The Efficiency of TB LAM Antigen Test to Xpert MTB/RIF Ultra Test for the Diagnosis of Tuberculous Pericarditis Using Pericardial Fluid Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Collection, Handling, and Analysis
2.2.1. Culture
2.2.2. Xpert MTB/RIF Ultra Test (Cepheid, Sunnyvale, CA, USA)
2.2.3. TB LAM Ag Test (Alere Determined TB LAM Ag test, Abbott Laboratories, Chicago, IL, USA)
Statistical Analysis
3. Results
3.1. Interpretation of Results
3.1.1. Characteristics of the Study Population
3.1.2. TBP Detection Rates
The Detection Rate of PCF Xpert MTB/RIF Ultra in Comparison to PCF Culture
The Detection Rate of PCF TB LAM Ag in Comparison to PCF Culture
The Detection Rate of PCF TB LAM Ag in Comparison to PCF Xpert MTB/RIF Ultra
3.1.3. Diagnostic Efficiency
Xpert MTB/RIF Ultra Test
TB LAM Ag Test
Combined Results from TB LAM Ag and Xpert MTB/RIF Ultra Assays
Comparison of Diagnostic Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Tuberculosis Fact Sheet; World Health Organisation: Geneva, Switzerland, 2022. [Google Scholar]
- Yates, T.A.; Khan, P.Y.; Knight, G.M.; Taylor, J.G.; McHugh, T.D.; Lipman, M.; White, R.G.; Cohen, T.; Cobelens, F.G.; Wood, R.; et al. The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect. Dis. 2016, 16, 227–238. [Google Scholar] [CrossRef] [PubMed]
- WHO. Tuberculosis Fact Sheet; World Health Organisation: Geneva, Switzerland, 2023. [Google Scholar]
- WHO. Global Tuberculosis Report 2022; World Health Organisation: Geneva, Switzerland, 2022. [Google Scholar]
- United Nations Development Programme. Sustainable Development Agenda-Sustainable Development Goals by 2030. Goal 3: Good Health and Well-Being; United Nations Development Programme: New York, NY, USA, 2015. [Google Scholar]
- Kohli, M.; Schiller, I.; Dendukuri, N.; Dheda, K.; Denkinger, C.M.; Schumacher, S.G.; Steingart, K.R. Xpert(®) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst. Rev. 2018, 8, Cd012768. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Bizzi, E.; Picchi, C.; Mastrangelo, G.; Imazio, M.; Brucato, A. Recent advances in pericarditis. Eur. J. Intern. Med. 2022, 95, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.A.; Stancic, C.; Vos, D.; Carmen Diaz Insua, M.M.D.; Lima, C.C.V.; de Castro, R.L.; Maravelas, R.; Melgar, T.A. Hospital admissions for tuberculous pericarditis in the United States 2002–2014. Int. J. Mycobacteriol. 2019, 8, 347–350. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.P.; Posada-Martínez, E.L.; Saldarriaga, C.; Wyss, F.; Ponte-Negretti, C.I.; Alexander, B.; Miranda-Arboleda, A.F.; Martínez-Sellés, M.; Baranchuk, A. Tuberculosis and the Heart. J. Am. Heart Assoc. 2021, 10, e019435. [Google Scholar] [CrossRef] [PubMed]
- Isiguzo, G.; Du Bruyn, E.; Howlett, P.; Ntsekhe, M. Diagnosis and Management of Tuberculous Pericarditis: What Is New? Curr. Cardiol. Rep. 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Meeting Report of a Technical Expert Consultation, Non-Inferiority Analysis of Xpert MTB/RIF Ultra Compared to Xpert MTB/RIF. 2017. Available online: http://www.who.int/tb/publications/2017/XpertUltra/en/ (accessed on 16 August 2023).
- Hamada, Y.; Getahun, H.; Tadesse, B.T.; Ford, N. HIV-associated tuberculosis. Int. J. STD AIDS 2021, 32, 780–790. [Google Scholar] [CrossRef] [PubMed]
- WHO. Xpert MTB/RIF and Xpert MTB/RIF Ultra Assays; TB Knowledge Sharing (tbksp.org); World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- Chakravorty, S.; Simmons, A.M.; Rowneki, M.; Parmar, H.; Cao, Y.; Ryan, J.; Banada, P.P.; Deshpande, S.; Shenai, S.; Gall, A.; et al. The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Dorman, S.E.; Schumacher, S.G.; Alland, D.; Nabeta, P.; Armstrong, D.T.; King, B.; Hall, S.L.; Chakravorty, S.; Cirillo, D.M.; Tukvadze, N.; et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: A prospective multicentre diagnostic accuracy study. Lancet Infect. Dis. 2018, 18, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Mekkaoui, L.; Hallin, M.; Mouchet, F.; Payen, M.C.; Maillart, E.; Clevenbergh, P.; Georgala, A.; Van den Wijngaert, S. Performance of Xpert MTB/RIF Ultra for diagnosis of pulmonary and extra-pulmonary tuberculosis, one year of use in a multi-centric hospital laboratory in Brussels, Belgium. PLoS ONE 2021, 16, e0249734. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tan, G.; Gao, R.; Yao, L.; Bi, D.; Guo, Y.; Yu, F.; Fan, L. Assessment of the Xpert MTB/RIF Ultra assay on rapid diagnosis of extrapulmonary tuberculosis. Int. J. Infect. Dis. 2019, 81, 91–96. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Use of Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis and Screening of Active Tuberculosis in People Living with HIV. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/193633/9789241509633_eng.pdf;jsessionid=9391ABAC38CE4F752F5A3FA74EC68556?sequence=1 (accessed on 16 August 2023).
- Mathabire Rucker, S.C.; Cossa, L.; Harrison, R.E.; Mpunga, J.; Lobo, S.; Kisaka Kimupelenge, P.; Mandar Kol’Ampwe, F.; Amoros Quiles, I.; Molfino, L.; Szumilin, E.; et al. Feasibility of using Determine TB-LAM to diagnose tuberculosis in HIV-positive patients in programmatic conditions: A multisite study. Glob. Health Action 2019, 12, 1672366. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, S.; Schiller, I.; Dendukuri, N.; Kohli, M.; Nathavitharana, R.R.; Zwerling, A.A.; Denkinger, C.M.; Steingart, K.R.; Shah, M. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst. Rev. 2019, 10, Cd011420. [Google Scholar] [CrossRef] [PubMed]
- WHO. Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis of Active Tuberculosis in People Living with HIV; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Pandie, S.; Peter, J.G.; Kerbelker, Z.S.; Meldau, R.; Theron, G.; Govender, U.; Ntsekhe, M.; Dheda, K.; Mayosi, B.M. The diagnostic accuracy of pericardial and urinary lipoarabinomannan (LAM) assays in patients with suspected tuberculous pericarditis. Sci. Rep. 2016, 6, 32924. [Google Scholar] [CrossRef] [PubMed]
- Pandie, S.; Peter, J.G.; Kerbelker, Z.S.; Meldau, R.; Theron, G.; Govender, U.; Ntsekhe, M.; Dheda, K.; Mayosi, B.M. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous pericarditis compared to adenosine deaminase and unstimulated interferon-γ in a high burden setting: A prospective study. BMC Med. 2014, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Arend, S.M.; van Soolingen, D. Performance of Xpert MTB/RIF Ultra: A matter of dead or alive. Lancet Infect. Dis. 2018, 18, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Kendall, E.A.; Schumacher, S.G.; Denkinger, C.M.; Dowdy, D.W. Estimated clinical impact of the Xpert MTB/RIF Ultra cartridge for diagnosis of pulmonary tuberculosis: A modeling study. PLoS Med. 2017, 14, e1002472. [Google Scholar] [CrossRef] [PubMed]
- Schramm, B.; Nganaboy, R.C.; Uwiragiye, P.; Mukeba, D.; Abdoubara, A.; Abdou, I.; Nshimiymana, J.C.; Sounna, S.; Hiffler, L.; Flevaud, L.; et al. Correction: Potential value of urine lateral-flow lipoarabinomannan (LAM) test for diagnosing tuberculosis among severely acute malnourished children. PLoS ONE 2021, 16, e0256717. [Google Scholar] [CrossRef] [PubMed]
- NDOH. Guidance on the Use of the Lateral Flow Urine LIpoarabinomannan Assay for the Diagnosis of Tuberculosis in People Living with HIV. A Clinical Reference Guide for Healthcare Providers in South Africa. 2021. Available online: https://knowledgehub.health.gov.za/system/files/elibdownloads/2023-04/TB%2520LAM%2520Guidelines%2520-%252008%2520Feb%25202021%2520%25282%2529.pdf (accessed on 16 August 2023).
- Simieneh, A.; Tadesse, M.; Kebede, W.; Gashaw, M.; Abebe, G. Combination of Xpert® MTB/RIF and DetermineTM TB-LAM Ag improves the diagnosis of extrapulmonary tuberculosis at Jimma University Medical Center, Oromia, Ethiopia. PLoS ONE 2022, 17, e0263172. [Google Scholar] [CrossRef] [PubMed]
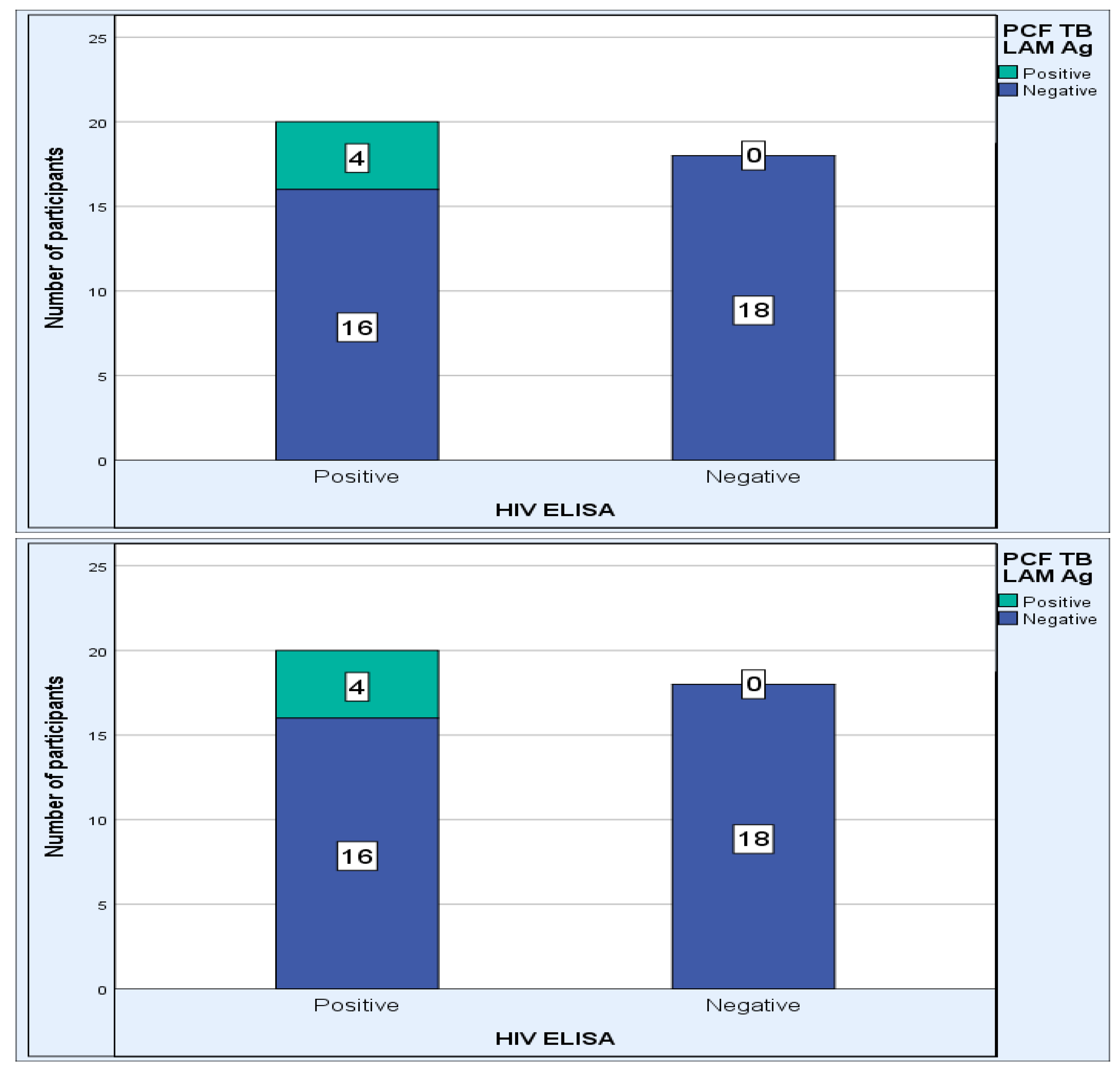



| Characteristics | n = 46 |
| Age year; median (interquartile range) | 48 (33.75–66.00) |
| Age year (min–max) | 14–80 |
| Male (n, %) | 24 (52) |
| Female (n, %) | 22 (48) |
| HIV-positive (n, %) N = 38 | 20 (52.6) |
| HIV-negative (n, %) N = 38 | 18 (47.4) |
| CD4 cell count; median (interquartile range) | 253 (142.25–463.25) |
| Viral load; median (interquartile range) | 2960 (922–121,350) |
| Study Population | TBP, n (%) | Non-TBP, n (%) | X2 p-Value |
|---|---|---|---|
| All, n = 44 | 15 (34.1) | 29 (65.9) | |
| Age group (years) | |||
| ≤45, n = 18 | 3 (16.7) | 15 (83.3) | 0.88 |
| >45, n = 26 | 12 (46.2) | 14 (53.8) | |
| HIV status | |||
| Positive, n = 20 | 7 (35) | 13 (65) | 0.99 |
| Negative, n = 18 | 6 (33.3) | 12 (66.7) | |
| Not done, n = 6 | 2 (33.3) | 4 (66.7) | |
| CD4 cell count categories (cells/mm3) | |||
| <200, n = 4 | 4 (100) | 0 (0.0) | 0.008 |
| 200–500, n = 8 | 3 (37.5) | 5 (62.5) | |
| >500, n = 2 | 0 (0.0) | 2 (100) | |
| No data, n = 6 | 0 (0.0) | 6 (100) | |
| Viral load categories (copies/mL) | |||
| Below detectable limit, n = 4 | 3 (75) | 1 (25) | 0.28 |
| Low, n = 1 | 0 (0.0) | 1 (100) | |
| High, n = 8 | 2 (25) | 6 (75) | |
| No data, n = 7 | 2 (28.6) | 5 (71.4) |
| Culture Results | Test Results | PCF Xpert MTB/RIF Ultra Results | PCF TB LAM Ag Results |
|---|---|---|---|
| Positive, n = 15 | True positives | 12 | 5 |
| False negative | 3 | 10 | |
| Negative, n = 29 | True negatives | 27 | 29 |
| False positives | 2 | 0 |
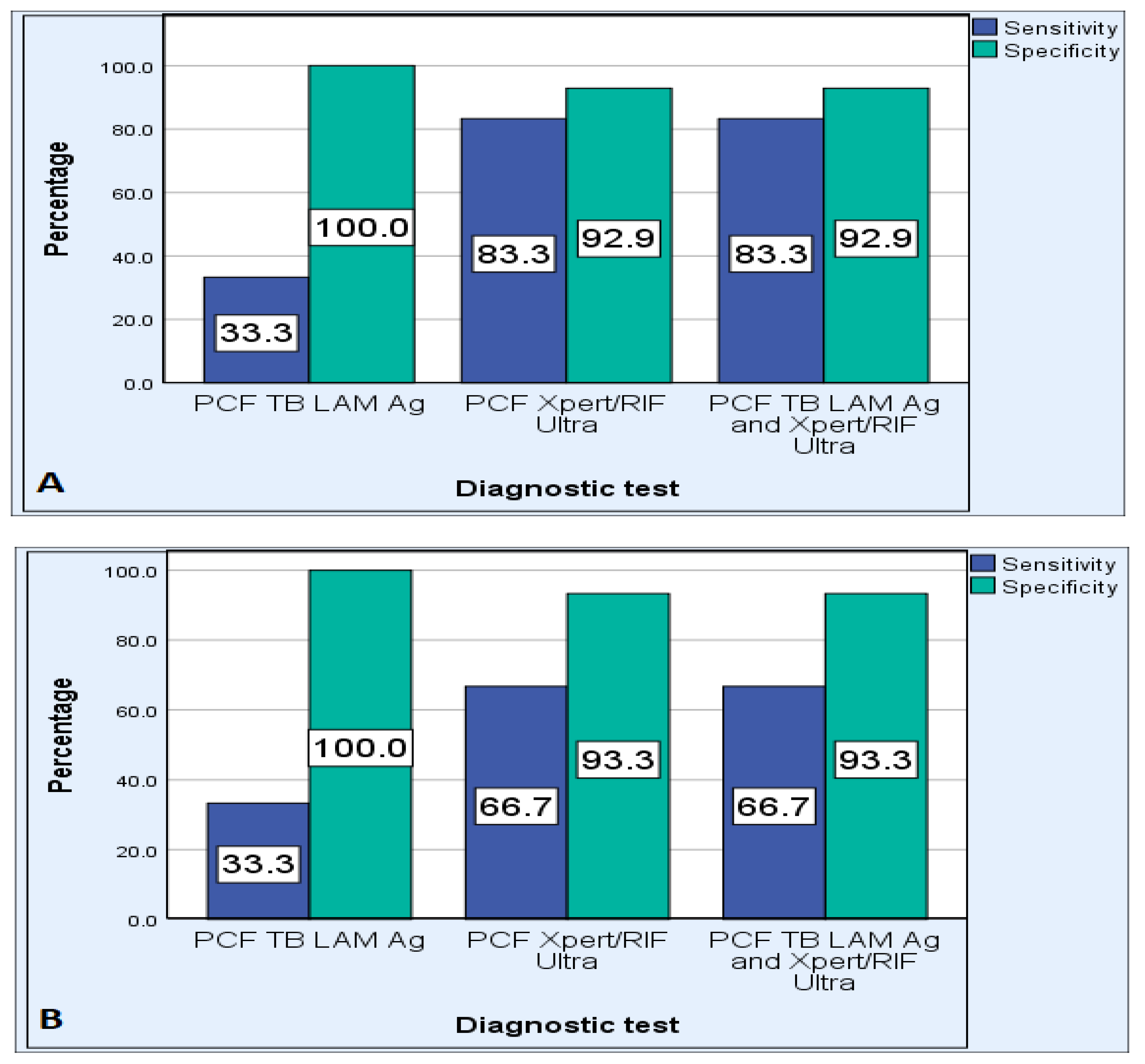
| Performance Measurements | PCF TB LAM Ag | PCF Xpert MTB/RIF Ultra | Combined TB-LAM and Xpert Ultra |
|---|---|---|---|
| Sensitivity | 33.3% | 80% | 80.0% |
| Specificity | 100% | 93.1% | 93.1% |
| PPV | 100% | 85.7% | 85.7% |
| NPV | 74.4% | 90.0% | 90.0% |
| PLR | 0.0 | 11.6 | 11.6 |
| NLR | 0.67 | 0.21 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomatu, S.; Vasaikar, S.; Thomas, K.; Dubula, T.; Moeketsi, K. The Efficiency of TB LAM Antigen Test to Xpert MTB/RIF Ultra Test for the Diagnosis of Tuberculous Pericarditis Using Pericardial Fluid Samples. Pathogens 2023, 12, 1175. https://doi.org/10.3390/pathogens12091175
Alomatu S, Vasaikar S, Thomas K, Dubula T, Moeketsi K. The Efficiency of TB LAM Antigen Test to Xpert MTB/RIF Ultra Test for the Diagnosis of Tuberculous Pericarditis Using Pericardial Fluid Samples. Pathogens. 2023; 12(9):1175. https://doi.org/10.3390/pathogens12091175
Chicago/Turabian StyleAlomatu, Samuel, Sandeep Vasaikar, Kandathil Thomas, Thozama Dubula, and Khulile Moeketsi. 2023. "The Efficiency of TB LAM Antigen Test to Xpert MTB/RIF Ultra Test for the Diagnosis of Tuberculous Pericarditis Using Pericardial Fluid Samples" Pathogens 12, no. 9: 1175. https://doi.org/10.3390/pathogens12091175
APA StyleAlomatu, S., Vasaikar, S., Thomas, K., Dubula, T., & Moeketsi, K. (2023). The Efficiency of TB LAM Antigen Test to Xpert MTB/RIF Ultra Test for the Diagnosis of Tuberculous Pericarditis Using Pericardial Fluid Samples. Pathogens, 12(9), 1175. https://doi.org/10.3390/pathogens12091175







