Antimicrobial Activity of Methylene Blue Associated with Photodynamic Therapy: In Vitro Study in Multi-Species Oral Biofilm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subgingival Biofilm Model
2.2. Biofilm Treatments
2.3. Biofilm Metabolic Activity
2.4. DNA-DNA Hybridization (Checkerboard DNA-DNA)
2.5. Statistical Analysis
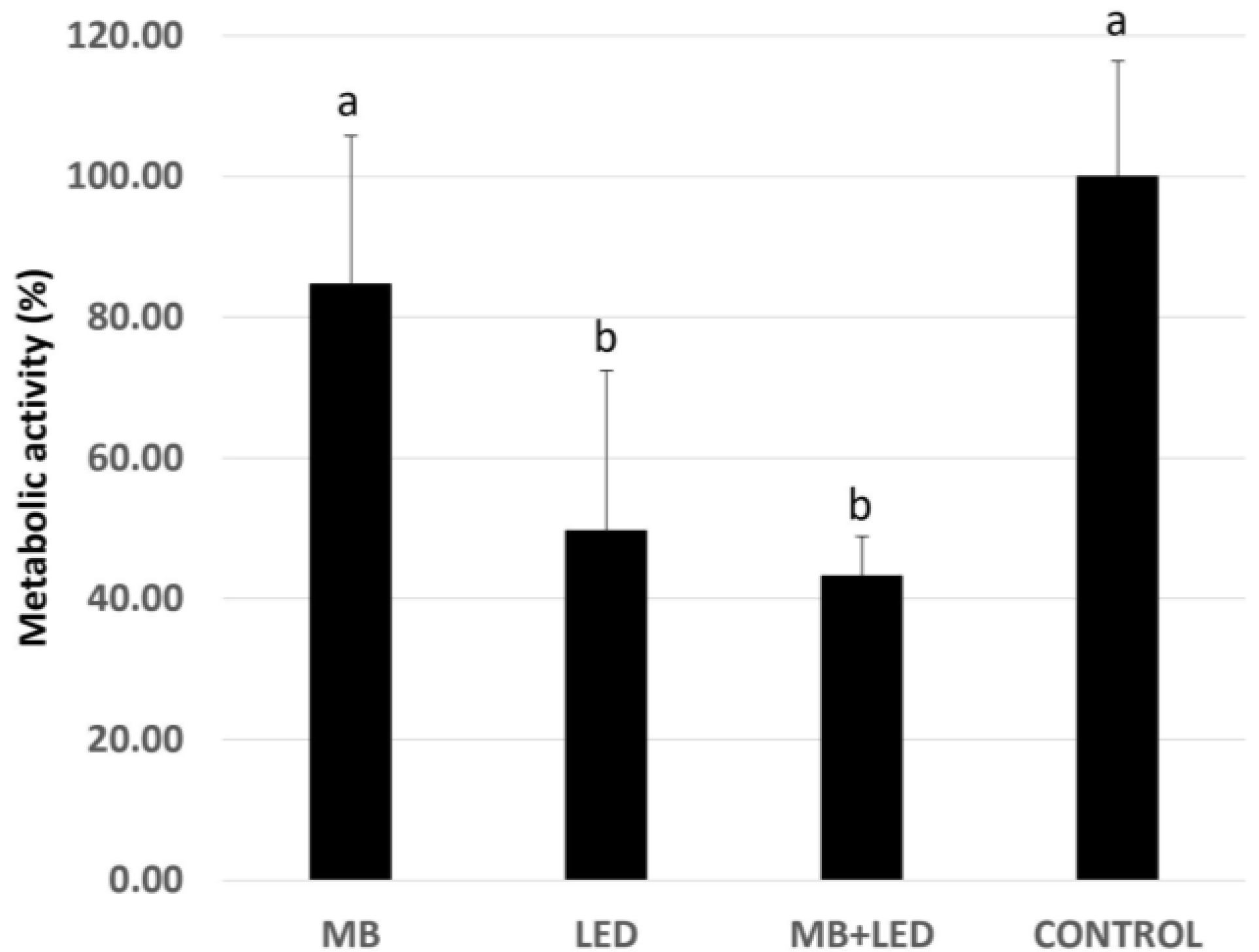
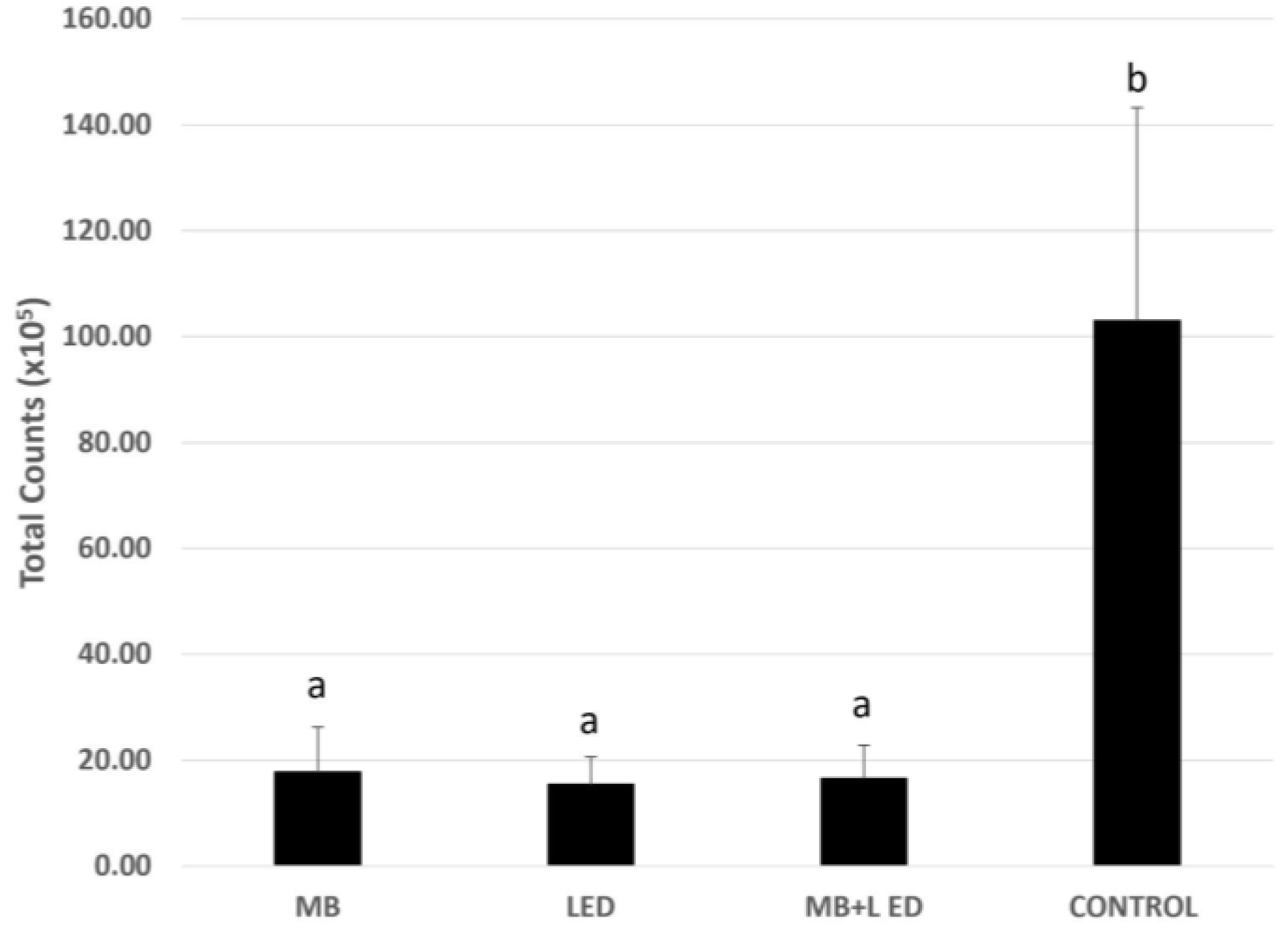
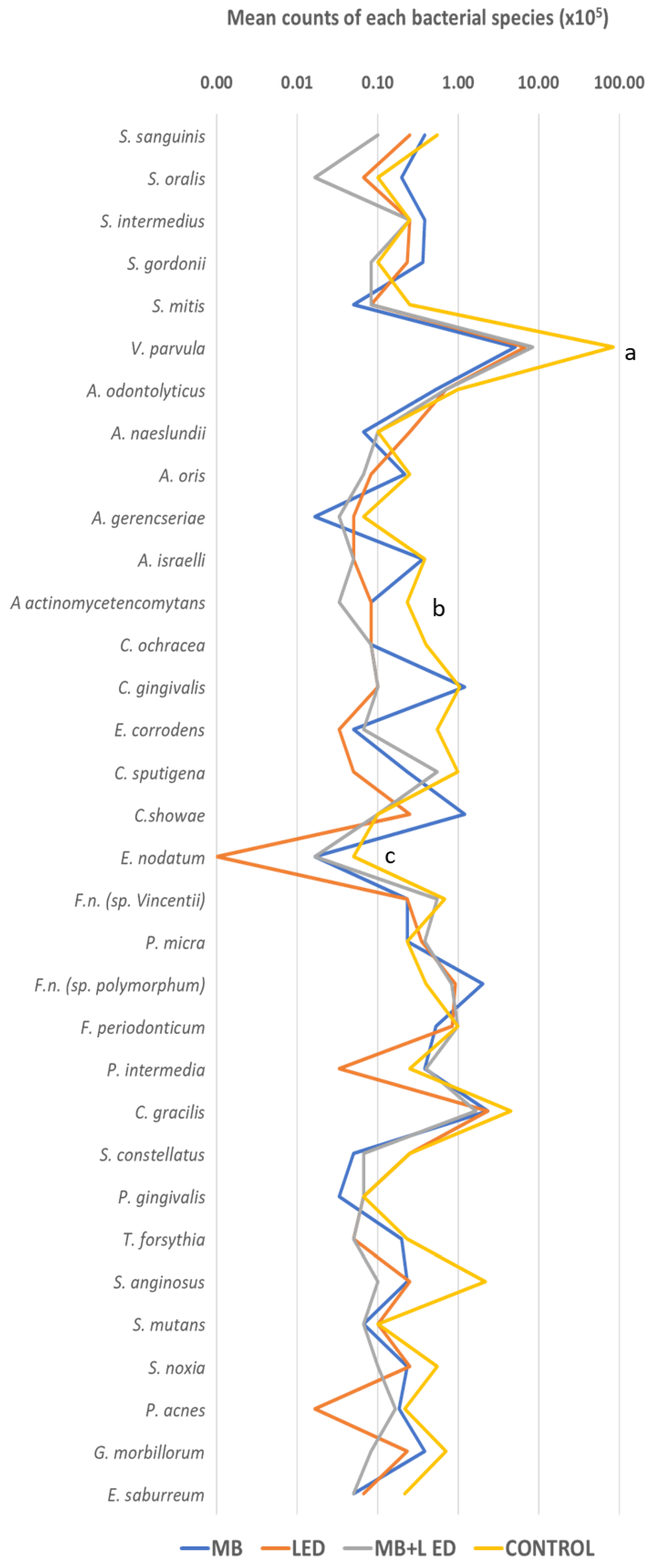
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies Against Microbial Biofilm Challenges. Front. Cell Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Nagasawa, M.A.; de Carvalho Formiga, M.; Moraschini, V.; Bertolini, M.; Souza, J.G.S.; Feres, M.; Figueiredo, L.C.; Shibli, J.A. Do the Progression of Experimentally Induced Gingivitis and Peri-Implant Mucositis Present Common Features? A Systematic Review of Clinical Human Studies. Biofouling 2022, 38, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Pallos, D.; Sousa, V.; Feres, M.; Retamal-Valdes, B.; Chen, T.; Curtis, M.; Boaventura, R.M.; Tanaka, M.H.; da Silva Salomão, G.V.; Zanella, L.; et al. Salivary Microbial Dysbiosis Is Associated with Peri-Implantitis: A Case-Control Study in a Brazilian Population. Front. Cell Infect. Microbiol. 2021, 11, 696432. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Feres, M.; Figueiredo, L.C.; Castro Dos Santos, N.; Retamal-Valdes, B. Decontamination and Biomodification of Periodontally Affected Root Surface for Successful Regeneration: Is There Room for Improvement? Dent. Clin. N. Am. 2022, 66, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, A.; Shapira, L.; Limones, A.; Martin, C. The Efficacy of Implant Surface Decontamination Using Chemicals during Surgical Treatment of Peri-Implantitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 336–358. [Google Scholar] [CrossRef] [PubMed]
- Guentsch, A. Antibiotics against Periodontal Biofilms. Monogr. Oral Sci. 2021, 29, 119–132. [Google Scholar] [CrossRef]
- Schwarz, S.R.; Hirsch, S.; Hiergeist, A.; Kirschneck, C.; Muehler, D.; Hiller, K.-A.; Maisch, T.; Al-Ahmad, A.; Gessner, A.; Buchalla, W.; et al. Limited Antimicrobial Efficacy of Oral Care Antiseptics in Microcosm Biofilms and Phenotypic Adaptation of Bacteria upon Repeated Exposure. Clin. Oral Investig. 2021, 25, 2939–2950. [Google Scholar] [CrossRef]
- Warrier, A.; Mazumder, N.; Prabhu, S.; Satyamoorthy, K.; Murali, T.S. Photodynamic Therapy to Control Microbial Biofilms. Photodiagnosis Photodyn. Ther. 2021, 33, 102090. [Google Scholar] [CrossRef]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive Laser or Antimicrobial Photodynamic Therapy to Non-surgical Mechanical Instrumentation in Patients with Untreated Periodontitis: A Systematic Review and Meta-analysis. J. Clin. Periodontol. 2020, 47, 176–198. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef]
- Nguyen, T.; Brody, H.; Lin, G.-H.; Rangé, H.; Kuraji, R.; Ye, C.; Kamarajan, P.; Radaic, A.; Gao, L.; Kapila, Y. Probiotics, Including Nisin-Based Probiotics, Improve Clinical and Microbial Outcomes Relevant to Oral and Systemic Diseases. Periodontology 2000 2020, 82, 173–185. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Górski, A. Bacteriophages and Lysins in Biofilm Control. Virol. Sin. 2020, 35, 125–133. [Google Scholar] [CrossRef]
- Szafrański, S.P.; Winkel, A.; Stiesch, M. The Use of Bacteriophages to Biocontrol Oral Biofilms. J. Biotechnol. 2017, 250, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.F.; Lo Svenningsen, S.; Røder, H.L.; Middelboe, M.; Burmølle, M. Big Impact of the Tiny: Bacteriophage-Bacteria Interactions in Biofilms. Trends Microbiol. 2019, 27, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Isola, G. The Impact of Diet, Nutrition and Nutraceuticals on Oral and Periodontal Health. Nutrients 2020, 12, 2724. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Brugnera Junior, A.; Theodoro, L.H. Antimicrobial Photodynamic Therapy for the Treatment of Periodontitis and Peri-Implantitis: What Are We Missing? Photobiomodul. Photomed. Laser Surg. 2021, 39, 502–503. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, R.; Qian, Y.; Shi, J.; Si, M. Antimicrobial Photodynamic Therapy versus Antibiotics as an Adjunct in the Treatment of Periodontitis and Peri-Implantitis: A Systematic Review and Meta-Analysis. Photodiagnosis Photodyn. Ther. 2021, 34, 102231. [Google Scholar] [CrossRef]
- Sculean, A.; Deppe, H.; Miron, R.; Schwarz, F.; Romanos, G.; Cosgarea, R. Effectiveness of Photodynamic Therapy in the Treatment of Periodontal and Peri-Implant Diseases. Monogr. Oral Sci. 2021, 29, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Wang, H.-L.; Romanos, G.E. Antimicrobial Photodynamic Therapy for the Treatment of Periodontitis and Peri-Implantitis: An American Academy of Periodontology Best Evidence Review. J. Periodontol. 2018, 89, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Almohareb, T.; Alhamoudi, N.; Al Deeb, M.; Bin-Shuwaish, M.S.; Mokeem, S.A.; Saad Shafqat, S.; Vohra, F.; Abduljabbar, T. Clinical Efficacy of Photodynamic Therapy as an Adjunct to Mechanical Debridement in the Treatment of Per-Implantitis with Abscess. Photodiagnosis Photodyn. Ther. 2020, 30, 101750. [Google Scholar] [CrossRef] [PubMed]
- Eldwakhly, E.; Saadaldin, S.; Aldegheishem, A.; Salah Mostafa, M.; Soliman, M. Antimicrobial Capacity and Surface Alterations Using Photodynamic Therapy and Light Activated Disinfection on Polymer-Infiltrated Ceramic Material Contaminated with Periodontal Bacteria. Pharmaceuticals 2020, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Azizi, B.; Budimir, A.; Mehmeti, B.; Jakovljević, S.; Bago, I.; Gjorgievska, E.; Gabrić, D. Antimicrobial Efficacy of Photodynamic Therapy and Light-Activated Disinfection Against Bacterial Species on Titanium Dental Implants. Int. J. Oral Maxillofac. Implant. 2018, 33, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Azizi, B.; Budimir, A.; Bago, I.; Mehmeti, B.; Jakovljević, S.; Kelmendi, J.; Stanko, A.P.; Gabrić, D. Antimicrobial Efficacy of Photodynamic Therapy and Light-Activated Disinfection on Contaminated Zirconia Implants: An in Vitro Study. Photodiagnosis Photodyn. Ther. 2018, 21, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Park, H.-W.; Lee, J.-H.; Seo, H.-W.; Lee, S.-Y. The Photodynamic Therapy on Streptococcus mutans Biofilms Using Erythrosine and Dental Halogen Curing Unit. Int. J. Oral Sci. 2012, 4, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Alsaif, A.; Tahmassebi, J.F.; Wood, S.R. Treatment of Dental Plaque Biofilms Using Photodynamic Therapy: A Randomised Controlled Study. Eur. Arch. Paediatr. Dent. 2021, 22, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Tahmassebi, J.F.; Drogkari, E.; Wood, S.R. A Study of the Control of Oral Plaque Biofilms via Antibacterial Photodynamic Therapy. Eur. Arch. Paediatr. Dent. 2015, 16, 433–440. [Google Scholar] [CrossRef]
- Biel, M.A. Antimicrobial Photodynamic Therapy for Treatment of Biofilm-Based Infections. Adv. Exp. Med. Biol. 2015, 831, 119–136. [Google Scholar] [CrossRef]
- do Rosario Palma, A.L.; de Paula-Ramos, L.; Domingues, N.; Back-Brito, G.N.; de Oliveira, L.D.; Pereira, C.A.; Jorge, A.O.C. Biofilms of Candida albicans and Streptococcus sanguinis and Their Susceptibility to Antimicrobial Effects of Photodynamic Inactivation. Photodiagnosis Photodyn. Ther. 2018, 24, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.H.; Pereira, E.S.; Rodrigues, L.K.A.; Saxena, D.; Duarte, S.; Zanin, I.C.J. Effect of Photodynamic Antimicrobial Chemotherapy on in Vitro and in Situ Biofilms. Caries Res. 2012, 46, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Zanin, I.C.J.; Gonçalves, R.B.; Junior, A.B.; Hope, C.K.; Pratten, J. Susceptibility of Streptococcus mutans Biofilms to Photodynamic Therapy: An in Vitro Study. J. Antimicrob. Chemother. 2005, 56, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Araújo, N.C.; Fontana, C.R.; Bagnato, V.S.; Gerbi, M.E.M. Photodynamic Antimicrobial Therapy of Curcumin in Biofilms and Carious Dentine. Lasers Med. Sci. 2014, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, M.T.M.; Soares, T.T.; Aragão, M.G.B.; Lima, R.A.; Duarte, S.; Zanin, I.C.J. Effect of Photodynamic Antimicrobial Chemotherapy on Mono- and Multi-Species Cariogenic Biofilms: A Literature Review. Photomed. Laser Surg. 2017, 35, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Erkiert-Polguj, A.; Halbina, A.; Polak-Pacholczyk, I.; Rotsztejn, H. Light-Emitting Diodes in Photodynamic Therapy in Non-Melanoma Skin Cancers--Own Observations and Literature Review. J. Cosmet. Laser Ther. 2016, 18, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.M.S.; Teles, F.; Starr, J.R.; Feres, M.; Patel, M.; Martin, L.; Teles, R. Effects of Azithromycin, Metronidazole, Amoxicillin, and Metronidazole plus Amoxicillin on an in Vitro Polymicrobial Subgingival Biofilm Model. Antimicrob. Agents Chemother. 2015, 59, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Dental Biofilms: Difficult Therapeutic Targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Microbiology of Dental Plaque Biofilms and Their Role in Oral Health and Caries. Dent. Clin. N. Am. 2010, 54, 441–454. [Google Scholar] [CrossRef]
- Abusleme, L.; Hoare, A.; Hong, B.-Y.; Diaz, P.I. Microbial Signatures of Health, Gingivitis, and Periodontitis. Periodontology 2000 2021, 86, 57–78. [Google Scholar] [CrossRef]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [PubMed]
- Haag, P.A.; Steiger-Ronay, V.; Schmidlin, P.R. The in Vitro Antimicrobial Efficacy of PDT against Periodontopathogenic Bacteria. Int. J. Mol. Sci. 2015, 16, 27327–27338. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-C.; Chen, C.-J.; Ding, S.-J.; Chen, C.-C. Antimicrobial Efficacy of Methylene Blue-Mediated Photodynamic Therapy on Titanium Alloy Surfaces in Vitro. Photodiagnosis Photodyn. Ther. 2019, 25, 7–16. [Google Scholar] [CrossRef]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Nosi, D.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. Effects of Photodynamic Laser and Violet-Blue Led Irradiation on Staphylococcus aureus Biofilm and Escherichia Coli Lipopolysaccharide Attached to Moderately Rough Titanium Surface: In Vitro Study. Lasers Med. Sci. 2017, 32, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Sharab, L.; Baier, R.E.; Ciancio, S.; Mang, T. Influence of Photodynamic Therapy on Bacterial Attachment to Titanium Surface. J. Oral Implantol. 2021, 47, 427–435. [Google Scholar] [CrossRef]
- Braham, P.; Herron, C.; Street, C.; Darveau, R. Antimicrobial Photodynamic Therapy May Promote Periodontal Healing through Multiple Mechanisms. J. Periodontol. 2009, 80, 1790–1798. [Google Scholar] [CrossRef]
- Cagno, V.; Medaglia, C.; Cerny, A.; Cerny, T.; Zwygart, A.C.-A.; Cerny, E.; Tapparel, C. Methylene Blue Has a Potent Antiviral Activity against SARS-CoV-2 and H1N1 Influenza Virus in the Absence of UV-Activation in Vitro. Sci. Rep. 2021, 11, 14295. [Google Scholar] [CrossRef]
- Lu, G.; Nagbanshi, M.; Goldau, N.; Mendes Jorge, M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Müller, O. Efficacy and Safety of Methylene Blue in the Treatment of Malaria: A Systematic Review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef]
- Hamidi-Alamdari, D.; Hafizi-Lotfabadi, S.; Bagheri-Moghaddam, A.; Safari, H.; Mozdourian, M.; Javidarabshahi, Z.; Peivandi-Yazdi, A.; Ali-Zeraati, A.; Sedaghat, A.; Poursadegh, F.; et al. Methylene Blue for Treatment of Hospitalized COVID-19 Patients: A Randomized, Controlled, Open-Label Clinical Trial, Phase 2. Rev. Investig. Clin. 2021, 73, 190–198. [Google Scholar] [CrossRef]
- Gazel, D.; Zanapalıoğlu Gazel, Ö. In Vitro Activity of Methylene Blue on Mycobacterium Tuberculosis Complex Isolates. Tuberk. Toraks 2021, 69, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Thesnaar, L.; Bezuidenhout, J.J.; Petzer, A.; Petzer, J.P.; Cloete, T.T. Methylene Blue Analogues: In Vitro Antimicrobial Minimum Inhibitory Concentrations and in Silico Pharmacophore Modelling. Eur. J. Pharm. Sci. 2021, 157, 105603. [Google Scholar] [CrossRef] [PubMed]
- Clifton, J.; Leikin, J.B. Methylene Blue. Am. J. Ther. 2003, 10, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.; Liu, H.; Chen, X.; Liu, Y.; Tan, H. Photodynamic Inactivation of Fibroblasts and Inhibition of Staphylococcus epidermidis Adhesion and Biofilm Formation by Toluidine Blue O. Mol. Med. Rep. 2017, 15, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Lavaee, F.; Yousefi, M.; Haddadi, P. Comparison of the Fungicidal Efficacy of Photodynamic Therapy with Methylene Blue, Silver Nanoparticle, and Their Conjugation on Oral Candida Isolates Using Cell Viability Assay. Curr. Med. Mycol. 2020, 6, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; Barrena-López, Y.; Gilaberte, Y.; Rezusta, A. In Vitro Effect of Photodynamic Therapy with Different Lights and Combined or Uncombined with Chlorhexidine on Candida spp. Pharmaceutics 2021, 13, 1176. [Google Scholar] [CrossRef]
- Fumes, A.C.; da Silva Telles, P.D.; Corona, S.A.M.; Borsatto, M.C. Effect of APDT on Streptococcus mutans and Candida albicans Present in the Dental Biofilm: Systematic Review. Photodiagnosis Photodyn. Ther. 2018, 21, 363–366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno-Silva, B.; Parma-Garcia, J.; Frigo, L.; Suárez, L.J.; Macedo, T.T.; Uyeda, F.H.; Melo, M.A.R.d.C.; Sacco, R.; Mourão, C.F.; Feres, M.; et al. Antimicrobial Activity of Methylene Blue Associated with Photodynamic Therapy: In Vitro Study in Multi-Species Oral Biofilm. Pathogens 2024, 13, 342. https://doi.org/10.3390/pathogens13040342
Bueno-Silva B, Parma-Garcia J, Frigo L, Suárez LJ, Macedo TT, Uyeda FH, Melo MARdC, Sacco R, Mourão CF, Feres M, et al. Antimicrobial Activity of Methylene Blue Associated with Photodynamic Therapy: In Vitro Study in Multi-Species Oral Biofilm. Pathogens. 2024; 13(4):342. https://doi.org/10.3390/pathogens13040342
Chicago/Turabian StyleBueno-Silva, Bruno, Javier Parma-Garcia, Lucio Frigo, Lina J. Suárez, Tatiane Tiemi Macedo, Fábio Hideaki Uyeda, Marcelo Augusto Ruiz da Cunha Melo, Roberto Sacco, Carlos Fernando Mourão, Magda Feres, and et al. 2024. "Antimicrobial Activity of Methylene Blue Associated with Photodynamic Therapy: In Vitro Study in Multi-Species Oral Biofilm" Pathogens 13, no. 4: 342. https://doi.org/10.3390/pathogens13040342





