Prevalence of Toxoplasma gondii in Wild American Mink (Neogale vison): The First Serological Study in Germany and Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Sample Collection
2.3. Determination of Antibodies to T. gondii by ELISA
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P. Toxoplasmosis of Animals and Humans; CRC Press: London, UK; New York, NY, USA, 2021. [Google Scholar]
- Tong, W.H.; Hlaváčová, J.; Abdulai-Saiku, S.; Kaňková, Š.; Flegr, J.; Vyas, A. Presence of Toxoplasma gondii tissue cysts in human semen: Toxoplasmosis as a potential sexually transmissible infection. J. Infect. 2023, 86, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Bowie, W.R.; King, A.S.; Werker, D.H.; Isaac-Renton, J.L.; Bell, A.; Eng, S.B.; Marion, S.A. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet 1997, 350, 173–177. [Google Scholar] [CrossRef] [PubMed]
- de Moura, L.; Bahia-Oliveira, L.M.G.; Wada, M.; Jones, J.; Tuboi, S.; Carmo, E.; Ramalho, W.M.; Camargo, N.; Trevisan, R.; Graça, R.M.T.; et al. Waterborne Toxoplasmosis, Brazil, from Field to Gene. Emerg. Infect. Dis. 2006, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, M.; Madhavan, B.; Balasundaram, M.B.; Andavar, R.; Venkatapathy, N. Outbreak of ocular toxoplasmosis in Coimbatore, India. Indian J. Ophthalmol. 2006, 54, 129–131. [Google Scholar] [CrossRef]
- Minuzzi, C.E.; Fernandes, F.D.A.; Portella, L.P.; Bräunig, P.; Sturza, D.A.F.; Giacomini, L.; Salvagni, E.; Ribeiro, J.d.S.; Silva, C.R.; Difante, C.M.; et al. Contaminated water confirmed as source of infection by bioassay in an outbreak of toxoplasmosis in South Brazil. Transb. Emerg. Dis. 2021, 68, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Mortari, A.P.G.; Tagarra, L.G.; de Souza, M.L.; Roman, I.J.; Ratzlaff, F.R.; Braunig, P.; de Andrade, C.M.; Cargnelutti, J.F.; Sangioni, L.A.; Vogel, F.S.F. Increased seroprevalence of anti-Toxoplasma gondii antibodies in dogs in southern Brazil after an outbreak of human toxoplasmosis. Parasitol. Res. 2023, 122, 1009–1014. [Google Scholar] [CrossRef]
- Miller, M.A.; Gardner, I.A.; Kreuder, C.; Paradies, D.M.; Worcester, K.R.; Jessup, D.A.; Dodd, E.; Harris, M.D.; Ames, J.A.; Packham, A.E.; et al. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. 2002, 32, 997–1006. [Google Scholar] [CrossRef]
- Conrad, P.A.; Miller, M.A.; Kreuder, C.; James, E.R.; Mazet, J.; Dabritz, H.; Jessup, D.A.; Gulland, F.; Grigg, M.E. Transmission of Toxoplasma: Clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment. Int. J. Parasitol. 2005, 35, 1155–1168. [Google Scholar] [CrossRef]
- Jones, J.L.; Dubey, J.P. Waterborne toxoplasmosis—Recent developments. Exp. Parasitol. 2010, 124, 10–25. [Google Scholar] [CrossRef]
- Shapiro, K.; Largier, J.; Mazet Jonna, A.K.; Bernt, W.; Ell John, R.; Melli Ann, C.; Conrad Patricia, A. Surface Properties of Toxoplasma gondii Oocysts and Surrogate Microspheres. Appl. Environ. Microbiol. 2009, 75, 1185–1191. [Google Scholar] [CrossRef]
- Shapiro, K.; Silver, M.W.; Largier, J.L.; Conrad, P.A.; Mazet, J.A.K. Association of Toxoplasma gondii oocysts with fresh, estuarine, and marine macroaggregates. Limnol. Oceanogr. 2012, 57, 449–456. [Google Scholar] [CrossRef]
- Ahlers, A.A.; Mitchell, M.A.; Dubey, J.P.; Schooley, R.L.; Heske, E.J. Risk Factors for Toxoplasma gondii Exposure in Semiaquatic Mammals in a Freshwater Ecosystem. J. Wildl. Dis. 2015, 51, 488–492. [Google Scholar] [CrossRef]
- Fredebaugh, S.L.; Mateus-Pinilla, N.E.; McAllister, M.; Warner, R.E.; Weng, H.-Y. Prevalence of antibody to Toxoplasma gondii in terestrial wildlife in a nutral area. J. Wildl. Dis. 2011, 47, 381–392. [Google Scholar] [CrossRef]
- Bossart, G.D. Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef] [PubMed]
- de Moura, J.F.; Hauser-Davis, R.A.; Lemos, L.; Emin-Lima, R.; Siciliano, S. Guiana Dolphins (Sotalia guianensis) as Marine Ecosystem Sentinels: Ecotoxicology and Emerging Diseases. Rev. Environ. Contam. Toxicol. 2014, 228, 1–29. [Google Scholar] [CrossRef]
- Benecke, H.-G. Freilassung von Nerzen (Mink, Mustela vision) bei Burg, Sachsen-Anhalt. Säugetierkd. Inf. 2007, 35, 127–128. [Google Scholar]
- Bonesi, L.; Palazon, S. The American mink in Europe: Status, impacts, and control. Biol. Conserv. 2007, 134, 470–483. [Google Scholar] [CrossRef]
- Heidemann, G. Über das Vorkommen des Farmnerzes (Mustela vison f. dom.) in Schleswig-Holstein. Z. Jagdwiss. 1983, 29, 120–122. [Google Scholar] [CrossRef]
- Brzeziński, M.; Marzec, M. The origin, dispersal and distribution of the American mink Mustela vison in Poland. Acta. Theriol. 2003, 48, 505–514. [Google Scholar] [CrossRef]
- Zschille, J.; Heidecke, D.; Stubbe, M. Distribution and ecology of feral American mink Mustela vison Schreber, 1777 (Carnivora, Mustelidae) in Saxony-Anhalt (Germany). Hercynia 2004, 37, 103–126. [Google Scholar]
- Vada, R.; Illanas, S.; Acevedo, P.; Adriaens, T.; Apollonio, M.; Belova, O.; Blanco-Aguiar, J.A.; Csányi, S.; Body, G.; Fernández-De-Mera, I.G.; et al. Feral American mink Neogale vison continues to expand its European range: Time to harmonise population monitoring and coordinate control. Mam. Rev. 2023, 53, 158–176. [Google Scholar] [CrossRef]
- Brzeziński, M.; Zarzycka, A.; Diserens, T.A.; Zalewski, A. Does the American mink displace the European polecat? A need for more research on interspecific competition between invasive and native species. Eur. J. Wildl. Res. 2021, 67, 64. [Google Scholar] [CrossRef]
- Dziech, A.; Wierzbicki, H.; Moska, M.; Zatoń-Dobrowolska, M. Invasive and Alien Mammal Species in Poland—A Review. Diversity 2023, 15, 138. [Google Scholar] [CrossRef]
- Nentwig, W.; Kühnel, E.; Bacher, S. A Generic Impact-Scoring System Applied to Alien Mammals in Europe. Conserv. Biol. 2010, 24, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Bevanger, K.; Henriksen, G. The distributional history and present status of the American mink (Mustela vison Schreber, 1777) in Norway. Ann. Zool. Fenn. 1995, 32, 11–14. [Google Scholar]
- Jędrzejewska, B.; Sidorovich, V.E.; Pikulik, M.M.; Jędrzejewski, W. Feeding habits of the otter and the American mink in Białowieża Primeval Forest (Poland) compared to other Eurasian populations. Ecography 2001, 24, 165–180. [Google Scholar] [CrossRef]
- Rodney, F.K. An Outbreak of Toxoplasmosis in Farmed Mink (Mustela vison S.). J. Vet. Diagn. Investig. 2001, 13, 245–249. [Google Scholar] [CrossRef]
- Śmielewska-Łoś, E.; Turniak, W. Toxoplasma gondii infection in Polish farmed mink. Vet. Parasitol. 2004, 122, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Shwab, E.K.; Zhu, X.-Q.; Majumdar, D.; Pena, H.F.J.; Gennari, S.M.; Dubey, J.P.; Su, C. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology 2014, 141, 453–461. [Google Scholar] [CrossRef]
- Henriksen, P.; Dietz, H.H.; Uttenthal, A.; Hansen, M. Seroprevalence of Toxoplasma gondii in Danish farmed mink (Mustela vison S.). Vet. Parasitol. 1994, 53, 1–5. [Google Scholar] [CrossRef]
- Zheng, W.-B.; Zhang, X.-X.; Ma, J.-G.; Li, F.-C.; Zhao, Q.; Huang, S.-Y.; Zhu, X.-Q. Molecular Detection and Genetic Characterization of Toxoplasma gondii in Farmed Minks (Neovison vison) in Northern China by PCR-RFLP. PLoS ONE 2016, 11, e0165308. [Google Scholar] [CrossRef] [PubMed]
- Shamaev, N.D.; Shuralev, E.A.; Petrov, S.V.; Kazaryan, G.G.; Aleksandrova, N.M.; Valeeva, A.R.; Khaertynov, K.S.; Mukminov, M.N.; Kitoh, K.; Takashima, Y. Seroprevalence and B1 gene genotyping of Toxoplasma gondii in farmed European mink in the Republic of Tatarstan, Russia. Parasitol. Int. 2020, 76, 102067. [Google Scholar] [CrossRef]
- Sengupta, M.E.; Pagh, S.; Stensgaard, A.-S.; Chriel, M.; Petersen, H.H. Prevalence of Toxoplasma gondii and Cryptosporidium in feral and farmed American Mink (Neovison vison) in Denmark. Acta. Parasitol. 2021, 66, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Beattie, C.P. Toxoplasmosis of Animals and Man; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H. Recent epidemiologic and clinical Toxoplasma gondii infections in wild canids and other carnivores: 2009–2020. Vet. Parasitol. 2021, 290, 109337. [Google Scholar] [CrossRef] [PubMed]
- Kornacka-Stackonis, A. Toxoplasma gondii infection in wild omnivorous and carnivorous animals in Central Europe—A brief overview. Vet. Parasitol. 2022, 304, 109701. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Genovesi, P.; Middleton, A. European Code of Conduct on Hunting and IAS. 2013, Volume 20. Available online: https://www.jagdverband.de/sites/default/files/Code%20of%20Conduct%20on%20Hunting%20and%20IAS%20%2012%202013.pdf (accessed on 27 December 2023).
- Nielsen, B.; Ekeroth, L.; Bager, F.; Lind, P. Use of muscle fluid as a source of antibodies for serologic detection of Salmonella infection in slaughter pig herds. J. Vet. Diagn. Investig. 1998, 10, 158–163. [Google Scholar] [CrossRef]
- Heddergott, M.; Pohl, D.; Steinbach, P.; Salazar, L.C.; Müller, F.; Frantz, A.C. Determinants and effects of sinus worm Skrjabingylus nasicola (Nematoda: Metastrongyloidae) infestation in invasive American mink Neovison vison in Germany. Parasit. Res. 2016, 115, 3449–3457. [Google Scholar] [CrossRef]
- Wingstrand, A.; Lind, P.; Haugegaard, J.; Henriksen, S.A.; Bille-Hansen, V.; Sørensen, V. Clinical observations, pathology, bioassay in mice and serological response at slaughter in pigs experimentally infected with Toxoplasma gondii. Vet. Parasitol. 1997, 72, 129–140. [Google Scholar] [CrossRef]
- Kornacka, A.; Cybulska, A.; Popiołek, M.; Kuśmierek, N.; Moskwa, B. Survey of Toxoplasma gondii and Neospora caninum in raccoons (Procyon lotor) from the Czech Republic, Germany and Poland. Vet. Parasitol. 2018, 262, 47–50. [Google Scholar] [CrossRef]
- Engel, L.; Hamedy, A.; Kornacka-Stackonis, A.; Langner, T.; Birka, S.; Koethe, M. Toxoplasma gondii in raccoons (Procyon lotor) in Germany: A serosurvey based on meat juice. Parasitol. Res. 2022, 121, 3417–3425. [Google Scholar] [CrossRef]
- Tizard, I.R.; Billett, J.B.; Ramsden, R.O. The prevalence of antibodies against Toxoplasma gondii in some Ontario mammals. J. Wildl. Dis. 1976, 12, 322–325. [Google Scholar] [CrossRef]
- Smith, D.D.; Frenkel, J.K. Prevalence of antibodies to Toxoplasma gondii in wild mammals of Missouri and east central Kansas: Biologic and ecologic considerations of transmission. J. Wildl. Dis. 1995, 31, 15–21. [Google Scholar] [CrossRef]
- Gerhold, R.W.; Saraf, P.; Chapman, A.; Zou, X.; Hickling, G.; Stiver, W.H.; Houston, A.; Souza, M.; Su, C. Toxoplasma gondii seroprevalence and genotype diversity in select wildlife species from the southeastern United States. Parasit. Vectors 2017, 10, 508. [Google Scholar] [CrossRef]
- Sepúlveda, M.A.; Muñoz-Zanzi, C.; Rosenfeld, C.; Jara, R.; Pelican, K.M.; Hill, D. Toxoplasma gondii in feral American minks at the Maullín river, Chile. Vet. Parasitol. 2011, 175, 60–65. [Google Scholar] [CrossRef]
- Goncharuk, M.S.; Kerley, L.L.; Naidenko, S.V.; Rozhnov, V.V. Prevalence of seropositivity to pathogens in small carnivores in adjacent areas of Lazovskii Reserve. Biol. Bull. Russ. Acad. Sci. 2012, 39, 708–713. [Google Scholar] [CrossRef]
- Martino, P.E.; Samartino, L.E.; Stanchi, N.O.; Radman, N.E.; Parrado, E.J. Serology and protein electrophoresis for evidence of exposure to 12 mink pathogens in free-ranging American mink (Neovison vison) in Argentina. Vet. Q. 2017, 37, 207–211. [Google Scholar] [CrossRef]
- Barros, M.; Cabezon, O.; Dubey, J.P.; Almeria, S.; Ribas, M.P.; Escobar, L.E.; Ramos, B.; Medina-Vogel, G. Toxoplasma gondii infection in wild mustelids and cats across an urban-rural gradient. PLoS ONE 2018, 13, e0199085. [Google Scholar] [CrossRef]
- Ribas, M.P.; Almería, S.; Fernández-Aguilar, X.; De Pedro, G.; Lizarraga, P.; Alarcia-Alejos, O.; Molina-López, R.; Obón, E.; Gholipour, H.; Temiño, C.; et al. Tracking Toxoplasma gondii in freshwater ecosystems: Interaction with the invasive American mink (Neovison vison) in Spain. Parasitol. Res. 2018, 117, 2275–2281. [Google Scholar] [CrossRef]
- Kornacka, A.; Cybulska, A.; Bień, J.; Goździk, K.; Moskwa, B. The usefulness of direct agglutination test, enzyme-linked immunosorbent assay and polymerase chain reaction for the detection of Toxoplasma gondii in wild animals. Vet. Parasitol. 2016, 228, 85–89. [Google Scholar] [CrossRef]
- Dubey, J.P.; Hamir, A.N.; Shen, S.K.; Thulliez, P.; Rupprecht, C.E. Experimental Toxoplasma gondii infection in raccoons (Procyon lotor). J. Parasitol. 1993, 79, 548–552. [Google Scholar] [CrossRef]
- Gamble, H.R.; Dubey, J.P.; Lambillotte, D.N. Comparison of a commercial ELISA with the modified agglutination test for detection of Toxoplasma infection in the domestic pig. Vet. Parasitol. 2005, 128, 177–181. [Google Scholar] [CrossRef]
- VanWormer, E.; Conrad, P.A.; Miller, M.A.; Melli, A.C.; Carpenter, T.E.; Mazet, J.A.K. Toxoplasma gondii, source to sea: Higher contribution of domestic felids to terrestrial parasite loading despite lower infection prevalence. EcoHealth 2013, 10, 277–289. [Google Scholar] [CrossRef]
- Heddergott, M.; Frantz, A.C.; Stubbe, M.; Stubbe, A.; Ansorge, H.; Osten-Sacken, N. Seroprevalence and risk factors of Toxoplasma gondii infection in invasive raccoons (Procyon lotor) in Central European. Parasitol. Res. 2017, 116, 2335–2340. [Google Scholar] [CrossRef]
- Herrmann, D.C.; Maksimov, P.; Maksimov, A.; Sutor, A.; Schwarz, S.; Jaschke, W.; Schliephake, A.; Denzin, N.; Conraths, F.J.; Schares, G. Toxoplasma gondii in foxes and rodents from the German Federal States of Brandenburg and Saxony-Anhalt: Seroprevalence and genotypes. Vet. Parasitol. 2012, 185, 78–85. [Google Scholar] [CrossRef]
- Zschille, J.; Stier, N.; Roth, M.; Mayer, R. Feeding habits of invasive American mink (Neovison vison) in northern Germany—Potential implications for fishery and waterfowl. Acta Theriol. 2014, 59, 25–34. [Google Scholar] [CrossRef]
- Krawczyk, A.J.; Bogdziewicz, M.; Czyż, M.J. Diet of the American mink Neovison vison in an agricultural landscape in western Poland. Folia Zool. 2013, 62, 304–310. [Google Scholar] [CrossRef]
- Massie, G.N.; Ware, M.W.; Villegas, E.N.; Black, M.W. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish. Vet. Parasitol. 2010, 169, 296–303. [Google Scholar] [CrossRef]
- Bartoszewicz, M.; Zalewski, A. American Mink, Mustela Vison Diet and Predation on Waterfowl in the Słońsk Reserve, Western Poland; Institute of Vertebrate Biology, Academy of Sciences of the Czech Republic: Brno, Czech Republic, 2003; Volume 52, pp. 225–238. [Google Scholar]
- Jones, J.L.; Kruszon-Moran, D.; Wilson, M.; McQuillan, G.; Navin, T.; McAuley, J.B. Toxoplasma gondii infection in the United States: Seroprevalence and risk factors. Am. J. Epidemiol. 2001, 154, 357–365. [Google Scholar] [CrossRef]
- Jones, Y.L.; Fitzgerald, S.D.; Sikarske, J.G.; Murphy, A.; Grosjean, N.; Kiupel, M. Toxoplasmosis in a free-ranging mink. J. Wildl. Dis. 2006, 42, 865–869. [Google Scholar] [CrossRef]
- Sroka, J.; Karamon, J.; Wójcik-Fatla, A.; Dutkiewicz, J.; Bilska-Zając, E.; Zając, V.; Piotrowska, W.; Cencek, T. Toxoplasma gondii infection in selected species of free-living animals in Poland. Ann. Agric. Environ. Med. 2019, 26, 656–660. [Google Scholar] [CrossRef]
- Dietz, H.H.; Henriksen, P.; Lebech, M.; Henriksen, S.A. Experimental infection with Toxoplasma gondii in farmed mink (Mustela vison S.). Vet. Parasitol. 1993, 47, 1–7. [Google Scholar] [CrossRef]
- Mel’nikov, V.D.; Rodyukov, A.P.; Berestov, A.A. Data of toxoplasmosis in fur-animals. In Adapattsionnye Reaktsii Pushnykh Zverei; Berestov, V.A., Ed.; Karel’skiĭ Filial Akademii Nauk SSR: Petrozavodsk, Russia, 1980; pp. 124–128. [Google Scholar]
- Gavier-Widén, D.; Bröjer, C.; Dietz, H.H.; Englund, L.; Hammer, A.S.; Hedlund, K.-O.; Hård af Segerstad, C.; Nilsson, K.; Nowotny, N.; Puurula, V.; et al. Investigations into shaking mink syndrome: An encephalomyelitis of unknown cause in farmed mink (Mustela Vison) kits in Scandinavia. J. Vet. Diagn. Investig. 2004, 16, 305–312. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R.; Dubey, J.P.; Lindsay, D.S. Zoonotic protozoa: From land to sea. Trends Parasitol. 2004, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, E.A.; Cable, J.; Chinchen, A.; Francis, J.; Guy, E.; Kean, E.F.; Paul, S.C.; Perkins, S.E.; Sherrard-Smith, E.; Wilkinson, C.; et al. Seroprevalence of Toxoplasma gondii in the Eurasian otter (Lutra lutra) in England and Wales. Parasit Vectors 2013, 6, 75. [Google Scholar] [CrossRef]
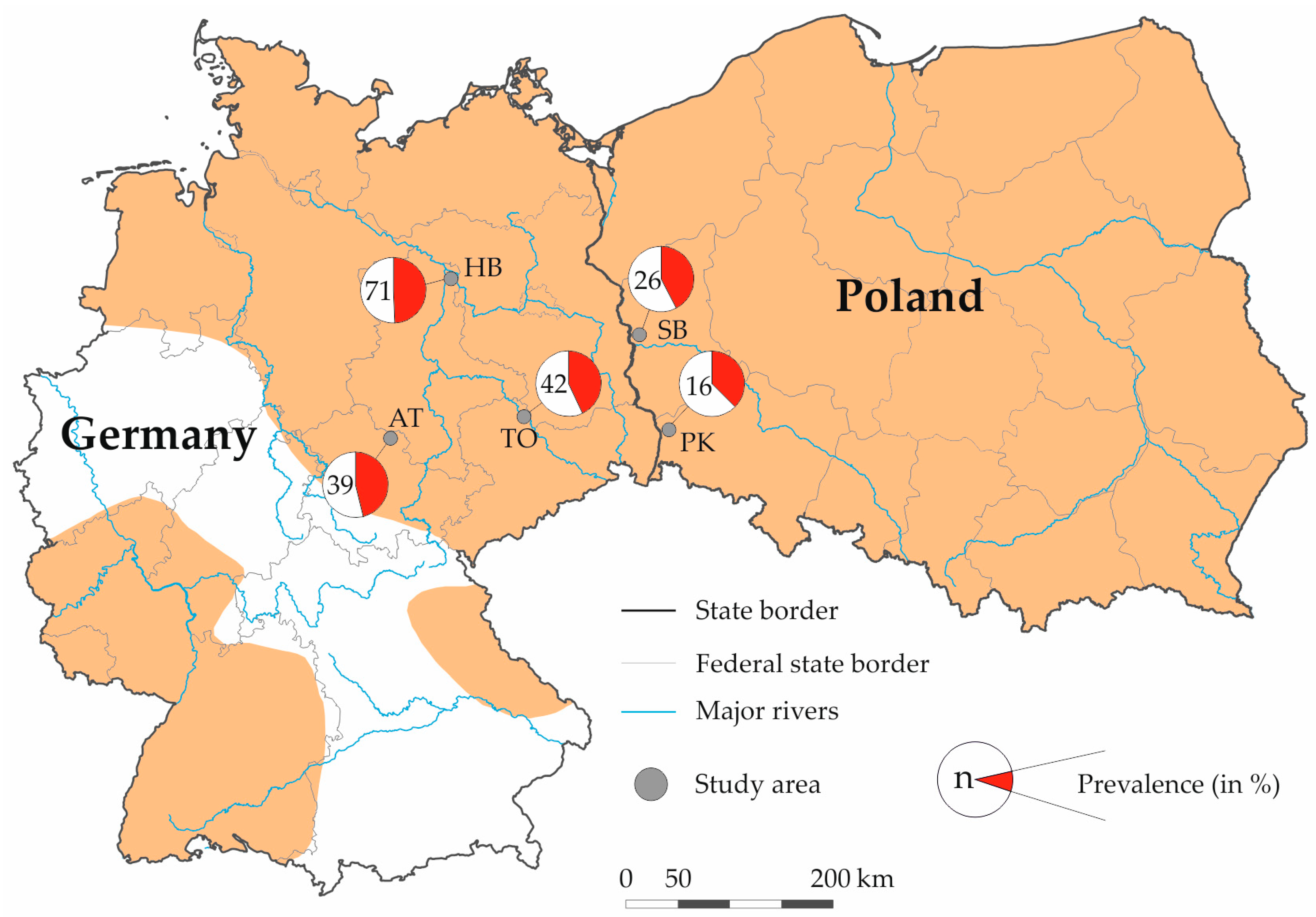
| State | Study Area | No. Tested | No. Positive | Prevalence in % (95% CI) 1 |
|---|---|---|---|---|
| Germany | Artern | 39 | 18 | 46.15 (30.54–61.86) |
| Torgau | 42 | 18 | 42.86 (27.93–57.87) | |
| Havelland | 71 | 35 | 49.30 (37.67–60.93) | |
| Poland | Pieńsk | 16 | 6 | 37.50 (13.78–61.22) |
| Słubice | 26 | 11 | 42.31 (23.31–61.29) | |
| Total | 194 | 88 | 45.36 (38.39–52.41) |
| Coefficients | Estimate | s.e. | Odds Ration | 95% CI 1 Odds Ration | z-Value | p-Value |
|---|---|---|---|---|---|---|
| (Intercept) | 0.707 | 0.273 | 2.028 | 1.199–3.516 | 2.589 | 0.010 |
| Sex—female | - | - | - | - | ||
| Sex—male | −0.069 | 0.310 | 0.933 | 0.508–1.718 | −0.224 | 0.823 |
| Age—adult | - | - | - | - | ||
| Age—juvenile | −1.531 | 0.312 | 0.216 | 0.116–0.395 | −4.904 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heddergott, M.; Pikalo, J.; Müller, F.; Osten-Sacken, N.; Steinbach, P. Prevalence of Toxoplasma gondii in Wild American Mink (Neogale vison): The First Serological Study in Germany and Poland. Pathogens 2024, 13, 153. https://doi.org/10.3390/pathogens13020153
Heddergott M, Pikalo J, Müller F, Osten-Sacken N, Steinbach P. Prevalence of Toxoplasma gondii in Wild American Mink (Neogale vison): The First Serological Study in Germany and Poland. Pathogens. 2024; 13(2):153. https://doi.org/10.3390/pathogens13020153
Chicago/Turabian StyleHeddergott, Mike, Jutta Pikalo, Franz Müller, Natalia Osten-Sacken, and Peter Steinbach. 2024. "Prevalence of Toxoplasma gondii in Wild American Mink (Neogale vison): The First Serological Study in Germany and Poland" Pathogens 13, no. 2: 153. https://doi.org/10.3390/pathogens13020153






