scholar] [CrossRef]
- McDonald, B.D.; Jabri, B.; Bendelac, A. Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 2018, 18, 514–525. [Google Scholar] [CrossRef]
- Sheridan, B.S.; Lefrançois, L. Intraepithelial lymphocytes: To serve and protect. Curr. Gastroenterol. Rep. 2010, 12, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Cheng, H.; Zhou, J.; Xu, H.; Han, J.; Zhang, D. Development and function of natural TCR+ CD8αα+ intraepithelial lymphocytes. Front. Immunol. 2022, 13, 1059042. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Wang, Y.; Wang, Q.; Li, X.; Bi, Y.; Liu, L.; Wei, X.; Li, T.; Chen, J. Gestational vitamin A deficiency reduces the intestinal immune response by decreasing the number of immune cells in rat offspring. Nutrition 2014, 30, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.C.; Noelle, R.J. Seeing through the dark: New insights into the immune regulatory functions of vitamin A. Eur. J. Immunol. 2015, 45, 1287–1295. [Google Scholar] [CrossRef]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.-Y. retinoic acid imprints gut-homing specificity on T cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Bruce, D.; Cantorna, M.T. Intrinsic requirement for the vitamin D receptor in the development of CD8αα-expressing T cells. J. Immunol. 2011, 186, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, L.; Leyssens, C.; Beullens, I.; Marcelis, S.; Mathieu, C.; De Clercq, P.; Verstuyf, A. The vitamin D analog TX527 ameliorates disease symptoms in a chemically induced model of inflammatory bowel disease. J. Steroid Biochem. Mol. Biol. 2013, 136, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Bruce, D.; Froicu, M.; Weaver, V.; Cantorna, M.T. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc. Natl. Acad. Sci. USA 2008, 105, 20834–20839. [Google Scholar] [CrossRef]
- Chen, L.; Cencioni, M.T.; Angelini, D.F.; Borsellino, G.; Battistini, L.; Brosnan, C.F. Transcriptional profiling of gamma delta T cells identifies a role for vitamin D in the immunoregulation of the V gamma 9V delta 2 response to phosphate-containing ligands. J. Immunol. 2005, 174, 6144–6152. [Google Scholar] [CrossRef]
- Veldhoen, M.; Brucklacher-Waldert, V. Dietary influences on intestinal immunity. Nat. Rev. Immunol. 2012, 12, 696–708. [Google Scholar] [CrossRef]
- Sullivan, Z.A.; Khoury-Hanold, W.; Lim, J.; Smillie, C.; Biton, M.; Reis, B.S.; Zwick, R.K.; Pope, S.D.; Israni-Winger, K.; Parsa, R.; et al. γδ T cells regulate the intestinal response to nutrient sensing. Science 2021, 371, eaba8310. [Google Scholar] [CrossRef] [PubMed]
- James, O.J.; Vandereyken, M.; Marchingo, J.M.; Singh, F.; Bray, S.E.; Wilson, J.; Love, A.G.; Swamy, M. IL-15 and PIM kinases direct the metabolic programming of intestinal intraepithelial lymphocytes. Nat. Commun. 2021, 12, 4290. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Arora, J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, X.; Zeng, B.; Liu, L.; Tardivel, A.; Wei, H.; Han, J.; MacDonald, H.R.; Tschopp, J.; Tian, Z.; et al. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J. Exp. Med. 2013, 210, 2465–2476. [Google Scholar] [CrossRef]
- Liu, L.; Gong, T.; Tao, W.; Lin, B.; Li, C.; Zheng, X.; Zhu, S.; Jiang, W.; Zhou, R. Commensal viruses maintain intestinal intraepithelial lymphocytes via noncanonical RIG-I signaling. Nat. Immunol. 2019, 20, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wu, G.; Alonso, S.; Zhao, C.; Lemenze, A.; Lam, Y.Y.; Zhao, L.; Edelblum, K.L. A transmissible γδ intraepithelial lymphocyte hyperproliferative phenotype is associated with the intestinal microbiota and confers protection against acute infection. Mucosal Immunol. 2022, 15, 772–782. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8αα(+) T cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the Molecular mechanisms underlying the protective effects of microbial SCFAs on intestinal tolerance and food allergy. Front. Immunol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Vandereyken, M.; James, O.J.; Swamy, M. Mechanisms of activation of innate-like intraepithelial T lymphocytes. Mucosal Immunol. 2020, 13, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Sugiyama, M.; Hemmi, H.; Yamazaki, C.; Okura, S.; Sasaki, I.; Fukuda, Y.; Orimo, T.; Ishii, K.J.; Hoshino, K.; et al. Crucial roles of XCR1-expressing dendritic cells and the XCR1-XCL1 chemokine axis in intestinal immune homeostasis. Sci. Rep. 2016, 6, 23505. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Qiu, Y.; Yang, H. CD4CD8αα IELs: They Have Something to Say. Front. Immunol. 2019, 10, 2269. [Google Scholar] [CrossRef] [PubMed]
- Luda, K.M.; Joeris, T.; Persson, E.K.; Rivollier, A.; Demiri, M.; Sitnik, K.M.; Pool, L.; Holm, J.B.; Melo-Gonzalez, F.; Richter, L.; et al. IRF8 Transcription-factor-dependent classical dendritic cells are essential for intestinal T cell homeostasis. Immunity 2016, 44, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Golovchenko, N.; Xu, W.; Galan, M.; Edelblum, K. Loss of γδ intraepithelial lymphocytes and reduced immunosurveillance of the epithelial barrier precedes the onset of Crohn’s disease-like ileitis. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Williams, J.M.; Duckworth, C.A.; Burkitt, M.D.; Watson, A.J.M.; Campbell, B.J.; Pritchard, D.M. Epithelial cell shedding and barrier function. Vet. Pathol. 2015, 52, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Van Kaer, L.; Itohara, S.; Tonegawa, S. Highly restricted expression of the thymus leukemia antigens on intestinal epithelial cells. J. Exp. Med. 1991, 174, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.K.; Glaccum, M.; Brown, S.N.; Butz, E.A.; Viney, J.L.; Embers, M.; Matsuki, N.; Charrier, K.; Sedger, L.; Willis, C.R.; et al. Reversible defects in natural killer and memory Cd8 T cell lineages in interleukin 15–deficient mice. J. Exp. Med. 2000, 191, 771–780. [Google Scholar] [CrossRef]
- Konkel, J.E.; Chen, W. Balancing acts: The role of TGF-β in the mucosal immune system. Trends Mol. Med. 2011, 17, 668–676. [Google Scholar] [CrossRef]
- Probert, C.S.; Saubermann, L.J.; Balk, S.; Blumberg, R.S. Repertoire of the alpha beta T-cell receptor in the intestine. Immunol. Rev. 2007, 215, 215–225. [Google Scholar] [CrossRef]
- Van Kerckhove, C.; Russell, G.J.; Deusch, K.; Reich, K.; Bhan, A.K.; Dersimonian, H.; Brenner, M.B. Oligoclonality of human intestinal intraepithelial T cells. J. Exp. Med. 1992, 175, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jabri, B.; Selby, J.M.; Negulescu, H.; Lee, L.; Roberts, A.I.; Beavis, A.; Lopez-Botet, M.; Ebert, E.C.; Winchester, R.J. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity 2002, 17, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, R.S.; Yockey, C.E.; Gross, G.G.; Ebert, E.C.; Balk, S.P. Human intestinal intraepithelial lymphocytes are derived from a limited number of T cell clones that utilize multiple V beta T cell receptor genes. J. Immunol. 1993, 150, 5144–5153. [Google Scholar] [CrossRef] [PubMed]
- Montufar-Solis, D.; Garza, T.; Klein, J.R. T-cell activation in the intestinal mucosa. Immunol. Rev. 2007, 215, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Shires, J.; Theodoridis, E.; Hayday, A.C. Biological Insights into TCRγδ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE). Immunity 2001, 15, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Melgar, S.; Bas, A.; Hammarström, S.; Hammarström, M.L. Human small intestinal mucosa harbours a small population of cytolytically active CD8+ αβ T lymphocytes. Immunology 2002, 106, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Cheroutre, H. Starting at the beginning: New perspectives on the biology of mucosal T cells. Annu. Rev. Immunol. 2004, 22, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Liu, Y.; Cheng, Y.; Glanville, J.; Zhang, D.; Lee, J.-Y.; Olshen, R.A.; Weyand, C.M.; Boyd, S.D.; Goronzy, J.J. Diversity and clonal selection in the human T-cell repertoire. Proc. Natl. Acad. Sci. USA 2014, 111, 13139–13144. [Google Scholar] [CrossRef]
- Mayassi, T.; Jabri, B. Human intraepithelial lymphocytes. Mucosal Immunol. 2018, 11, 1281–1289. [Google Scholar] [CrossRef]
- Joannou, K.; Baldwin, T.A. Destined for the intestine: Thymic selection of TCRαβ CD8αα intestinal intraepithelial lymphocytes. Clin. Exp. Immunol. 2023, 213, 67–75. [Google Scholar] [CrossRef]
- Bandeira, A.; Mota-Santos, T.; Itohara, S.; Degermann, S.; Heusser, C.; Tonegawa, S.; Coutinho, A. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J. Exp. Med. 1990, 172, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Nanno, M.; Umesaki, Y.; Matsumoto, S.; Okada, Y.; Cai, Z.; Shimamura, T.; Matsuoka, Y.; Ohwaki, M.; Ishikawa, H. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing gamma delta T-cell antigen receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 8591–8594. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.; El Guindy, A.; Panwala, C.M.; Hagan, P.M.; Camerini, V. Differential appearance of T cell subsets in the large and small intestine of neonatal mice. Pediatr. Res. 2001, 49, 543–551. [Google Scholar] [CrossRef]
- Umesaki, Y.; Setoyama, H.; Matsumoto, S.; Okada, Y. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology 1993, 79, 32–37. [Google Scholar]
- Yamagata, T.; Mathis, D.; Benoist, C. Self-reactivity in thymic double-positive cells commits cells to a CD8 alpha alpha lineage with characteristics of innate immune cells. Nat. Immunol. 2004, 5, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Melandri, D.; Zlatareva, I.; Chaleil, R.A.G.; Dart, R.J.; Chancellor, A.; Nussbaumer, O.; Polyakova, O.; Roberts, N.A.; Wesch, D.; Kabelitz, D.; et al. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 2018, 19, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Ruscher, R.; Kummer, R.L.; Lee, Y.J.; Jameson, S.C.; Hogquist, K.A. CD8αα intraepithelial lymphocytes arise from two main thymic precursors. Nat. Immunol. 2017, 18, 771–779. [Google Scholar] [CrossRef]
- Rocha, B.; Vassalli, P.; Guy-Grand, D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J. Exp. Med. 1994, 180, 681–686. [Google Scholar] [CrossRef]
- Golec, D.P.; Hoeppli, R.E.; Henao Caviedes, L.M.; Mccann, J.; Levings, M.K.; Baldwin, T.A. Thymic progenitors of TCRαβ+ CD8αα intestinal intraepithelial lymphocytes require RasGRP1 for development. J. Exp. Med. 2017, 214, 2421–2435. [Google Scholar] [CrossRef]
- Lambolez, F.; Kronenberg, M.; Cheroutre, H. Thymic differentiation of TCRαβ+CD8αα+IELs. Immunol. Rev. 2007, 215, 178–188. [Google Scholar] [CrossRef]
- Wojciech, L.; Szurek, E.; Kuczma, M.; Cebula, A.; Elhefnawy, W.R.; Pietrzak, M.; Rempala, G.; Ignatowicz, L. Non-canonicaly recruited TCRαβCD8αα IELs recognize microbial antigens. Sci. Rep. 2018, 8, 10848. [Google Scholar] [CrossRef] [PubMed]
- Ruscher, R.; Hogquist, K.A. Development, ontogeny, and maintenance of TCRαβ(+) CD8αα IEL. Curr. Opin Immunol. 2019, 58, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Regnault, A.; Levraud, J.-P.; Lim, A.; Six, A.; Moreau, C.; Cumano, A.; Kourilsky, P. The expansion and selection of T cell receptor αβ intestinal intraepithelial T cell clones. Eur. J. Immunol. 1996, 26, 914–921. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.D.; Bunker, J.J.; Ishizuka, I.E.; Jabri, B.; Bendelac, A. Elevated T cell receptor signaling identifies a thymic precursor to the TCRαβ+CD4−CD8β− intraepithelial lymphocyte lineage. Immunity 2014, 41, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, T.; Karunakaran, M.M. Butyrophilins: γδ T cell receptor ligands, immunomodulators and more. Front. Immunol. 2022, 13, 876493. [Google Scholar] [CrossRef] [PubMed]
- Poussier, P.; Ning, T.; Banerjee, D.; Julius, M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J. Exp. Med. 2002, 195, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Denning, T.L.; Granger, S.W.; Mucida, D.; Graddy, R.; Leclercq, G.; Zhang, W.; Honey, K.; Rasmussen, J.P.; Cheroutre, H.; Rudensky, A.Y.; et al. Mouse TCRalphabeta+CD8alphaalpha intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J. Immunol. 2007, 178, 4230–4239. [Google Scholar] [CrossRef]
- Cheroutre, H.; Lambolez, F.; Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 2011, 11, 445–456. [Google Scholar] [CrossRef]
- Roberts, S.J.; Smith, A.L.; West, A.B.; Wen, L.; Findly, R.C.; Owen, M.J.; Hayday, A.C. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc. Natl. Acad. Sci. USA 1996, 93, 11774–11779. [Google Scholar] [CrossRef]
- Inagaki-Ohara, K.; Sakamoto, Y.; Dohi, T.; Smith, A.L. γδ T cells play a protective role during infection with nippostrongylus brasiliensis by promoting goblet cell function in the small intestine. Immunology 2011, 134, 448–458. [Google Scholar] [CrossRef]
- Hu, M.D.; Edelblum, K.L. Sentinels at the frontline: The role of intraepithelial lymphocytes in inflammatory bowel disease. Curr. Pharmacol. Rep. 2017, 3, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Kapp, J.A.; Kapp, L.M.; McKenna, K.C.; Lake, J.P. Gammadelta T-cell clones from intestinal intraepithelial lymphocytes inhibit development of CTL responses ex vivo. Immunology 2004, 111, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Kühl, A.A.; Pawlowski, N.N.; Grollich, K.; Loddenkemper, C.; Zeitz, M.; Hoffmann, J.C. Aggravation of intestinal inflammation by depletion/deficiency of γδ T cells in different types of IBD animal models. J. Leukoc. Biol. 2007, 81, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Fukuda, S.; Hamada, H.; Nakamura, A.; Kohama, Y.; Ishikawa, H.; Tsujikawa, K.; Yamamoto, H. Role of γδT cells in the inflammatory response of experimental colitis mice. J. Immunol. 2003, 171, 5507–5513. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chou, K.; Fuchs, E.; Havran, W.L.; Boismenu, R. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc. Natl. Acad. Sci. USA 2002, 99, 14338–14343. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Hayday, A.C. An αβ T-cell-independent immunoprotective response towards gut coccidia is supported by γδ cells. Immunology 2000, 101, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ladel, C.H.; Blum, C.; Kaufmann, S.H. Control of natural killer cell-mediated innate resistance against the intracellular pathogen Listeria monocytogenes by gamma/delta T lymphocytes. Infect. Immun. 1996, 64, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.J.; Leach, M.W.; Fort, M.M.; Thompson-Snipes, L.; Kühn, R.; Müller, W.; Berg, D.J.; Rennick, D.M. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J. Exp. Med. 1996, 184, 241–251. [Google Scholar] [CrossRef]
- Das, G.; Augustine, M.M.; Das, J.; Bottomly, K.; Ray, P.; Ray, A. An important regulatory role for CD4+CD8αα T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2003, 100, 5324–5329. [Google Scholar] [CrossRef]
- Muller, S.; Buhler-Jungo, M.; Mueller, C. Intestinal intraepithelial lymphocytes exert potent protective cytotoxic activity during an acute virus infection1. J. Immunol. 2000, 164, 1986–1994. [Google Scholar] [CrossRef]
- Lepage, A.C.; Buzoni-Gatel, D.; Bout, D.T.; Kasper, L.H. Gut-derived intraepithelial lymphocytes induce long term immunity against toxoplasma gondii1. J. Immunol. 1998, 161, 4902–4908. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.G.; Yu, L.C.; Buret, A.G. Role of CD8+ and CD4+ T lymphocytes in jejunal mucosal injury during murine giardiasis. Infect Immun 2004, 72, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Keselman, A.; Li, E.; Maloney, J.; Singer, S.M. The microbiota contributes to CD8+ T cell activation and nutrient malabsorption following intestinal infection with giardia duodenalis. Infect. Immun. 2016, 84, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Helgeland, L.; Dissen, E.; Dai, K.-Z.; Midtvedt, T.; Brandtzaeg, P.; Vaage, J.T. Microbial colonization induces oligoclonal expansions of intraepithelial CD8 T cells in the gut. Eur. J. Immunol. 2004, 34, 3389–3400. [Google Scholar] [CrossRef] [PubMed]
- Jabri, B.; De Serre, N.P.M.; Cellier, C.; Evans, K.; Gache, C.; Carvalho, C.; Mougenot, J.F.; Allez, M.; Jian, R.; Desreumaux, P.; et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E–specific natural killer receptor CD94 in celiac disease. Gastroenterology 2000, 118, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Kandel, A.; Masello, M.; Xiao, Z. CD4+ T cell responses to pathogens in cattle. In Bovine Science—Challenges and Advances; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Brown, W.C.; Rice-Ficht, A.C.; Estes, D.M. Bovine type 1 and type 2 responses. Vet. Immunol. Immunopathol. 1998, 63, 45–55. [Google Scholar] [CrossRef]
- Rambault, M.; Doz-Deblauwe, É.; Le Vern, Y.; Carreras, F.; Cunha, P.; Germon, P.; Rainard, P.; Winter, N.; Remot, A. Neutrophils encompass a regulatory subset suppressing T cells in apparently healthy cattle and mice. Front. Immunol. 2021, 12, 625244. [Google Scholar] [CrossRef]
- Baldwin, C.L.; Damani-Yokota, P.; Yirsaw, A.; Loonie, K.; Teixeira, A.F.; Gillespie, A. Special features of γδ T cells in ruminants. Mol. Immunol. 2021, 134, 161–169. [Google Scholar] [CrossRef]
- Kandel, A.; Li, L.; Hada, A.; Xiao, Z. Differential expression of CD45RO and CD45RA in bovine T cells. Cells 2022, 11, 1844. [Google Scholar] [CrossRef]
- Mackay, C.R.; Hein, W.R. A large proportion of bovine T cells express the γδ T cell receptor and show a distinct tissue distribution and surface phenotype. Int. Immunol. 1989, 1, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.N.; VanBuren, D.G.; Hedblom, E.E.; Tilahun, M.E.; Telfer, J.C.; Baldwin, C.L. γδ T cell function varies with the expressed WC1 coreceptor 1. J. Immunol. 2005, 174, 3386–3393. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.C.; Brown, W.C.; Hamilton, M.J.; Wyatt, C.R.; Orden, J.A.; Khalid, A.M.; Naessens, J. Analysis of monoclonal antibodies specific for the γδ TcR. Vet. Immunol. Immunopathol. 1996, 52, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, B.E.; Gasbarre, L.C.; Waite, A.; Bechtol, D.T.; Brown, M.S.; Robinson, N.A.; Olson, E.J.; Newcomb, H. Cooperia punctata: Effect on cattle productivity? Vet. Parasitol. 2012, 183, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Hedges, J.F.; Buckner, D.L.; Rask, K.M.; Kerns, H.M.M.; Jackiw, L.O.; Trunkle, T.C.; Pascual, D.W.; Jutila, M.A. Mucosal lymphatic-derived γδ T cells respond early to experimental salmonella enterocolitis by increasing expression of IL-2Rα. Cell. Immunol. 2007, 246, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.R.; Brackett, E.J.; Perryman, L.E.; Rice-Ficht, A.C.; Brown, W.C.; O’Rourke, K.I. Activation of intestinal intraepithelial T lymphocytes in calves infected with cryptosporidium parvum. Infect. Immun. 1997, 65, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tian, F.; Yu, Y.; Luo, J.; Hu, Q.; Bequette, B.J.; Baldwin VI, R.L.; Liu, G.; Zan, L.; Scott Updike, M.; et al. Muscle transcriptomic analyses in angus cattle with divergent tenderness. Mol. Biol. Rep. 2012, 39, 4185–4193. [Google Scholar] [CrossRef]
- Carrillo, J.A.; He, Y.; Li, Y.; Liu, J.; Erdman, R.A.; Sonstegard, T.S.; Song, J. Integrated metabolomic and transcriptome analyses reveal finishing forage affects metabolic pathways related to beef quality and animal welfare. Sci. Rep. 2016, 6, 25948. [Google Scholar] [CrossRef]
- Li, L.; Si, H.; Wu, S.-W.; Mendez, J.O.; Zarlenga, D.; Tuo, W.; Xiao, Z. Characterization of IL-10-producing neutrophils in cattle infected with ostertagia ostertagi. Sci. Rep. 2019, 9, 20292. [Google Scholar] [CrossRef]
- Mendez, J.; Sun, D.; Tuo, W.; Xiao, Z. Bovine neutrophils form extracellular traps in response to the gastrointestinal parasite ostertagia ostertagi. Sci. Rep. 2018, 8, 17598. [Google Scholar] [CrossRef]
- Anmol, K.; Akanksha, H.; Zhengguo, X. Are CD45RO+ and CD45RA- genuine markers for bovine memory T cells? Anim. Dis. 2022, 2, 23. [Google Scholar] [CrossRef]
- Mandala, W.; Harawa, V.; Munyenyembe, A.; Soko, M.; Longwe, H. Optimization of stimulation and staining conditions for intracellular cytokine staining (ICS) for determination of cytokine-producing T cells and monocytes. Curr. Res. Immunol. 2021, 2, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Li, H.; Song, N.; Li, L.; Chen, H. Optimal method to stimulate cytokine production and its use in immunotoxicity assessment. Int. J. Environ. Res. Public Health 2013, 10, 3834–3842. [Google Scholar] [CrossRef]
- Nylander, S.; Kalies, I. Brefeldin A, but not monensin, completely blocks CD69 expression on mouse lymphocytes: Efficacy of inhibitors of protein secretion in protocols for intracellular cytokine staining by flow cytometry. J. Immunol. Methods 1999, 224, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Zeller, J.; Bogner, B.; Hörbrand, I.A.; Lang, F.; Deiss, E.; Winninger, O.; Fricke, M.; Kreuzaler, S.; Smudde, E.; et al. An unbiased flow cytometry-based approach to assess subset-specific circulating monocyte activation and cytokine profile in whole blood. Front. Immunol. 2021, 12, 641224. [Google Scholar] [CrossRef] [PubMed]
- Ellmeier, W.; Sunshine, M.J.; Losos, K.; Littman, D.R. Multiple developmental stage–specific enhancers regulate CD8 Expression in developing thymocytes and in thymus-independent T cells. Immunity 1998, 9, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Janeway, C.A., Jr. Development of CD8α/α and CD8α/β T cells in major histocompatibility complex class I–deficient mice. J. Exp. Med. 1999, 190, 881–884. [Google Scholar] [CrossRef]
- Guzman, E.; Hope, J.; Taylor, G.; Smith, A.L.; Cubillos-Zapata, C.; Charleston, B. Bovine gammadelta T cells are a major regulatory T cell subset. J. Immunol. 2014, 193, 208–222. [Google Scholar] [CrossRef]
- Hoek, A.; Rutten, V.P.M.G.; Kool, J.; Arkesteijn, G.J.A.; Bouwstra, R.J.; Van Rhijn, I.; Koets, A.P. Subpopulations of bovine WC1+γδ T cells rather than CD4+CD25highFoxp3+T cells act as immune regulatory cells ex vivo. Vet. Res. 2009, 40, 06. [Google Scholar] [CrossRef]
- Rhodes, S.G.; Hewinson, R.G.; Vordermeier, H.M. Antigen Recognition and Immunomodulation by γδ T Cells in Bovine Tuberculosis. J. Immunol. 2001, 166, 5604–5610. [Google Scholar] [CrossRef]
- Albarrak, S.M.; Waters, W.R.; Stabel, J.R.; Hostetter, J.M. Evaluating the cytokine profile of the WC1+ γδ T cell subset in the ileum of cattle with the subclinical and clinical forms of MAP infection. Vet. Immunol. Immunopathol. 2018, 201, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.L.; Telfer, J.C. The bovine model for elucidating the role of γδ T cells in controlling infectious diseases of importance to cattle and humans. Mol. Immunol. 2015, 66, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.L.; Yirsaw, A.; Gillespie, A.; Le Page, L.; Zhang, F.; Damani-Yokota, P.; Telfer, J.C. γδ T cells in livestock: Responses to pathogens and vaccine potential. Transbound. Emerg. Dis. 2020, 67, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Bilate, A.M.; London, M.; Castro, T.B.R.; Mesin, L.; Bortolatto, J.; Kongthong, S.; Harnagel, A.; Victora, G.D.; Mucida, D. T Cell receptor is required for differentiation, but not maintenance, of intestinal CD4(+) intraepithelial lymphocytes. Immunity 2020, 53, 1001–1014.e20. [Google Scholar] [CrossRef] [PubMed]
- Sujino, T.; London, M.; Hoytema van Konijnenburg, D.P.; Rendon, T.; Buch, T.; Silva, H.M.; Lafaille, J.J.; Reis, B.S.; Mucida, D. Tissue adaptation of regulatory and intraepithelial CD4+ T cells controls gut inflammation. Science 2016, 352, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Mizoguchi, E.; Allen, D.; Bhan, A.K.; Terhorst, C. Evidence that CD4+, but not CD8+ T cells are responsible for murine interleukin-2-deficient colitis. Eur. J. Immunol. 1995, 25, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Pahar, B.; Lackner, A.A.; Veazey, R.S. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur. J. Immunol. 2006, 36, 583–592. [Google Scholar] [CrossRef]
- Mackay, L.K.; Braun, A.; Macleod, B.L.; Collins, N.; Tebartz, C.; Bedoui, S.; Carbone, F.R.; Gebhardt, T. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 2015, 194, 2059–2063. [Google Scholar] [CrossRef]
- Radulovic, K.; Niess, J.H. CD69 is the crucial regulator of intestinal inflammation: A new target molecule for IBD treatment? J. Immunol. Res. 2015, 2015, 497056. [Google Scholar] [CrossRef]
- Zheng, M.Z.M.; Wakim, L.M. Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 2022, 15, 379–388. [Google Scholar] [CrossRef]
- Li, X.; Garcia, K.; Sun, Z.; Xiao, Z. Temporal regulation of rapamycin on memory CTL programming by IL-12. PLoS ONE 2011, 6, e25177. [Google Scholar] [CrossRef] [PubMed]
- Mescher, M.F.; Agarwal, P.; Casey, K.A.; Hammerbeck, C.D.; Xiao, Z.; Curtsinger, J.M. Molecular basis for checkpoints in the CD8 T cell response: Tolerance versus activation. Semin. Immunol. 2007, 19, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Lauvau, G.; Soudja, S.M. Mechanisms of Memory T Cell Activation and Effective Immunity. Adv. Exp. Med. Biol. 2015, 850, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, G.; Karjalainen, K.; Lanzavecchia, A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 1998, 8, 89–95. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, Y.; Xu, Y. Follicular regulatory T cells infiltrated the ovarian carcinoma and resulted in CD8 T cell dysfunction dependent on IL-10 pathway. Int. Immunopharmacol. 2019, 68, 81–87. [Google Scholar] [CrossRef]
- Liu, Y.; Rhoads, J.M. How does metabolism of an “immuno acid”(tryptophan) by commensal Lactobacillus reuteri educate resident intestinal intraepithelial lymphocytes? J. Lab. Precis. Med. 2018, 3, 46. [Google Scholar] [CrossRef]
- Pai, M.H.; Liu, J.J.; Yeh, S.L.; Chen, W.J.; Yeh, C.L. Glutamine modulates acute dextran sulphate sodium-induced changes in small-intestinal intraepithelial γδ-T-lymphocyte expression in mice. Br. J. Nutr. 2014, 111, 1032–1039. [Google Scholar] [CrossRef]
- Tung, J.N.; Lee, W.Y.; Pai, M.H.; Chen, W.J.; Yeh, C.L.; Yeh, S.L. Glutamine modulates CD8αα(+) TCRαβ(+) intestinal intraepithelial lymphocyte expression in mice with polymicrobial sepsis. Nutrition 2013, 29, 911–917. [Google Scholar] [CrossRef]
- Horio, Y.; Osawa, S.; Takagaki, K.; Hishida, A.; Furuta, T.; Ikuma, M. Glutamine supplementation increases Th1-cytokine responses in murine intestinal intraepithelial lymphocytes. Cytokine 2008, 44, 92–95. [Google Scholar] [CrossRef]
- Ishizuka, S.; Tanaka, S. Modulation of CD8+ intraepithelial lymphocyte distribution by dietary fiber in the rat large intestine1. Exp. Biol. Med. 2002, 227, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Robles, E.F.; Vázquez, V.P.; Emiliano, J.R.; Amaro, R.G.; Briones, S.L. High fat diet induces alterations to intraepithelial lymphocyte and cytokine mRNA in the small intestine of C57BL/6 mice. RSC Adv. 2017, 7, 5322–5330. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Ma, H.; Lu, B.; Wang, J.; Li, Y.; Li, J. Inhibitory effect of dietary n-3 polyunsaturated fatty acids to intestinal IL-15 expression is associated with reduction of TCRαβ+CD8α+CD8β− intestinal intraepithelial lymphocytes. J. Nutr. Biochem. 2008, 19, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Ferreira, C. Influence of nutrient-derived metabolites on lymphocyte immunity. Nat. Med. 2015, 21, 709–718. [Google Scholar] [CrossRef]
- Chen, W.; Pu, A.; Sheng, B.; Zhang, Z.; Li, L.; Liu, Z.; Wang, Q.; Li, X.; Ma, Y.; Yu, M.; et al. Aryl hydrocarbon receptor activation modulates CD8αα(+)TCRαβ(+) IELs and suppression of colitis manifestations in mice. Biomed. Pharm. 2017, 87, 127–134. [Google Scholar] [CrossRef]
- Pinto, C.J.G.; Ávila-Gálvez, M.Á.; Lian, Y.; Moura-Alves, P.; Nunes dos Santos, C. Targeting the aryl hydrocarbon receptor by gut phenolic metabolites: A strategy towards gut inflammation. Redox Biol. 2023, 61, 102622. [Google Scholar] [CrossRef]
- Li, Y.; Innocentin, S.; Withers, D.R.; Roberts, N.A.; Gallagher, A.R.; Grigorieva, E.F.; Wilhelm, C.; Veldhoen, M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 2011, 147, 629–640. [Google Scholar] [CrossRef]
- Zhang, Z.; Pu, A.; Yu, M.; Xiao, W.; Sun, L.; Cai, Y.; Yang, H. Aryl hydrocarbon receptor activation modulates γδ intestinal intraepithelial lymphocytes and protects against ischemia/reperfusion injury in the murine small intestine. Mol. Med. Rep. 2019, 19, 1840–1848. [Google Scholar] [CrossRef]
- Nagafuchi, S.; Totsuka, M.; Hachimura, S.; Goto, M.; Takahashi, T.; Yajima, T.; Kuwata, T.; Kaminogawa, S. Dietary nucleotides increase the proportion of a tcrγδ+ Subset of intraepithelial lymphocytes (iel) and il-7 production by intestinal epithelial cells (iec); implications for modification of cellular and molecular cross-talk between iel and iec by dietary nucleotides. Biosci. Biotechnol. Biochem. 2000, 64, 1459–1465. [Google Scholar] [CrossRef]
- Minton, K. Negative regulation of AHR essential for intestinal homeostasis. Nat. Rev. Immunol. 2023, 23, 202. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.R.; Brackett, E.J.; Perryman, L.E.; Davis, W.C. Identification of γδT lymphocyte subsets that populate calf ileal mucosa after birth. Vet. Immunol. Immunopathol. 1996, 52, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Ogawa, D.; Nasu, T.; Yamaguchi, T.; Murakami, T. Kinetics and distribution of bovine γδ T-lymphocyte in the intestine: γδ T cells accumulate in the dome region of Peyer’s patch during prenatal development. Dev. Comp. Immunol. 2005, 29, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Sakaguchi, S.; Tenno, M.; Kopf, A.; Boucheron, N.; Carpenter, A.C.; Egawa, T.; Taniuchi, I.; Ellmeier, W. Cd8 enhancer E8I and Runx factors regulate CD8α expression in activated CD8+ T cells. Proc. Natl. Acad. Sci. USA 2011, 108, 18330–18335. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.; Marrinan, E.; Muenchhoff, M.; Fergusson, J.; Kloverpris, H.; Cheroutre, H.; Barnes, E.; Goulder, P.; Klenerman, P. CD8αα expression marks terminally differentiated human CD8+ T cells expanded in chronic viral infection. Front. Immunol. 2013, 4, 223. [Google Scholar] [CrossRef] [PubMed]
- Cheroutre, H.; Lambolez, F. Doubting the TCR coreceptor function of CD8αα. Immunity 2008, 28, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Hedges, J.F.; Butcher, E.C.; Briskin, M.; Jutila, M.A. Bovine γδ T cell subsets express distinct patterns of chemokine responsiveness and adhesion molecules: A mechanism for tissue-specific γδ T cell subset accumulation1. J. Immunol. 2002, 169, 4970–4975. [Google Scholar] [CrossRef]
- Costes, L.M.M.; Lindenbergh-Kortleve, D.J.; Van Berkel, L.A.; Veenbergen, S.; Raatgeep, H.C.; Simons-Oosterhuis, Y.; Van Haaften, D.H.; Karrich, J.J.; Escher, J.C.; Groeneweg, M.; et al. IL-10 signaling prevents gluten-dependent intraepithelial CD4+ cytotoxic T lymphocyte infiltration and epithelial damage in the small intestine. Mucosal Immunol. 2019, 12, 479–490. [Google Scholar] [CrossRef]
- Reis, B.S.; Rogoz, A.; Costa-Pinto, F.A.; Taniuchi, I.; Mucida, D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat. Immunol. 2013, 14, 271–280. [Google Scholar] [CrossRef]
- Konkel, J.E.; Maruyama, T.; Carpenter, A.C.; Xiong, Y.; Zamarron, B.F.; Hall, B.E.; Kulkarni, A.B.; Zhang, P.; Bosselut, R.; Chen, W. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat. Immunol. 2011, 12, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.S.; van Konijnenburg, D.P.H.; Grivennikov, S.I.; Mucida, D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity 2014, 41, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Carton, J.; Byrne, B.; Madrigal-Estebas, L.; O’Donoghue, D.P.; O’Farrelly, C. CD4+CD8+ human small intestinal T cells are decreased in coeliac patients, with CD8 expression downregulated on intra-epithelial T cells in the active disease. Eur. J. Gastroenterol. Hepatol. 2004, 16, 961–968. [Google Scholar] [CrossRef] [PubMed]
- London, M.; Bilate, A.M.; Castro, T.B.R.; Sujino, T.; Mucida, D. Stepwise chromatin and transcriptional acquisition of an intraepithelial lymphocyte program. Nat. Immunol. 2021, 22, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, Z.A.; Rahman, M.A.; Maradana, M.R.; Mehdi, A.M.; Bergot, A.-S.; Simone, D.; El-Kurdi, M.; Garrido-Mesa, J.; Cai, C.B.B.; Cameron, A.J.; et al. Genetically encoded Runx3 and CD4+ intestinal epithelial lymphocyte deficiencies link SKG mouse and human predisposition to spondyloarthropathy. Clin. Immunol. 2023, 247, 109220. [Google Scholar] [CrossRef] [PubMed]
- Germain, R.N. T-cell development and the CD4–CD8 lineage decision. Nat. Rev. Immunol. 2002, 2, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Bankovich, A.J.; Shiow, L.R.; Cyster, J.G. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 2010, 285, 22328–22337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Xiang, H.; Liang, Z.; Lu, H. Role of sphingosine-1-phosphate receptors in vascular injury of inflammatory bowel disease. J. Cell. Mol. Med. 2021, 25, 2740–2749. [Google Scholar] [CrossRef]
- Ji, T.; Xu, C.; Sun, L.; Yu, M.; Peng, K.; Qiu, Y.; Xiao, W.; Yang, H. Aryl hydrocarbon receptor activation down-regulates IL-7 and reduces inflammation in a mouse model of dss-induced colitis. Dig. Dis. Sci. 2015, 60, 1958–1966. [Google Scholar] [CrossRef]
- Grailer, J.J.; Kodera, M.; Steeber, D.A. L-selectin: Role in regulating homeostasis and cutaneous inflammation. J. Dermatol. Sci. 2009, 56, 141–147. [Google Scholar] [CrossRef]
- La Scaleia, R.; Barba, M.; Di Nardo, G.; Bonamico, M.; Oliva, S.; Nenna, R.; Valitutti, F.; Mennini, M.; Barbato, M.; Montuori, M.; et al. Size and dynamics of mucosal and peripheral IL-17A+ T-cell pools in pediatric age, and their disturbance in celiac disease. Mucosal Immunol. 2012, 5, 513–523. [Google Scholar] [CrossRef]
- Paroni, M.; Magarotto, A.; Tartari, S.; Nizzoli, G.; Larghi, P.; Ercoli, G.; Gianelli, U.; Pagani, M.; Elli, L.; Abrignani, S.; et al. Uncontrolled IL-17 production by intraepithelial lymphocytes in a case of non-IPEX Autoimmune enteropathy. Clin. Transl. Gastroenterol. 2016, 7, e182. [Google Scholar] [CrossRef] [PubMed]
- Ruberti, M.; Fernandes, L.G.R.; Simioni, P.U.; Gabriel, D.L.; Yamada, A.T.; Tamashiro, W.M.d.S.C. Phenotypical and functional analysis of intraepithelial lymphocytes from small intestine of mice in oral tolerance. J. Immunol. Res. 2012, 2012, 208054. [Google Scholar]
- Monteleone, I.; Sarra, M.; Del Vecchio Blanco, G.; Paoluzi, O.A.; Franzè, E.; Fina, D.; Fabrizi, A.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Characterization of IL-17A-producing cells in celiac disease mucosa. J. Immunol. 2010, 184, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, C.; Melgar, S.; Yeung, M.M.; Hammarström, S.; Hammarström, M.L. Intraepithelial lymphocytes in human gut have lytic potential and a cytokine profile that suggest T helper 1 and cytotoxic functions. J. Immunol. 1996, 157, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Wilharm, A.; Tabib, Y.; Nassar, M.; Reinhardt, A.; Mizraji, G.; Sandrock, I.; Heyman, O.; Barros-Martins, J.; Aizenbud, Y.; Khalaileh, A.; et al. Mutual interplay between IL-17–producing γδT cells and microbiota orchestrates oral mucosal homeostasis. Proc. Natl. Acad. Sci. USA 2019, 116, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kiristioglu, I.; Fan, Y.; Forbush, B.; Bishop, D.K.; Antony, P.A.; Zhou, H.; Teitelbaum, D.H. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann. Surg. 2002, 236, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.A.; Gajewski, T.F.; Danielpour, D.; Chang, E.B.; Beagley, K.W.; Bluestone, J.A. Differential function of intestinal intraepithelial lymphocyte subsets. J. Immunol. 1992, 149, 1124–1130. [Google Scholar] [CrossRef]
- Mennechet, F.J.D.; Kasper, L.H.; Rachinel, N.; Minns, L.A.; Luangsay, S.; Vandewalle, A.; Buzoni-Gatel, D. Intestinal intraepithelial lymphocytes prevent pathogen-driven inflammation and regulate the Smad/T-bet pathway of lamina propria CD4+ T cells. Eur. J. Immunol. 2004, 34, 1059–1067. [Google Scholar] [CrossRef]
- Howe, K.L.; Reardon, C.; Wang, A.; Nazli, A.; Mckay, D.M. Transforming growth factor-β regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic escherichia coli O157:H7-induced increased permeability. Am. J. Pathol. 2005, 167, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Ihara, S.; Hirata, Y.; Serizawa, T.; Suzuki, N.; Sakitani, K.; Kinoshita, H.; Hayakawa, Y.; Nakagawa, H.; Ijichi, H.; Tateishi, K.; et al. TGF-β signaling in dendritic cells governs colonic homeostasis by controlling epithelial differentiation and the luminal microbiota. J. Immunol. 2016, 196, 4603–4613. [Google Scholar] [CrossRef]
- Li, G.; Ren, J.; Hu, Q.; Deng, Y.; Chen, G.; Guo, K.; Li, R.; Li, Y.; Wu, L.; Wang, G.; et al. Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-β signaling in a murine colitis model. Biochem. Pharm. 2016, 117, 57–67. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hada, A.; Li, L.; Kandel, A.; Jin, Y.; Xiao, Z. Characterization of Bovine Intraepithelial T Lymphocytes in the Gut. Pathogens 2023, 12, 1173. https://doi.org/10.3390/pathogens12091173
Hada A, Li L, Kandel A, Jin Y, Xiao Z. Characterization of Bovine Intraepithelial T Lymphocytes in the Gut. Pathogens. 2023; 12(9):1173. https://doi.org/10.3390/pathogens12091173
Chicago/Turabian StyleHada, Akanksha, Lei Li, Anmol Kandel, Younggeon Jin, and Zhengguo Xiao. 2023. "Characterization of Bovine Intraepithelial T Lymphocytes in the Gut" Pathogens 12, no. 9: 1173. https://doi.org/10.3390/pathogens12091173









