Abstract
Human Metapneumovirus (hMPV) is a leading respiratory viral pathogen associated with bronchiolitis, pneumonia, and asthma exacerbation in young children, the elderly and immunocompromised individuals. The development of a potential vaccine against hMPV requires detailed understanding of the host immune system, which plays a significant role in hMPV pathogenesis, susceptibility and vaccine efficacy. As a result, animal models have been developed to better understand the mechanisms by which hMPV causes disease. Several animal models have been evaluated and established so far to study the host immune responses and pathophysiology of hMPV infection. However, inbred laboratory mouse strains have been one of the most used animal species for experimental modeling and therefore used for the studies of immunity and immunopathogenesis to hMPV. This review summarizes the contributions of the mouse model to our understanding of the immune response against hMPV infection.
1. Introduction
Human metapneumovirus (hMPV), belongs to the Paramyxoviridae family and represents the first human member of the genus Metapneumovirus. hMPV is a leading respiratory viral pathogen causing acute respiratory tract infection (ARTI) in young children, the elderly and immunocompromised individuals []. hMPV was first isolated in the Netherlands in 2001 from respiratory specimens of young children suffering with acute respiratory tract illness [] and represents a major respiratory pathogen worldwide. Epidemiological studies show that hMPV is responsible for 5%–15% of pediatric hospitalizations for respiratory tract infections [,,,,]. It induces clinical syndromes ranging from mild disease to more severe disease, with high fever, wheezing, severe cough, difficulty in breathing, tachypnea, bronchiolitis and pneumonia [,,].
hHMPV is an enveloped, negative sense single-stranded RNA virus (Figure 1). Based on phylogenetic analysis, hMPV is classified into four genetic lineages, named A1, A2, B1 and B2 that divide into the A and B antigenic subgroups that belong to one serotype [,]. hMPV genome size is approximately 13,000 nt as it varies depending on the strain. Examples of the subgroup A indicate that the strain CAN97-83 is 13,335 nt and NL/00/1 is 13,350 nt, and for the subgroup B: CAN98-75 is 13,280 nt and NL/1/99 is 13,293 nt [,]. The hMPV sequence includes eight genes encoding nine proteins: nucleocapsid (N), phosphoprotein (P), matrix (M), second matrix (M2-1, M2-2), fusion (F), small hydrophobic (SH), attachment (G) and RNA-dependent RNA polymerase (L). The gene order in hMPV is represented as 3′-N-P-M-F-M2-SH-G-L-5′ (Figure 1). The attachment (G) and small hydrophobic (SH) genes are found to be highly variable while a high level of sequence conservation has been observed for the fusion (F) gene []. The G protein is a transmembrane surface glycoprotein, which initiates the virus-host cell membrane attachment and so considered as a key player in viral replication. The fusion (F) protein is required for the fusion of virus with host cell membrane and is capable of being accessed by neutralizing antibodies. The nucleocapsid (N), phosphoprotein (P) and RNA-dependent RNA polymerase (L) proteins along with M2 protein are involved in RNA synthesis [,,].
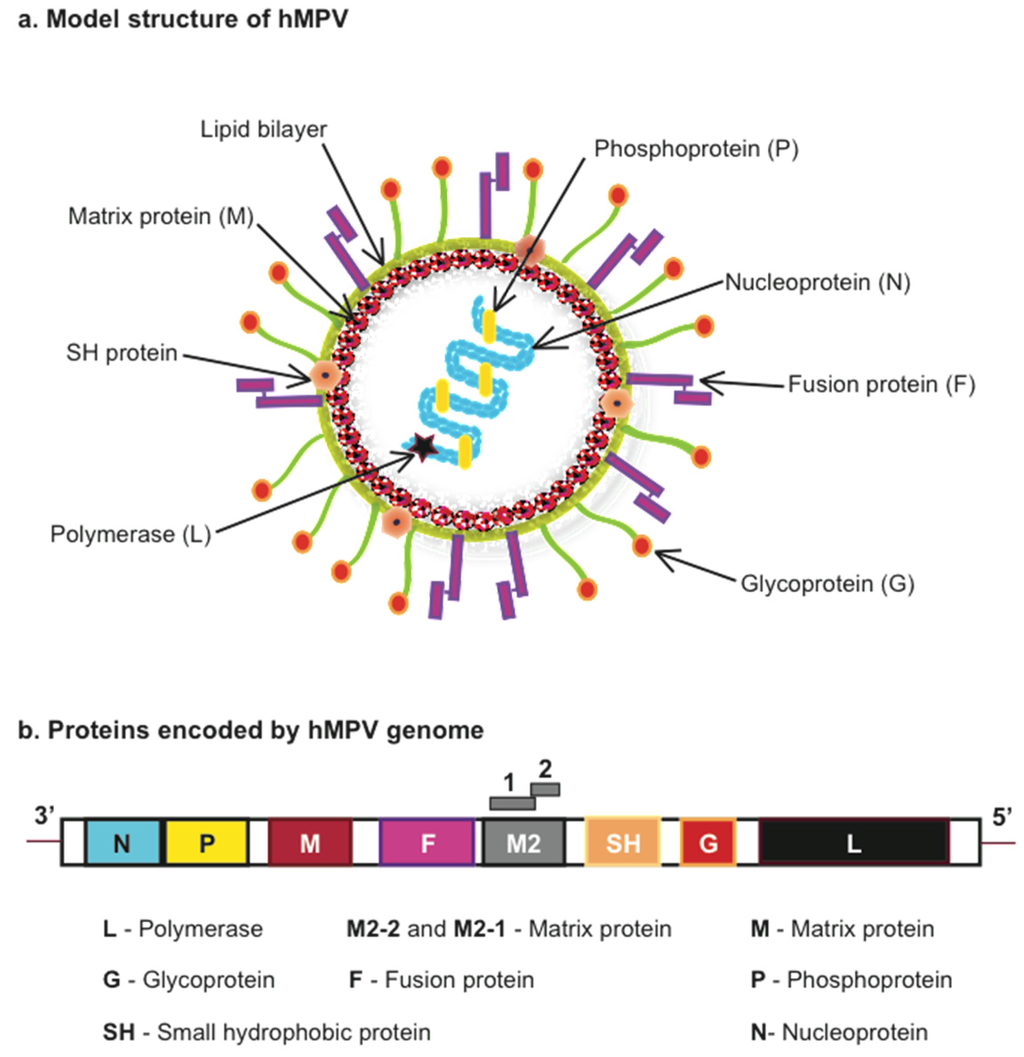
Figure 1.
Model structure and proteins encoded by Human Metapneumovirus (hMPV). (a) hMPV model structure indicating viral proteins encoded by (b) the viral genome.
Several animal models including mouse (see Table 1), cotton rat [,,,], hamster [,,], ferret [] and nonhuman primate models [,,] have been established to date to study the immunopathology occurring after hMPV infection. Among them, the mouse model has provided considerable knowledge towards our understanding of the hMPV-host interaction. Thus this review focuses on the current knowledge of the immunity and immunopathology induced by hMPV in the experimental mouse model of infection.

Table 1.
Different conditions for mouse infection with hMPV.
| Mice Strain | Mice Age | (Group) Strain | Virus Dose | Refs. |
|---|---|---|---|---|
| BALB/c | F 6–8 week-old | (A) NL 00-01 | 3.3 × 105 PFU | [] |
| BALB/c | F 4–6-week-old | (A) C-85473 | 1.5 × 105–108 TCID50 | [,,,,,,,] |
| BALB/c | F 6–8-week-old | (A) C4-CJP05 | 106 PFU | [] |
| BALB/c | F 4–6-week-old | (B) CAN98-75 | 0.8–1 × 106 PFU | [,,] |
| BALB/c | F 5–7 week-old | (A) NL/1/00 | 106–107 PFU | [,] |
| BALB/c | F 6–7 week-old | (B) NL/1/99 | 107 PFU | [] |
| BALB/c | F 6–10 week-old | (A) CAN97-83 | 106–107 PFU/TCID50 | [,,,,,] |
| BALB/c | F 5–6 week-old | (A) CZ0107 | 106 PFU | [] |
| BALB/c | M 19 month-old | (A) CAN97-83 | 2 × 107 geq | [] |
| BALB/c | F 8–10 week-old | (A) D03-574 | 2 × 105 PFU | [] |
| C57BL/6 | 6–10 week-old | (A) CAN97-83 | 106–107 PFU | [,,,,,] |
| C57BL/6 | F 6–12 week-old | (A) TN/94-49 | 0.6–1.5 × 106 PFU | [,,,] |
| DBA/2 | 5–6 week-old | (A) TN/94-49 | 105.9 PFU | [] |
| SCID | F 6–8 week-old | (A) NL/1/00 | 6.5 × 106 PFU | [] |
PFU = Plaque Forming Units; geq = genome equivalents; TCID50 = 50% tissue culture infective dose.
2. hMPV Infection in Mice
The experimental mouse model of hMPV infection has been established in several mouse backgrounds using different hMPV strains at diverse inoculum concentrations, as shown in Table 1.
Intranasal inoculation of mice with hMPV induces pulmonary inflammation characterized by interstitial inflammation and/or peribronchiolar and perivascular cellular infiltration [,,,], body weight loss with a peak of 15%–25% [,,,,], altered respiratory function characterized by a significant increase in airway obstruction on day 5 after hMPV infection that could persist until day 21 [], and lung viral titers that peak between day 3 to day 14 after hMPV infection [,,,].
However, some variations can be observed depending on the different experimental conditions. For instance, intranasal inoculation of BALB/c mice with hMPV CAN98-75 resulted in a biphasic lung viral replication with peaks at day 7 and day 14 [,] while infection of BALB/c mice with any other hMPV strain led to a one-peak only of viral titer on or before day 5 after infection (Table 2). Based on the data from the reports included in Table 2, BALB/c mice appear to be more permissive than C57BL/6 mice. Although, shedding of infectious virus beyond the recovery phase has been rarely reported [], detection of hMPV transcripts have been found at day 154 [] and 180 [] after infection, suggesting that hMPV could persist in the lung of infected animals since hMPV infection has been characterized as a localized infection affecting just the airways but no other organs [].

Table 2.
Mouse susceptibility and permissibility to hMPV.
| Mice Strain | Virus Strain | Virus Inoculum | Peak Viral Titer | Ref. |
|---|---|---|---|---|
| BALB/c | NL/1/00 | 3.3 × 105 PFU | Day 4 (Log10 2.37 PFU/g) | [] |
| BALB/c | CAN97-83 | 107 TCID50 | Day 4 (105 TCID50/g) | [] |
| BALB/c | C85473 | 1.5 × 105 TCID50 | Day 6 (~104 TCID50/lung) | [] |
| BALB/c | C85473 | 1 × 108 TCID50 | Day 5 (7 × 106 TCID50/lung) | [] |
| BALB/c | C85473 | 1 × 108 TCID50 | Day 5 (1.92 × 107 TCID50/g) | [] |
| BALB/c | C85473 | 5.8 × 105 TCID50 | Day 5 (~105 TCID50/g) | [] |
| BALB/c | NL/1/00 | 1.5 × 105 PFU | Day 5 (5.1 × 105 PFU/g) | [] |
| BALB/c | D03-574 | 2 × 105 PFU | Day 4 (~103.6 PFU/lung) | [] |
| C57BL/6 | CAN97-83 | 5 × 106 PFU | Day 5 (104.9 PFU/g) | [] |
| C57BL/6 | TN/94-49 | 1 × 106 PFU | Day 5 (~4.7 Log10 PFU/g) | [] |
| C57BL/6 | CAN97-83 | 1 × 107 PFU | Day 5 (~4.1 Log10 PFU/g) | [] |
| C57BL/6 | TN/94-49 | 6 × 105 PFU | Day 5 (~4.2 Log10 PFU/g) | [] |
4. Conclusions
The experimental mouse model represents a valuable tool for in vivo research on hMPV infection and has provided important information regarding the hMPV-induced disease and detailed aspects of the immune response induced by hMPV infection. Although, inherent limitations are observed in the mouse model when data are extrapolated to the natural human infection, due to the availability of several gene deficient mice strains and multiple murine specific antibodies, it provides a valued experimental small animal model that allows answering critical questions that are necessary to our better understanding of the immune response and disease pathogenesis of hMPV.
Acknowledgments
Research in Antonieta Guerrero-Plata’s laboratory has been supported by Grants from the National Center for Research Resources (P20RR020159-09), and the National Institute of General Medical Sciences (P20GM103458-09) from the National Institutes of Health, the LSU Competitive Organized Research program (LAV-3489), and the Louisiana Experimental Program from the National Science Foundation and Louisiana Board of Regents (41884).
Author Contributions
Antonieta Guerrero-Plata and Nagarjuna R. Cheemarla performed the literature research and wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feuillet, F.; Lina, B.; Rosa-Calatrava, M.; Boivin, G. Ten years of human metapneumovirus research. J. Clin. Virol. 2012, 53, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001, 7, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; de Serres, G.; Cote, S.; Gilca, R.; Abed, Y.; Rochette, L.; Bergeron, M.G.; Dery, P. Human metapneumovirus infections in hospitalized children. Emerg. Infect. Dis. 2003, 9, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, S.; Minini, C.; Colombrita, D.; Rossi, D.; Miglietti, N.; Vettore, E.; Caruso, A.; Fiorentini, S. Human metapneumovirus infection in young children hospitalized with acute respiratory tract disease: Virologic and clinical features. Pediatr. Infect. Dis. J. 2008, 27, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.E., Jr. Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr. Infect. Dis. J. 2004, 23, S215–S221. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.S. Epidemiology of human metapneumovirus. Clin. Microbiol. Rev. 2006, 19, 546–557. [Google Scholar] [CrossRef]
- Mullins, J.A.; Erdman, D.D.; Weinberg, G.A.; Edwards, K.; Hall, C.B.; Walker, F.J.; Iwane, M.; Anderson, L.J. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg. Infect. Dis. 2004, 10, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Esper, F.; Boucher, D.; Weibel, C.; Martinello, R.A.; Kahn, J.S. Human metapneumovirus infection in the United States: Clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics 2003, 111, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.S. Human metapneumovirus, a newly emerging respiratory virus. Pediatr. Infect. Dis. J. 2003, 22, 923–924. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.V.; Wang, C.K.; Yang, C.F.; Tollefson, S.J.; House, F.S.; Heck, J.M.; Chu, M.; Brown, J.B.; Lintao, L.D.; Quinto, J.D.; et al. The role of human metapneumovirus in upper respiratory tract infections in children: A 20-year experience. J. Infect. Dis. 2006, 193, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Crowe, J. Respiratory Syncytial Virus and Metapneumovirus. In Fileds Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2007; Volume 2, pp. 1601–1646. [Google Scholar]
- Van den Hoogen, B.G.; Herfst, S.; Sprong, L.; Cane, P.A.; Forleo-Neto, E.; de Swart, R.L.; Osterhaus, A.D.; Fouchier, R.A. Antigenic and genetic variability of human metapneumoviruses. Emerg. Infect. Dis. 2004, 10, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Biacchesi, S.; Skiadopoulos, M.H.; Boivin, G.; Hanson, C.T.; Murphy, B.R.; Collins, P.L.; Buchholz, U.J. Genetic diversity between human metapneumovirus subgroups. Virology 2003, 315, 1–9. [Google Scholar] [CrossRef]
- Mackay, I.M.; Jacob, K.C.; Woolhouse, D.; Waller, K.; Syrmis, M.W.; Whiley, D.M.; Siebert, D.J.; Nissen, M.; Sloots, T.P. Molecular assays for detection of human metapneumovirus. J. Clin. Microbiol. 2003, 41, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Easton, A.J.; Domachowske, J.B.; Rosenberg, H.F. Animal pneumoviruses: Molecular genetics and pathogenesis. Clin. Microbiol. Rev. 2004, 17, 390–412. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, M.E.; Yim, K.; Kuhn, K.H.; Cragin, R.P.; Boukhvalova, M.; Blanco, J.C.; Prince, G.A.; Boivin, G. Pathogenesis of human metapneumovirus lung infection in BALB/c mice and cotton rats. J. Virol. 2005, 79, 8894–8903. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Tollefson, S.J.; Podsiad, A.B.; Shepherd, B.E.; Polosukhin, V.V.; Johnston, R.E.; Williams, J.V.; Crowe, J.E., Jr. An alphavirus replicon-based human metapneumovirus vaccine is immunogenic and protective in mice and cotton rats. J. Virol. 2008, 82, 11410–11418. [Google Scholar] [CrossRef] [PubMed]
- Green, M.G.; Huey, D.; Niewiesk, S. The cotton rat (Sigmodon hispidus) as an animal model for respiratory tract infections with human pathogens. Lab Animal 2013, 42, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalova, M.S.; Prince, G.A.; Blanco, J.C. The cotton rat model of respiratory viral infections. Biologicals 2009, 37, 152–159. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, M.; Schickli, J.H.; Tang, R.S.; Kaur, J.; Robinson, C.; Fouchier, R.A.; Osterhaus, A.D.; Spaete, R.R.; Haller, A.A. Identification of small-animal and primate models for evaluation of vaccine candidates for human metapneumovirus (hMPV) and implications for hMPV vaccine design. J. Gen. Virol. 2004, 85, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Herfst, S.; de Graaf, M.; Schrauwen, E.J.; Sprong, L.; Hussain, K.; van den Hoogen, B.G.; Osterhaus, A.D.; Fouchier, R.A. Generation of temperature-sensitive human metapneumovirus strains that provide protective immunity in hamsters. J. Gen. Virol. 2008, 89, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Schickli, J.H.; Kaur, J.; Macphail, M.; Guzzetta, J.M.; Spaete, R.R.; Tang, R.S. Deletion of human metapneumovirus M2-2 increases mutation frequency and attenuates growth in hamsters. Virol. J. 2008. [Google Scholar] [CrossRef] [PubMed]
- Biacchesi, S.; Pham, Q.N.; Skiadopoulos, M.H.; Murphy, B.R.; Collins, P.L.; Buchholz, U.J. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J. Virol. 2005, 79, 12608–12613. [Google Scholar] [CrossRef] [PubMed]
- Biacchesi, S.; Pham, Q.N.; Skiadopoulos, M.H.; Murphy, B.R.; Collins, P.L.; Buchholz, U.J. Modification of the trypsin-dependent cleavage activation site of the human metapneumovirus fusion protein to be trypsin independent does not increase replication or spread in rodents or nonhuman primates. J. Virol. 2006, 80, 5798–5806. [Google Scholar] [CrossRef] [PubMed]
- Darniot, M.; Petrella, T.; Aho, S.; Pothier, P.; Manoha, C. Immune response and alteration of pulmonary function after primary human metapneumovirus (hMPV) infection of BALB/c mice. Vaccine 2005, 23, 4473–4480. [Google Scholar] [CrossRef] [PubMed]
- Kukavica-Ibrulj, I.; Hamelin, M.E.; Prince, G.A.; Gagnon, C.; Bergeron, Y.; Bergeron, M.G.; Boivin, G. Infection with human metapneumovirus predisposes mice to severe pneumococcal pneumonia. J. Virol. 2009, 83, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, M.E.; Prince, G.A.; Boivin, G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob. Agents Chemother. 2006, 50, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, M.E.; Couture, C.; Sackett, M.K.; Boivin, G. Enhanced lung disease and Th2 response following human metapneumovirus infection in mice immunized with the inactivated virus. J. Gen. Virol. 2007, 88, 3391–3400. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Aerts, L.; Hamelin, M.E.; Granier, C.; Szecsi, J.; Lavillette, D.; Boivin, G.; Cosset, F.L. Virus-like particle vaccine induces cross-protection against human metapneumovirus infections in mice. Vaccine 2013, 31, 2778–2785. [Google Scholar] [CrossRef] [PubMed]
- Hamelin, M.E.; Prince, G.A.; Gomez, A.M.; Kinkead, R.; Boivin, G. Human metapneumovirus infection induces long-term pulmonary inflammation associated with airway obstruction and hyperresponsiveness in mice. J. Infect. Dis. 2006, 193, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Ludewick, H.P.; Aerts, L.; Hamelin, M.E.; Boivin, G. Long-term impairment of Streptococcus pneumoniae lung clearance is observed after initial infection with influenza A virus but not human metapneumovirus in mice. J. Gen. Virol. 2011, 92, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Aerts, L.; Hamelin, M.E.; Rheaume, C.; Lavigne, S.; Couture, C.; Kim, W.; Susan-Resiga, D.; Prat, A.; Seidah, N.G.; Vergnolle, N.; et al. Modulation of protease activated receptor 1 influences human metapneumovirus disease severity in a mouse model. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Darniot, M.; Schildgen, V.; Schildgen, O.; Sproat, B.; Kleines, M.; Ditt, V.; Pitoiset, C.; Pothier, P.; Manoha, C. RNA interference in vitro and in vivo using DsiRNA targeting the nucleocapsid N mRNA of human metapneumovirus. Antiviral Res. 2012, 93, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Harrod, K.S.; Shieh, W.J.; Zaki, S.; Tripp, R.A. Human metapneumovirus persists in BALB/c mice despite the presence of neutralizing antibodies. J. Virol. 2004, 78, 14003–14011. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Tripp, R.A. The immune response to human metapneumovirus is associated with aberrant immunity and impaired virus clearance in BALB/c mice. J. Virol. 2005, 79, 5971–5978. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shu, Z.; Qin, X.; Dou, Y.; Zhao, Y.; Zhao, X. A live attenuated human metapneumovirus vaccine strain provides complete protection against homologous viral infection and cross-protection against heterologous viral infection in BALB/c mice. Clin. Vaccine Immunol. 2013, 20, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A.; Casola, A.; Garofalo, R.P. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J. Virol. 2005, 79, 14992–14997. [Google Scholar] [CrossRef] [PubMed]
- Herd, K.A.; Mahalingam, S.; Mackay, I.M.; Nissen, M.; Sloots, T.P.; Tindle, R.W. Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice. J. Virol. 2006, 80, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A.; Baron, S.; Poast, J.S.; Adegboyega, P.A.; Casola, A.; Garofalo, R.P. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J. Virol. 2005, 79, 10190–10199. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A.; Kolli, D.; Hong, C.; Casola, A.; Garofalo, R.P. Subversion of pulmonary dendritic cell function by paramyxovirus infections. J. Immunol. 2009, 182, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Kolli, D.; Bataki, E.L.; Spetch, L.; Guerrero-Plata, A.; Jewell, A.M.; Piedra, P.A.; Milligan, G.N.; Garofalo, R.P.; Casola, A. T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection. J. Virol. 2008, 82, 8560–8569. [Google Scholar] [CrossRef] [PubMed]
- Palavecino, C.E.; Cespedes, P.F.; Gomez, R.S.; Kalergis, A.M.; Bueno, S.M. Immunization with a recombinant bacillus Calmette-Guerin strain confers protective Th1 immunity against the human metapneumovirus. J. Immunol. 2014, 192, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ditt, V.; Lusebrink, J.; Tillmann, R.L.; Schildgen, V.; Schildgen, O. Respiratory infections by HMPV and RSV are clinically indistinguishable but induce different host response in aged individuals. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed]
- Huck, B.; Neumann-Haefelin, D.; Schmitt-Graeff, A.; Weckmann, M.; Mattes, J.; Ehl, S.; Falcone, V. Human metapneumovirus induces more severe disease and stronger innate immune response in BALB/c mice as compared with respiratory syncytial virus. Respir. Res. 2007. [Google Scholar] [CrossRef] [PubMed]
- Banos-Lara Mdel, R.; Ghosh, A.; Guerrero-Plata, A. Critical role of MDA5 in the interferon response induced by human metapneumovirus infection in dendritic cells and in vivo. J. Virol. 2013, 87, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Zhou, Z.; Wakamatsu, N.; Guerrero-Plata, A. Interleukin-12p40 modulates human metapneumovirus-induced pulmonary disease in an acute mouse model of infection. PLoS ONE 2012. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Kolli, D.; Deng, J.; Fang, R.; Gong, B.; Xue, M.; Casola, A.; Garofalo, R.P.; Wang, T.; Bao, X. MyD88 controls human metapneumovirus-induced pulmonary immune responses and disease pathogenesis. Virus Res. 2013, 176, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Velayutham, T.S.; Kolli, D.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Critical role of TLR4 in human metapneumovirus mediated innate immune responses and disease pathogenesis. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Banos-Lara Mdel, R.; Harvey, L.; Mendoza, A.; Simms, D.; Chouljenko, V.N.; Wakamatsu, N.; Kousoulas, K.G.; Guerrero-Plata, A. Impact and regulation of lambda interferon response in human metapneumovirus infection. J. Virol. 2015, 89, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.G.; Erickson, J.J.; Hastings, A.K.; Becker, J.C.; Johnson, M.; Craven, R.E.; Tollefson, S.J.; Boyd, K.L.; Williams, J.V. Human metapneumovirus virus-like particles induce protective B and T cell responses in a mouse model. J. Virol. 2014, 88, 6368–6379. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.C.; Tollefson, S.J.; Johnson, M.; Gilchuk, P.; Boyd, K.L.; Shepherd, B.; Joyce, S.; Williams, J.V. Acute clearance of human metapneumovirus occurs independently of natural killer cells. J. Virol. 2014, 88, 10963–10969. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.J.; Rogers, M.C.; Hastings, A.K.; Tollefson, S.J.; Williams, J.V. Programmed death-1 impairs secondary effector lung CD8(+) T cells during respiratory virus reinfection. J. Immunol. 2014, 193, 5108–5117. [Google Scholar] [CrossRef] [PubMed]
- Hastings, A.K.; Erickson, J.J.; Schuster, J.E.; Boyd, K.L.; Tollefson, S.J.; Johnson, M.; Gilchuk, P.; Joyce, S.; Williams, J.V. Role of type I interferon signaling in human metapneumovirus pathogenesis and control of viral replication. J. Virol. 2015, 89, 4405–4420. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Li, R.P.; Chen, X.; Liu, P.; Zhao, X.D. Replication and pathogenicity of attenuated human metapneumovirus F mutants in severe combined immunodeficiency mice. Vaccine 2012, 30, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dou, Y.; Wu, J.; She, W.; Luo, L.; Zhao, Y.; Liu, P.; Zhao, X. Effects of N-linked glycosylation of the fusion protein on replication of human metapneumovirus in vitro and in mouse lungs. J. Gen. Virol. 2011, 92, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Reikine, S.; Nguyen, J.B.; Modis, Y. Pattern Recognition and Signaling Mechanisms of RIG-I and MDA5. Front. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Spann, K.M.; Loh, Z.; Lynch, J.P.; Ullah, A.; Zhang, V.; Baturcam, E.; Werder, R.B.; Khajornjiraphan, N.; Rudd, P.; Loo, Y.M.; et al. IRF-3, IRF-7, and IPS-1 promote host defense against acute human metapneumovirus infection in neonatal mice. Am. J. Pathol. 2014, 184, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Chen, Y.; Liu, G.; Ren, J.; Go, C.; Ivanciuc, T.; Deepthi, K.; Casola, A.; Garofalo, R.P.; Bao, X. MAVS plays an essential role in host immunity against human metapneumovirus. J. Gen. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Laham, F.R.; Israele, V.; Casellas, J.M.; Garcia, A.M.; Lac Prugent, C.M.; Hoffman, S.J.; Hauer, D.; Thumar, B.; Name, M.I.; Pascual, A.; et al. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J. Infect. Dis. 2004, 189, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Aherne, W.; Bird, T.; Court, S.D.; Gardner, P.S.; McQuillin, J. Pathological changes in virus infections of the lower respiratory tract in children. J. Clin. Pathol. 1970, 23, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Darniot, M.; Pitoiset, C.; Petrella, T.; Aho, S.; Pothier, P.; Manoha, C. Age-associated aggravation of clinical disease after primary metapneumovirus infection of BALB/c mice. J. Virol. 2009, 83, 3323–3332. [Google Scholar] [CrossRef] [PubMed]
- Stumbles, P.A.; Upham, J.W.; Holt, P.G. Airway dendritic cells: Co-ordinators of immunological homeostasis and immunity in the respiratory tract. APMIS 2003, 111, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A.; Casola, A.; Suarez, G.; Yu, X.; Spetch, L.; Peeples, M.E.; Garofalo, R.P. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 2006, 34, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Plata, A. Dendritic cells in human Pneumovirus and Metapneumovirus infections. Viruses 2013, 5, 1553–1570. [Google Scholar] [CrossRef] [PubMed]
- Le Nouen, C.; Munir, S.; Losq, S.; Winter, C.C.; McCarty, T.; Stephany, D.A.; Holmes, K.L.; Bukreyev, A.; Rabin, R.L.; Collins, P.L.; et al. Infection and maturation of monocyte-derived human dendritic cells by human respiratory syncytial virus, human metapneumovirus, and human parainfluenza virus type 3. Virology 2009, 385, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Pribul, P.K.; Harker, J.; Wang, B.; Wang, H.; Tregoning, J.S.; Schwarze, J.; Openshaw, P.J. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol. 2008, 82, 4441–4448. [Google Scholar] [CrossRef] [PubMed]
- Lohmann-Matthes, M.L.; Steinmuller, C.; Franke-Ullmann, G. Pulmonary macrophages. Eur. Respir. J. 1994, 7, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Kolli, D.; Gupta, M.R.; Sbrana, E.; Velayutham, T.S.; Hong, C.; Casola, A.; Garofalo, R.P. Alveolar Macrophages Contribute to the Pathogenesis of hMPV Infection While Protecting Against RSV Infection. Am. J. Respir. Cell Mol. Biol. 2014, 51, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Benoit, A.; Huang, Y.; Proctor, J.; Rowden, G.; Anderson, R. Effects of alveolar macrophage depletion on liposomal vaccine protection against respiratory syncytial virus (RSV). Clin. Exp. Immunol. 2006, 145, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Herd, K.A.; Nelson, M.; Mahalingam, S.; Tindle, R.W. Pulmonary infection of mice with human metapneumovirus induces local cytotoxic T-cell and immunoregulatory cytokine responses similar to those seen with human respiratory syncytial virus. J. Gen. Virol. 2010, 91, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.J.; Gilchuk, P.; Hastings, A.K.; Tollefson, S.J.; Johnson, M.; Downing, M.B.; Boyd, K.L.; Johnson, J.E.; Kim, A.S.; Joyce, S.; et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J. Clin. Investig. 2012, 122, 2967–2982. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.C.; Schuster, J.E.; Gilchuk, P.; Boyd, K.L.; Joyce, S.; Williams, J.V. Lung CD8+ T cell impairment occurs during human metapneumovirus infection despite Virus-Like Particle (VLP) induction of functional CD8+ T cells. J. Virol. 2015, 89, 8713–8726. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).