Helminth Prevalence in European Deer with a Focus on Abomasal Nematodes and the Influence of Livestock Pasture Contact: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
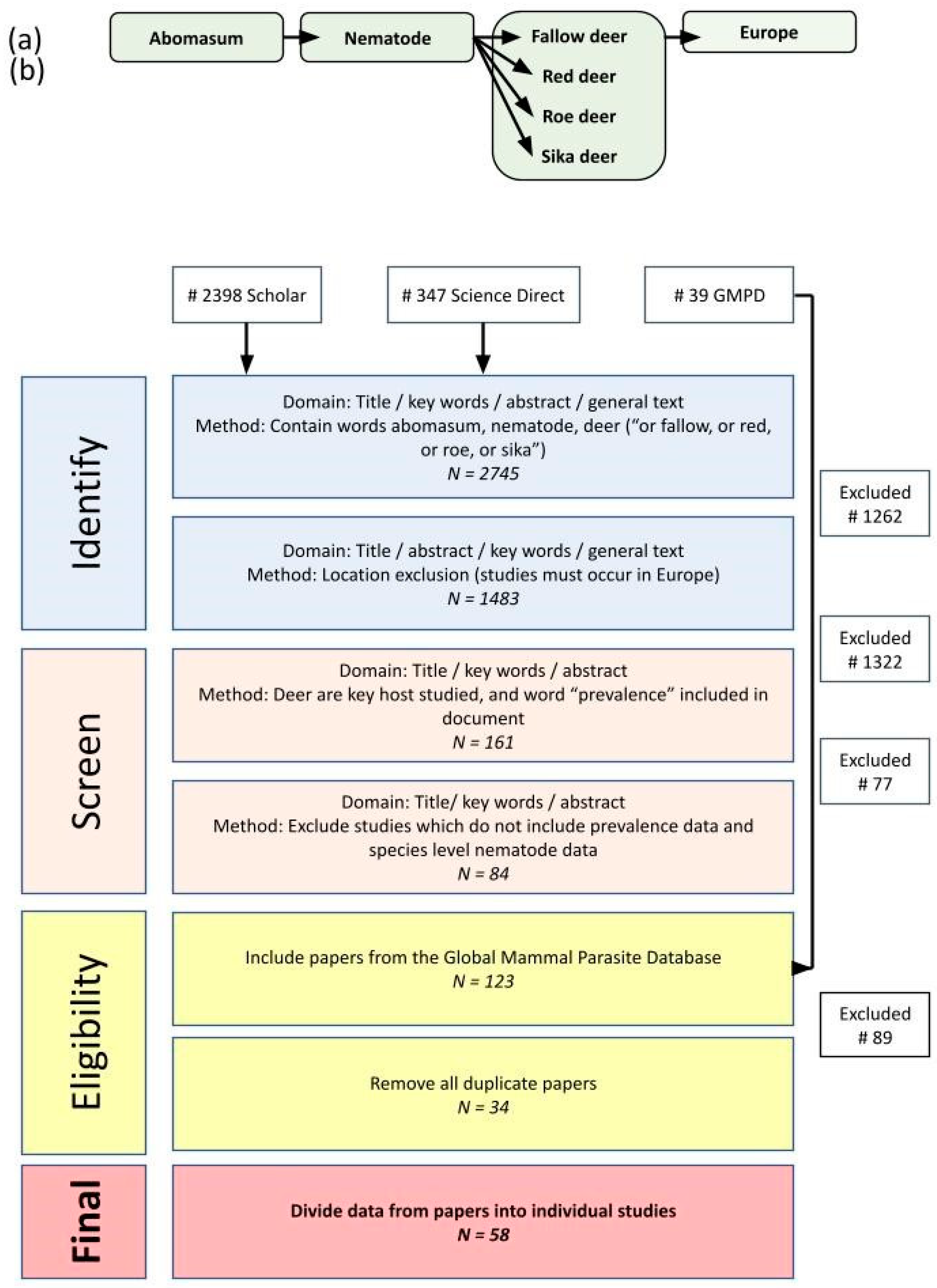
2.1. Review Protocol and Data Collection
2.2. Determining Livestock Contact
2.3. Data Analysis and Visualization
3. Results
3.1. Information on Studies
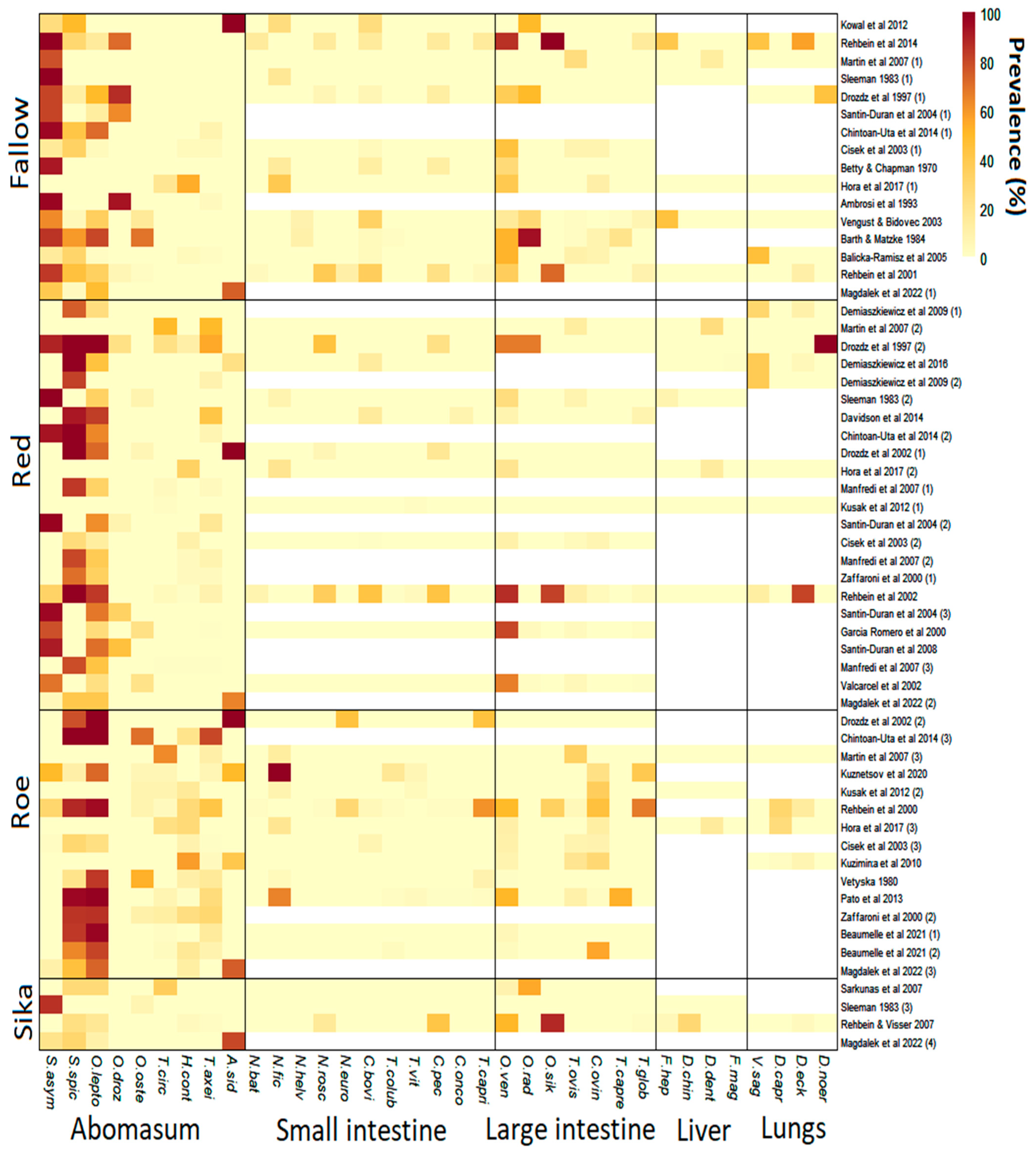
3.2. Abomasal Nematodes
3.3. Intestinal Nematodes
3.4. Liver Fluke and Lungworm
3.5. Impact of Livestock Contact on Abomasal Nematode Prevalence
4. Discussion
4.1. Host Range and Ecology
4.2. Seasonal Patterns
4.3. Abomasal Nematodes as Epidemiological Indicators
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Chartier, C.; Hinney, B.; von Samson-Himmelstjerna, G.; Bacescu, B. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej-Sobocińska, M.; Demiaszkiewicz, A.W.; Pyziel, A.M.; Marczuk, B.; Kowalczyk, R. Does the blood-sucking nematode Ashworthius sidemi (Trichostrongylidae) cause deterioration of blood parameters in European bison (Bison bonasus)? Eur. J. Wildl. Res. 2016, 62, 781–785. [Google Scholar] [CrossRef]
- Vineer, H.R.; Morgan, E.R.; Hertzberg, H.; Bartley, D.J.; Bosco, A.; Charlier, J.; Chartier, C.; Claerebout, E.; de Waal, T.; Hendrickx, G. Increasing importance of anthelmintic resistance in European livestock : Creation and meta-analysis of an open database. Parasite 2020, 27, 2020062. [Google Scholar] [CrossRef] [PubMed]
- Rehbein, S.; Visser, M.; Jekel, I.; Silaghi, C. Endoparasites of the fallow deer (Dama dama) of the Antheringer Au in Salzburg, Austria. Wien. Klin. Wochenschr. 2014, 126, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Santín-Durán, M.; Alunda, J.M.; Hoberg, E.P.; de la Fuente, C. Age Distribution and Seasonal Dynamics of Abomasal Helminths in Wild Red Deer from Central Spain. J. Parasitol. 2008, 94, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, J.; Banul, R.; Jurkiewicz-Azab, J.; Hołdyński, C.; Święczkowska, J.; Nasiadko, M.; Załuski, D. There is only one winner: The negative impact of red deer density on roe deer numbers and distribution in the Słowiński National Park and its vicinity. Ecol. Evol. 2021, 11, 6889–6899. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B. Cervus nippon, Sika Deer. The IUCN Red List of Threatened Species. Mammal Rev. 2015, 41, 313–325. [Google Scholar]
- Masseti, M.; Mertzanidou, D. Dama dama, Fallow Deer. The IUCN Red List of Threatened Species. Mamm. Species 2008, 317, 1–8. [Google Scholar] [CrossRef]
- Pato, F.J.; Vázquez, L.; Díez-Baños, N.; López, C.; Sánchez-Andrade, R.; Fernández, G.; Díez-Baños, P.; Panadero, R.; Díaz, P.; Morrondo, P. Gastrointestinal nematode infections in roe deer (Capreolus capreolus) from the NW of the Iberian Peninsula: Assessment of some risk factors. Vet. Parasitol. 2013, 196, 136–142. [Google Scholar] [CrossRef]
- Rehbein, S.; Visser, M. The endoparasites of Sika deer (Cervus nippon) in Austria. Wien. Klin. Wochenschr. 2007, 119, 96–101. [Google Scholar] [CrossRef]
- Santín-Durán, M.; Alunda, J.M.; Hoberg, E.P.; de la Fuente, C. Abomasal parasites in wild sympatric cervids, Red Deer, Cervus elaphus and Fallow Deer, Dama dama, from three localities across Central and Western Spain: Relationship to host density and park management. J. Parasitol. 2004, 90, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Hora, F.S.; Genchi, C.; Ferrari, N.; Morariu, S.; Mederle, N.; Dărăbuș, G. Frequency of gastrointestinal and pulmonary helminth infections in wild deer from western Romania. Vet. Parasitol. Region. Stud. Rep. 2017, 8, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Kusak, J.; Špičić, S.; Slijepčević, V.; Bosnić, S.; Rajković Janje, R.; Duvnjak, S.; Sindičić, M.; Majnarić, D.; Cvetnić, Ž.; Huber, Đ. Health status of red deer and roe deer in Gorski Kotar, Croatia. Vet. Arh. 2012, 82, 59–73. [Google Scholar]
- Chintoan-Uta, C.; Morgan, E.R.; Skuce, P.J.; Coles, G.C. Wild deer as potential vectors of anthelmintic-resistant abomasal nematodes between cattle and sheep farms. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132985. [Google Scholar] [CrossRef] [PubMed]
- Csivincsik, Á.; Nagy, G.; Halász, T.; Zsolnai, A. Shared pastures and anthelmintic resistance in wildlife and livestock. Agric. Conspec. Sci. 2017, 82, 189–191. [Google Scholar]
- Nagy, G.; Csivincsik, Á.; Sugár, L.; Zsolnai, A. Benzimidazole resistance within red deer, roe deer and sheep populations within a joint habitat in Hungary. Small Rumin. Res. 2017, 149, 172–175. [Google Scholar] [CrossRef]
- Grenfell, B.T. Maximum-likelihood estimates of the mortality and migration rates of the infective larvae of Ostertagia ostertagi and Cooperia oncophora. Parasitology 1986, 92, 643–652. [Google Scholar] [CrossRef]
- O’Connor, L.J.; Walkden-Brown, S.W.; Kahn, L.P. Ecology of the free-living stages of major trichostrongylid parasites of sheep. Vet. Parasitol. 2006, 142, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.R.; Milner-Gulland, E.J.; Torgerson, P.R.; Medley, G.F. Ruminating on complexity: Macroparasites of wildlife and livestock. Trends Ecol. Evol. 2004, 19, 181–188. [Google Scholar] [CrossRef]
- Cerutti, M.C.; Citterio, C.V.; Bazzocchi, C.; Epis, S.; D'Amelio, S.; Ferrari, N.; Lanfranchi, P. Genetic variability of Haemonchus contortus (Nematoda: Trichostrongyloidea) in alpine ruminant host species. J. Helminthol. 2010, 84, 276–283. [Google Scholar] [CrossRef]
- Davidson, R.K.; Kutz, S.J.; Madslien, K.; Hoberg, E.; Handeland, K. Gastrointestinal parasites in an isolated Norwegian population of wild red deer (Cervus elaphus). Acta Vet. Scand. 2014, 56, 59. [Google Scholar] [CrossRef] [PubMed]
- Demiaszkiewicz, A.W.; Merta, D.; Kobielski, J.; Filip, K.J.; Pyziel, A.M. Expansion of Ashworthius sidemi in red deer and roe deer from the Lower Silesian Wilderness and its impact on infection with other gastrointestinal nematodes. Acta Parasitol. 2017, 62, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.R.; Pappalardo, P.; Huang, S.; Byers, J.E.; Farrell, M.J.; Gehman, A.; Ghai, R.R.; Haas, S.E.; Han, B.; Park, A.W.; et al. Global mammal parasite database version 2.0. Ecology 2017, 98, 1476. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.A.; Gasser, R.B.; Chilton, N.B. The ITS-2 rDNA of Teladorsagia circumcincta, T. trifurcata and T. davtiani (Nematoda: Trichostrongylidae) indicates that these taxa are one species. Int. J. Parasitol. 1996, 26, 1123–1126. [Google Scholar] [CrossRef]
- Lienard, E.; Depaquit, J.; Ferté, H. Spiculopteragia mathevossiani Ruchliadev, 1948 is the minor morph of Spiculopteragia spiculoptera (Gushanskaya, 1931): Molecular evidence. Vet. Res. 2006, 37, 683–694. [Google Scholar] [CrossRef]
- Kolde, R. Package “pheatmap”: Pretty heatmaps. Version 1012 1–8. 2019. Available online: https://cran.ms.unimelb.edu.au/web/packages/pheatmap/pheatmap.pdf (accessed on 20 June 2023).
- Wickham, H. Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Kuzmina, T.; Kharchenko, V.; Malega, A. Helminth fauna of roe deer (Capreolus capreolus) in Ukraine: Biodiversity and parasite community. Vestn. Zool. 2010, 44, 12–19. [Google Scholar] [CrossRef]
- Vetýška, V. Endoparasites of Roe Deer in the Strakonice region. Acta Vet. Brno 1980, 49, 91–103. [Google Scholar] [CrossRef]
- Cisek, A.; Balicka-Ramisz, A.; Ramisz, A.; Pilarczyk, B. Occurrence of gastro-intestinal nematodes in cervids (Cervidae) of north-western Poland. Electr. J. Pol. Agric. Univ. 2003, 6, 1–7. [Google Scholar]
- Beaumelle, C.; Redman, E.M.; de Rijke, J.; Wit, J.; Benabed, S.; Debias, F.; Duhayer, J.; Pardonnet, S.; Poirel, M.T.; Capron, G.; et al. Metabarcoding in two isolated populations of wild roe deer (Capreolus capreolus) reveals variation in gastrointestinal nematode community composition between regions and among age classes. Parasites Vectors 2021, 14, 594. [Google Scholar] [CrossRef]
- Kowal, J.; Nosal, P.; Bonczar, Z.; Wajdzik, M. Parasites of captive fallow deer (Dama dama L.) from southern Poland with special emphasis on Ashworthius sidemi. Ann. Parasitol. 2012, 58, 23–26. [Google Scholar] [PubMed]
- Ramajo Martín, V.; Pérez Sánchez, R.; Ramajo Hernández, A.; Oleaga, A. Preliminary data about the parasitism caused by protozoa, helminths and ticks in cervids and wild bovids from Salamanca (western Spain). Res. Rev. Parasitol. 2007, 67, 69–77. [Google Scholar]
- Sleeman, D.P. Parasites of Deer in Ireland. J. Life Sci. R. Dublin Soc. 1983, 4, 203–210. [Google Scholar]
- Drózdz, J.; Malczewski, A.; Demiaszkiewicz, A.W.; Lachowicz, J. The helminthofauna of farmed deer (Cervidae) in Poland. Acta Parasitol. 1997, 42, 225–229. [Google Scholar]
- Batty, A.F.; Chapman, D.I. Gastro-intestinal parasites of wild Fallow Deer (Dama dama L.). J. Helminthol. 1970, 44, 57–61. [Google Scholar] [CrossRef]
- Ambrosi, M.; Manfredi, M.T.; Lanfranchi, P. Pattern of abomasal helminths in fallow deer farming in Umbria (central Italy). Vet. Parasitol. 1993, 47, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Vengušt, G.; Bidovec, A. Parasites of fallow deer (Dama dama) in Slovenia. Helminthologia 2003, 40, 161–164. [Google Scholar]
- Barth, D.; Matzke, P. Gastro-intestinal nematodes of fallow deer (Dama dama L.) in Germany. Vet. Parasitol. 1984, 16, 173–176. [Google Scholar] [CrossRef]
- Balicka-Ramisz, A.; Pilarczyk, B.; Ramisz, A.; Cisek, A. Occurrence of gastrointestinal and pulmonary nematodes of fallow deer (Dama dama L.) in North-West Poland. Acta Parasitol. 2005, 50, 94–96. [Google Scholar]
- Rehbein, S.; Lutz, W.; Visser, M.; Winter, R. Beiträge zur Kenntnis der Parasitenfauna des Wildes in Nordrhein-Westfalen. 2. Der Endoparasitenbefall des Damwildes. Eur. J. Wildl. Res. 2001, 47, 1. [Google Scholar] [CrossRef]
- Magdálek, J.; Bourgoin, G.; Vadlejch, J. Non-native nematode Ashworthius sidemi currently dominates the abomasal parasite community of cervid hosts in the Czech Republic. Front. Vet. Sci. 2022, 9, 862092. [Google Scholar] [CrossRef]
- Demiaszkiewicz, A.W.; Pyziel, A.; Lachowicz, J. Abomasum and lung nematodes of red deer in Strzałowo Forest District (Piska Primeval Forest). Sylwan 2009, 153, 57–61. [Google Scholar]
- Demiaszkiewicz, A.W.; Merta, D.; Kobielski, J. Infection of red deer by parasites in South-Western Poland (Lower Silesian Wilderness). Med. Weter. 2016, 72, 317–320. [Google Scholar]
- Drozdz, J.; Demiaszkiewicz, A.W.; Lachowicz, J. Exchange of gastrointestinal nematodes between roe and red deer [Cervidae] and European bison [Bovidae] in the Bieszczady Mountains [Carpathians, Poland]. Acta Parasitol. 2002, 47, 314–317. [Google Scholar]
- Manfredi, M.T.; Di Cerbo, A.R.; Tranquillo, V.; Nassuato, C.; Pedrotti, L.; Piccolo, G. Abomasal nematodes of the red deer Cervus elaphus in north-eastern Italy. J. Helminthol. 2007, 81, 247–253. [Google Scholar] [CrossRef]
- Zaffaroni, E.; Manfredi, M.T.; Citterio, C.; Sala, M.; Piccolo, G.; Lanfranchi, P. Host specificity of abomasal nematodes in free ranging alpine ruminants. Vet. Parasitol. 2000, 90, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Rehbein, S.; Lutz, W.; Visser, M.; Winter, R. Contributions à la connaissance de la faune parasitaire des espèces-gibier en Rhénanie-Westphalie 3. Endoparasitoses chez le Cerf. Eur. J. Wildl. Res. 2002, 48, 69. [Google Scholar] [CrossRef]
- García-Romero, C.; Valcárcel, F.; Corchero, J.M.; Olmeda, A.S.; Pérez-Jiménez, J.M. A contribution to the study of parasites of red deer (Cervus elaphus) in the provinces of Toledo and Ciudad Real (Castille-La Mancha, Spain). Ecología 2000, 14, 235–249. [Google Scholar]
- Valcárcel, F.; Corchero, J.; Olmeda, A.S.; Rojo Vázquez, F.A.; García Romero, C. Gastrointestinal nematode infections of Cervus elaphus in Castilla-La Mancha (Central Spain). Rev. Ibérica De Parasitol. 2002, 62, 108–113. [Google Scholar]
- Kuznetsov, D.N.; Romashova, N.B.; Romashov, B.V. Gastrointestinal nematodes of European roe deer (Capreolus capreolus) in Russia. Russ. J. Theriol. 2020, 19, 85–93. [Google Scholar] [CrossRef]
- Rehbein, S.; Lutz, W.; Visser, M.; Winter, R. Investigation of the parasite fauna of wildlife in North Rhine-Westphalia. 1. Endoparasites of roe deer. Zeitschrift für Jagdwissenschaft 2000, 46, 248–269. [Google Scholar] [CrossRef]
- Šarkūnas, M.; Veličkaitė, S.; Bružinskaitė, R.; Malakauskas, A.; Petkevičius, S. Faecal egg output and herbage contamination with infective larvae of species of Ostertagia and Oesophagostomum from naturally infected farmed sika deer Cervus nippon in Lithuania. J. Helminthol. 2007, 81, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Barone, C.D.; Wit, J.; Hoberg, E.P.; Gilleard, J.S.; Zarlenga, D.S. Wild ruminants as reservoirs of domestic livestock gastrointestinal nematodes. Vet. Parasitol. 2020, 279, 109041. [Google Scholar] [CrossRef]
- Borgsteede, F.H.M.; Tibben, J.; Cornelissen, J.B.W.; Agneessens, J.; Gaasenbeek, C.P. Nematode parasites of adult dairy cattle in the Netherlands. Vet. Parasitol. 2000, 89, 287–296. [Google Scholar] [CrossRef]
- Charlier, J.; Höglund, J.; Morgan, E.R.; Geldhof, P.; Vercruysse, J.; Claerebout, E. Biology and epidemiology of gastrointestinal nematodes in cattle. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 1–15. [Google Scholar] [CrossRef]
- Wyrobisz-Papiewska, A.; Kowalm, J.; Nosal, P.; Chovancová, G.; Rehbein, S. Host specificity and species diversity of the Ostertagiinae Lopez-Neyra, 1947 in ruminants: A European perspective. Parasites Vectors 2018, 11, 369. [Google Scholar] [CrossRef]
- Justine, J.-L.; Ferté, H. Redescription de Capillaria bovis (Schnyder, 1906) (Nematoda, Capillariinae), parasite du mouflon, du daim et du chevreuil en France. Bull. Mus. Natn. Hist. Nat. Paris 1989, 4, 79–96. [Google Scholar]
- Van Dijk, J.; Morgan, E.R. Hatching behaviour of Nematodirus filicollis in a flock co-infected with Nematodirus battus. Parasitology 2009, 136, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Herlich, H. The development of Cooperia pectinata, a nematode parasite of cattle. Am. J. Vet. Res. 1965, 26, 1026–1031. [Google Scholar]
- Sissay, M.M.; Uggla, A.; Waller, P.J. Prevalence and seasonal incidence of nematode parasites and fluke infections of sheep and goats in eastern Ethiopia. Trop. Anim. Health Prod. 2007, 39, 521–531. [Google Scholar] [CrossRef]
- Goldberg, A. Effects of the nematode Oesophagostomum venulosum on sheep and goats. J. Parasitol. 1952, 38, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Herd, R.P. The Pathogenic Importance of Chaberia Ovina (Fabricius, 1788) in Experimentally Infected Sheep; Pergamon Press: Oxford, UK, 1971. [Google Scholar]
- Ross, J.G.; Dow, C.; Purcell, D.A. A study of Chabertia ovina infections in lambs. Br. Vet. J. 1969, 125, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.A.; Sabourin, E.; Alda, P.; Leroy, C.; Leray, C.; Carron, E.; Mulero, S.; Caty, C.; Hasfia, S.; Boisseau, M.; et al. Genetic diversity and relationships of the liver fluke Fasciola hepatica (Trematoda) with native and introduced definitive and intermediate hosts. Transbound. Emerg. Dis. 2021, 68, 2274–2286. [Google Scholar] [CrossRef] [PubMed]
- Majidi-Rad, M.; Meshgi, B.; Bokaie, S. The prevalence and intensity rate of Dicrocoelium dendriticum infection in ruminants of three provinces in coastal regions of the Caspian Sea. Iran. J. Vet. Med. 2018, 12, 27–33. [Google Scholar] [CrossRef]
- Leontovyč, R.; Košťáková, M.; Siegelová, V.; Melounová, K.; Pankrác, J.; Vrbová, K.; Horák, P.; Kašný, M. Highland cattle and Radix labiata, the hosts of Fascioloides magna. BMC Vet. Res. 2014, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Rehbein, S.; Visser, M.; Hamel, D.; Reindl, H. Occurrence of the giant liver fluke, Fascioloides magna, in sympatric wild ungulates in one area in the Upper Palatinate Forest (northeastern Bavaria, Germany). Parasitol. Res. 2021, 120, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Rehbein, S.; Weigl, S.; Cantacessi, C.; Parisi, A.; Lia, R.P.; Olson, P.D. Morphological and molecular differentiation between Dicrocoelium dendriticum (Rudolphi, 1819) and Dicrocoelium chinensis (Sudarikov and Ryjikov, 1951) Tang and Tang, 1978 (Platyhelminthes: Digenea). Acta Trop. 2007, 104, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Mackintosh, C.G.; Labes, R.E.; Taylor, M.J. Dictyocaulus eckerti, lungworm infecting farmed red deer in new zealand. N. Z. Vet. J. 2001, 49, 34–35. [Google Scholar] [CrossRef]
- Bangoura, B.; Brinegar, B.; Creekmore, T.E. Dictyocaulus cervi-like lungworm infection in a rocky mountain elk (Cervus canadensis nelsoni) from Wyoming, USA. J. Wildl. Dis. 2021, 57, 71–81. [Google Scholar] [CrossRef]
- Divina, B.P.; Wilhelmsson, E.; Mattsson, J.G.; Waller, P.; Höglund, J. Identification of Dictyocaulus spp. in ruminants by morphological and molecular analyses. Parasitology 2000, 121, 193–201. [Google Scholar] [CrossRef]
- Bolukbas, C.S.; Gurler, A.T.; Beyhan, Y.E.; Acici, M.; Umur, S. Helminths of roe deer (Capreolus capreolus) in the Middle Black Sea Region of Turkey. Parasitol. Int. 2012, 61, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Panayotova-Pencheva, M.S.; Alexandrov, M.T. Etiopathological aspects of Elaphostrongylus cervi and Varestrongylus sagittatus infections in red deer in Bulgaria. Acta Vet. Brno 2011, 80, 349–352. [Google Scholar] [CrossRef]
- Morellet, N.; van Moorter, B.; Cargnelutti, B.; Angibault, J.M.; Lourtet, B.; Merlet, J.; Ladet, S.; Hewison, A.M. Landscape composition influences roe deer habitat selection at both home range and landscape scales. Landsc. Ecol. 2011, 26, 999–1010. [Google Scholar] [CrossRef]
- Hofmann, R.R. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive system. Oecologia 1989, 78, 443–457. [Google Scholar] [CrossRef]
- Ferron, E.S.; Verheyden, H.; Hummel, J.; Cargnelutti, B.; Lourtet, B.; Merlet, J.; Gonzalez-Candela, M.; Angibault, J.M.; Hewison, A.J.; Clauss, M. Digestive plasticity as a response to woodland fragmentation in roe deer. Ecol. Res. 2012, 27, 77–82. [Google Scholar] [CrossRef]
- Verheyden, H.; Richomme, C.; Sevila, J.; Merlet, J.; Lourtet, B.; Chaval, Y.; Hoste, H. Relationship between the excretion of eggs of parasitic helminths in roe deer and local livestock density. J. Helminthol. 2020, 94, 1–6. [Google Scholar] [CrossRef]
- Laca Megyesi, Š.; Königová, A.; Babják, M.; Molnár, L.; Rajský, M.; Szestáková, E.; Major, P.; Soroka, J.; Urda Dolinská, M.; Komáromyová, M.; et al. Wild ruminants as a potential risk factor for transmission of drug resistance in the abomasal nematode Haemonchus contortus. Eur. J. Wildl. Res. 2020, 66, 9. [Google Scholar] [CrossRef]
- Walker, J.G.; Evans, K.E.; Rose Vineer, H.; van Wyk, J.A.; Morgan, E.R. Prediction and attenuation of seasonal spillover of parasites between wild and domestic ungulates in an arid mixed-use system. J. Appl. Ecol. 2018, 55, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Verheyden, H.; Aubry, L.; Merlet, J.; Petibon, P.; Chauveau-Duriot, B.; Guillon, N.; Duncan, P. Faecal nitrogen, an index of diet quality in roe deer Capreolus capreolus? Wildl. Biol. 2011, 17, 166–175. [Google Scholar] [CrossRef]
- Hewison, A.J.M.; Morellet, N.; Verheyden, H.; Daufresne, T.; Angibault, J.M.; Cargnelutti, B.; Merlet, J.; Picot, D.; Rames, J.L.; Joachim, J.; et al. Landscape fragmentation influences winter body mass of roe deer. Ecography 2009, 32, 1062–1070. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, N.; Verheyden, H.; Hoste, H.; Cargnelutti, B.; Lourtet, B.; Merlet, J.; Daufresne, T.; Lavín, S.; Hewison, A.M.; Morand, S.; et al. Diet quality and immunocompetence influence parasite load of roe deer in a fragmented landscape. Eur. J. Wildl. Res. 2011, 57, 639–645. [Google Scholar] [CrossRef]
- Jerina, K. Roads and supplemental feeding affect home-range size of Slovenian red deer more than natural factors. J. Mammal. 2012, 93, 1139–1148. [Google Scholar] [CrossRef]
- Nugent, G. Home range size and its development for fallow deer in the Blue Mountains, New Zealand. Acta Theriol. 1994, 39, 159–175. [Google Scholar] [CrossRef]
- Stephens, P.A.; Zaumyslova, O.Y.; Miquelle, D.G.; Myslenkov, A.I.; Hayward, G.D. Estimating population density from indirect sign: Track counts and the Formozov-Malyshev-Pereleshin formula. Anim. Conserv. 2006, 9, 339–348. [Google Scholar] [CrossRef]
- Lovari, S.; Serrao, G.; Mori, E. Woodland features determining home range size of roe deer. Behav. Process. 2017, 140, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hoem, S.A.; Melis, C.; Linnell, J.D.C.; Andersen, R. Fighting behaviour in territorial male roe deer Capreolus capreolus: The effects of antler size and residence. Eur. J. Wildl. Res. 2007, 53, 1–8. [Google Scholar] [CrossRef]
- Maublanc, M.L.; Bideau, E.; Willemet, R.; Bardonnet, C.; Gonzalez, G.; Desneux, L.; Cèbe, N.; Gerard, J.F. Ranging behaviour of roe deer in an experimental high-density population: Are females territorial? Comptes Rendus–Biol. 2012, 335, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Apollonio, M.; Putman, R.; Grignolio, S.; Bartoš, L. Hunting Seasons in Relation to Biological Breeding Seasons and the Implications for the Control or Regulation of Ungulate Populations; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- O’Connor, L.J.; Kahn, L.P.; Walkden-Brown, S.W. Moisture requirements for the free-living development of Haemonchus contortus: Quantitative and temporal effects under conditions of low evaporation. Vet. Parasitol. 2007, 150, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Connan, R.M. Hypobiosis in the ostertagids of red deer and the efficacy of ivermectin and fenbendazole against them. Vet. Rec. 1997, 140, 203–205. [Google Scholar] [CrossRef]
- Gibbs, H.C. Hypobiosis in parasitic nematodes—An update. Adv. Parasitol. 1986, 25, 129–174. [Google Scholar] [CrossRef]
- Sargison, N.D.; Wilson, D.J.; Bartley, D.J.; Penny, C.D.; Jackson, F. Haemonchosis and teladorsagiosis in a Scottish sheep flock putatively associated with the overwintering of hypobiotic fourth stage larvae. Vet. Parasitol. 2007, 147, 326–331. [Google Scholar] [CrossRef]
- Allen, A.R.; Ford, T.; Skuce, R.A. Does Mycobacterium tuberculosis var. bovis survival in the environment confound bovine tuberculosis control and eradication? A literature review. Vet. Med. Int. 2021, 2021, 8812898. [Google Scholar] [CrossRef] [PubMed]
- Crispell, J.; Cassidy, S.; Kenny, K.; McGrath, G.; Warde, S.; Cameron, H.; Rossi, G.; MacWhite, T.; White, P.C.; Lycett, S.; et al. Mycobacterium bovis genomics reveals transmission of infection between cattle and deer in Ireland. Microb. Genom. 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- García-Bocanegra, I.; de Val, B.B.; Arenas-Montes, A.; Paniagua, J.; Boadella, M.; Gortázar, C.; Arenas, A. Seroprevalence and risk factors associated to mycobacterium bovis in wild artiodactyl species from southern spain, 2006–2010. PLoS ONE 2012, 7, 2006–2010. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Mullen, E.; Good, M. Bovine tuberculosis: The emergence of a new wildlife maintenance host in Ireland. Front. Vet. Sci. 2021, 8, 632525. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.V.; Waters, W.R.; Whipple, D.L. Investigation of the transmission of Mycobacterium bovis from deer to cattle through indirect contact. Am. J. Vet. Res. 2004, 65, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Byrne, A.W.; Menzies, F.D.; McBride, K.R.; McCormick, C.M.; Scantlebury, M.; Reid, N. Interspecific visitation of cattle and badgers to fomites: A transmission risk for bovine tuberculosis? Ecol. Evol. 2019, 9, 8479–8489. [Google Scholar] [CrossRef] [PubMed]
- Cowie, C.E.; Hutchings, M.R.; Barasona, J.A.; Gortázar, C.; Vicente, J.; White, P.C. Interactions between four species in a complex wildlife: Livestock disease community: Implications for Mycobacterium bovis maintenance and transmission. Eur. J. Wildl. Res. 2016, 62, 51–64. [Google Scholar] [CrossRef]
- Houe, H. Epidemiology of bovine viral diarrhea virus. Vet. Clin. N. Am. Food Anim. Pract. 1995, 11, 521–547. [Google Scholar] [CrossRef]
- Casaubon, J.; Vogt, H.R.; Stalder, H.; Hug, C.; Ryser-Degiorgis, M.P. Bovine viral diarrhea virus in free-ranging wild ruminants in Switzerland: Low prevalence of infection despite regular interactions with domestic livestock. BMC Vet. Res. 2012, 8, 204. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Du, R.; Wang, Q.; Sun, C.; Wang, N.; Zhang, P.; Zhang, L. Isolation and identification of a bovine viral diarrhea virus from sika deer in China. Virol. J. 2011, 8, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.A.; Gallagher, C.; Carden, R.F.; Lozano, J.M.; Moriarty, J.; O’Neill, R. A survey of free-ranging deer in Ireland for serological evidence of exposure to bovine viral diarrhoea virus, bovine herpes virus-1, bluetongue virus and Schmallenberg virus. Ir. Vet. J. 2017, 70, 13. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, V.; Kukielka, D.; Rivera-Arroyo, B.; Martínez-López, B.; de las Heras, A.I.; Sánchez-Vizcaíno, J.M.; Vicente, J. Evidence of shared bovine viral diarrhea infections between red deer and extensively raised cattle in south-central Spain. BMC Vet. Res. 2016, 12, 1–11. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.C.; Golovljova, I.; Jaenson, T.G.; Jensen, J.K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Lacasta, D.; Lorenzo, M.; González, J.M.; Ruiz de Arcaute, M.; Benito, A.Á.; Baselga, C.; Milian, M.E.; Lorenzo, N.; Jiménez, C.; Villanueva-Saz, S.; et al. Epidemiological study related to the first outbreak of ovine anaplasmosis in spain. Animals 2021, 11, 2036. [Google Scholar] [CrossRef]
- Matei, I.A.; Estrada-Peña, A.; Cutler, S.J.; Vayssier-Taussat, M.; Varela-Castro, L.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 2019, 12, 599. [Google Scholar] [CrossRef]
- Robinson, M.T.; Shaw, S.E.; Morgan, E.R. Anaplasma phagocytophilum infection in a multi-species deer community in the New Forest, England. Eur. J. Wildl. Res. 2009, 55, 439–442. [Google Scholar] [CrossRef]
- Woldehiwet, Z. Anaplasma phagocytophilum in ruminants in Europe. Ann. N. Y. Acad. Sci. 2006, 1078, 446–460. [Google Scholar] [CrossRef]
- Grassi, L.; Franzo, G.; Martini, M.; Mondin, A.; Cassini, R.; Drigo, M.; Pasotto, D.; Vidorin, E.; Menandro, M.L. Ecotyping of Anaplasma Phagocytophilum from wild ungulates and ticks shows circulation of zoonotic strains in northeastern Italy. Animals 2021, 11, 310. [Google Scholar] [CrossRef]
- Brown, T.L.; Airs, P.M.; Porter, S.; Caplat, P.; Morgan, E.R. Understanding the role of wild ruminants in anthelmintic resistance in livestock. Biol. Lett. 2022, 18, 20220057. [Google Scholar] [CrossRef]


| p-Values with Bonferroni Correction | |||
|---|---|---|---|
| Nematodes | Fallow-Red (n = 39) | Fallow-Roe (n = 31) | Red-Roe (n = 38) |
| Spiculopteragia asymmetrica | 0.08 | <0.001 * | 0.74 |
| Spiculopteragia spiculoptera | 0.61 | 0.48 | 1.00 |
| Ostertagia leptospicularis | 0.40 | 0.10 | 0.61 |
| Ostertagia drozdi | 1.00 | 0.11 | 0.49 |
| Ostertagia ostertagi | 1.00 | 1.00 | 0.74 |
| Teladorsagia circumcincta | 0.81 | 0.02 ** | 0.19 |
| Haemonchus contortus | 1.00 | 0.02 ** | 0.01 ** |
| Trichostrongylus axei | 0.35 | 0.01 ** | 0.86 |
| Ashworthius sidemi | 1.00 | 1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, T.L.; Morgan, E.R. Helminth Prevalence in European Deer with a Focus on Abomasal Nematodes and the Influence of Livestock Pasture Contact: A Meta-Analysis. Pathogens 2024, 13, 378. https://doi.org/10.3390/pathogens13050378
Brown TL, Morgan ER. Helminth Prevalence in European Deer with a Focus on Abomasal Nematodes and the Influence of Livestock Pasture Contact: A Meta-Analysis. Pathogens. 2024; 13(5):378. https://doi.org/10.3390/pathogens13050378
Chicago/Turabian StyleBrown, Tony L., and Eric R. Morgan. 2024. "Helminth Prevalence in European Deer with a Focus on Abomasal Nematodes and the Influence of Livestock Pasture Contact: A Meta-Analysis" Pathogens 13, no. 5: 378. https://doi.org/10.3390/pathogens13050378






