How Chemotherapy Increases the Risk of Systemic Candidiasis in Cancer Patients: Current Paradigm and Future Directions
Abstract
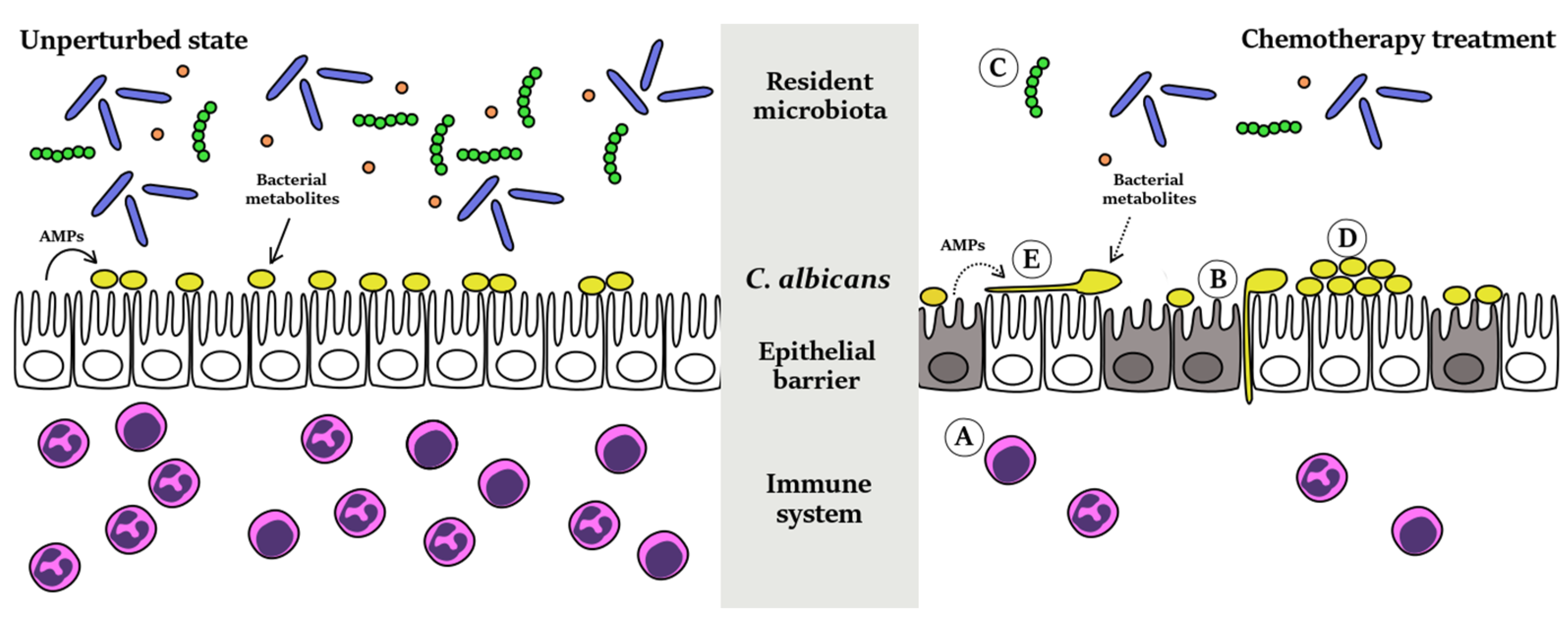
:1. Introduction
2. Epidemiology of Systemic Candidiasis in Cancer Patients
3. Effects of Chemotherapy on the Immune System
3.1. Effects on Innate Immunity
3.1.1. Cellular Factors
3.1.2. Humoral Factors
3.2. Effects on Adaptive Immunity
3.2.1. Cellular Factors
3.2.2. Humoral Factors
4. Effect of Chemotherapy on the Gut Epithelial Barrier and Resident Microbiota
4.1. Epithelial Barrier
4.2. Gut Microbiota
5. Effect of Chemotherapy on Candida Albicans
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. World Cancer Report; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Frei, E., 3rd; Karon, M.; Levin, R.H.; Freireich, E.J.; Taylor, R.J.; Hananian, J.; Selawry, O.; Holland, J.F.; Hoogstraten, B.; Wolman, I.J.; et al. The effectiveness of combinations of antileukemic agents in inducing and maintaining remission in children with acute leukemia. Blood 1965, 26, 642–656. [Google Scholar]
- Moxley, J.H., 3rd; De Vita, V.T.; Brace, K.; Frei, E., 3rd. Intensive combination chemotherapy and X-irradiation in Hodgkin’s disease. Cancer Res. 1967, 27, 1258–1263. [Google Scholar] [PubMed]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Goodman, P.J.; Ungerleider, J.S.; Emerson, W.A.; Tormey, D.C.; Glick, J.H.; et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med. 1990, 322, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Carelle, N.; Piotto, E.; Bellanger, A.; Germanaud, J.; Thuillier, A.; Khayat, D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 2002, 95, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, J.; Aoun, M. Opportunistic infections in patients with cancer. Ann. Oncol. 2004, 15 (Suppl. 4), iv329–iv335. [Google Scholar] [PubMed]
- Kunisaki, K.M.; Janoff, E.N. Influenza in immunosuppressed populations: A review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect. Dis. 2009, 9, 493–504. [Google Scholar] [CrossRef]
- Elting, L.S.; Cooksley, C.; Chambers, M.; Cantor, S.B.; Manzullo, E.; Rubenstein, E.B. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 2003, 98, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Wingard, J.R. Infection and mucosal injury in cancer treatment. J. Natl. Cancer Inst. Monogr. 2001, 31–36. [Google Scholar] [CrossRef]
- Pfaller, M.; Neofytos, D.; Diekema, D.; Azie, N.; Meier-Kriesche, H.U.; Quan, S.P.; Horn, D. Epidemiology and outcomes of candidemia in 3648 patients: Data from the Prospective Antifungal Therapy (PATH Alliance(R)) registry, 2004-2008. Diagn. Microbiol. Infect. Dis. 2012, 74, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Ebbers, J.; Geurtz, L.; Stefanik, D.; Major, Y.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Candida spp. in the USA: Species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agents 2014, 43, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20 (Suppl. 6), 5–10. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Merelli, M.; Ansaldi, F.; de Florentiis, D.; Sartor, A.; Scarparo, C.; Callegari, A.; Righi, E. Clinical and therapeutic aspects of candidemia: A five year single centre study. PLoS ONE 2015, 10, e0127534. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Nightingale, P.; Patel, M.; Jumaa, P. Epidemiology, clinical characteristics, and outcome of candidemia: Experience in a tertiary referral center in the UK. Int. J. Infect. Dis. 2011, 15, e759–e763. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.; Marco, F.; Soriano, A.; Almela, M.; Martinez, J.A.; Lopez, J.; Pitart, C.; Mensa, J. Candida species bloodstream infection: Epidemiology and outcome in a single institution from 1991 to 2008. J. Hosp. Infect. 2011, 77, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.W.; Yang, Y.S.; Shang, S.T.; Chen, K.H.; Yeh, K.M.; Chang, F.Y.; Lin, J.C. Candida albicans versus non-albicans bloodstream infections: The comparison of risk factors and outcome. J. Microbiol. Immunol. Infect. 2011, 44, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of candidemia in Latin America: A laboratory-based survey. PLoS ONE 2013, 8, e59373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underhill, D.M.; Iliev, I.D. The mycobiota: Interactions between commensal fungi and the host immune system. Nat. Rev. Immunol. 2014, 14, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Anaissie, E. Revisiting the source of candidemia: Skin or gut? Clin. Infect. Dis. 2001, 33, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.N.; van der Heijden, I.M.; Costa, S.F.; Sousa, A.P.; Sienra, R.A.; Gobara, S.; Santos, C.R.; Lobo, R.D.; Pessoa, V.P., Jr.; Levin, A.S. Candida colonisation as a source for candidaemia. J. Hosp. Infect. 2009, 72, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Merelli, M.; Righi, E.; Diaz-Martin, A.; Rosello, E.M.; Luzzati, R.; Parra, A.; Trecarichi, E.M.; Sanguinetti, M.; Posteraro, B.; et al. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J. Clin. Microbiol. 2013, 51, 4167–4172. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef] [PubMed]
- Rentz, A.M.; Halpern, M.T.; Bowden, R. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin. Infect. Dis. 1998, 27, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.; Grussemeyer, C.A.; Spalding, J.R.; Benjamin, D.K., Jr.; Reed, S.D. Comparison of costs, length of stay, and mortality associated with Candida glabrata and Candida albicans bloodstream infections. Am. J. Infect. Control 2010, 38, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-de-Cerio, A.; Hernando, F.L. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol. 2014, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.S.; Brandt, M.E.; Pruitt, W.R.; Conn, L.A.; Perkins, B.A.; Stephens, D.S.; Baughman, W.S.; Reingold, A.L.; Rothrock, G.A.; Pfaller, M.A.; et al. The epidemiology of candidemia in two United States cities: Results of a population-based active surveillance. Clin. Infect. Dis. 1999, 29, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Garbino, J.; Pittet, D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 2003, 3, 685–702. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, E.P.; Arber, D.A. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am. J. Clin. Pathol. 2013, 139, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Dale, D.C.; Lyman, G.H. Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer 2004, 100, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Nesher, L.; Rolston, K.V. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 2014, 42, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Depuydt, P.O.; Salluh, J.I. Mechanical ventilation in cancer patients: Clinical characteristics and outcomes. Crit. Care Clin. 2010, 26, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Leroy, O.; Gangneux, J.P.; Montravers, P.; Mira, J.P.; Gouin, F.; Sollet, J.P.; Carlet, J.; Reynes, J.; Rosenheim, M.; Regnier, B.; et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: A multicenter, prospective, observational study in France (2005–2006). Crit. Care Med. 2009, 37, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Soiffer, R.J.; Magee, C.C. Renal failure associated with cancer and its treatment: An update. J. Am. Soc. Nephrol. 2005, 16, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; van der Meer, J.W.; Kullberg, B.J.; van de Veerdonk, F.L. Immune defence against Candida fungal infections. Nat. Rev. Immunol. 2015, 15, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Rudkin, F.M.; Bain, J.M.; Walls, C.; Lewis, L.E.; Gow, N.A.; Erwig, L.P. Altered dynamics of Candida albicans phagocytosis by macrophages and PMNs when both phagocyte subsets are present. MBio 2013, 4, e00810–e00813. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.J.; Kohler, J.R.; DiDomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef]
- Kaposzta, R.; Marodi, L.; Hollinshead, M.; Gordon, S.; da Silva, R.P. Rapid recruitment of late endosomes and lysosomes in mouse macrophages ingesting Candida albicans. J. Cell Sci. 1999, 112 Pt 19, 3237–3248. [Google Scholar] [PubMed]
- Fulurija, A.; Ashman, R.B.; Papadimitriou, J.M. Neutrophil depletion increases susceptibility to systemic and vaginal candidiasis in mice, and reveals differences between brain and kidney in mechanisms of host resistance. Microbiology 1996, 142 Pt 12, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.Y.; Kohler, J.R.; Coggshall, K.T.; van Rooijen, N.; Pier, G.B. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 2008, 4, e35. [Google Scholar] [CrossRef] [PubMed]
- Andrews, T.; Sullivan, K.E. Infections in patients with inherited defects in phagocytic function. Clin. Microbiol. Rev. 2003, 16, 597–621. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Baldock, S.C.; Ghoneim, A.T.; Child, J.A. Defective neutrophil function and microbicidal mechanisms in the myelodysplastic disorders. J. Clin. Pathol. 1983, 36, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Gandossini, M.; Souhami, R.L.; Babbage, J.; Addison, I.E.; Johnson, A.L.; Berenbaum, M.C. Neutrophil function during chemotherapy for Hodgkin's disease. Br. J. Cancer 1981, 44, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Damiani, M.T.; Colombo, M.I. Microfilaments and microtubules regulate recycling from phagosomes. Exp. Cell Res. 2003, 289, 152–161. [Google Scholar] [CrossRef]
- Malech, H.L.; Root, R.K.; Gallin, J.I. Structural analysis of human neutrophil migration. Centriole, microtubule, and microfilament orientation and function during chemotaxis. J. Cell Biol. 1977, 75, 666–693. [Google Scholar] [CrossRef] [PubMed]
- Stroka, K.M.; Hayenga, H.N.; Aranda-Espinoza, H. Human neutrophil cytoskeletal dynamics and contractility actively contribute to trans-endothelial migration. PLoS ONE 2013, 8, e61377. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, M.A.; Souto, F.O.; Micheli, D.C.; Alves-Filho, J.C.; Cunha, F.Q.; Murta, E.F.; Tavares-Murta, B.M. Mechanisms affecting neutrophil migration capacity in breast cancer patients before and after chemotherapy. Cancer Chemother. Pharmacol. 2014, 73, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Swystun, L.L.; Mukherjee, S.; Liaw, P.C. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J. Thromb. Haemost. 2011, 9, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed]
- Halverson, T.W.; Wilton, M.; Poon, K.K.; Petri, B.; Lewenza, S. DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 2015, 11, e1004593. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Jutila, M.A.; van Rooijen, N.; Cutler, J.E. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J. Immunol. 1994, 152, 5000–5008. [Google Scholar] [PubMed]
- Ngo, L.Y.; Kasahara, S.; Kumasaka, D.K.; Knoblaugh, S.E.; Jhingran, A.; Hohl, T.M. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J. Infect. Dis. 2014, 209, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Saeed, S.; Martens, J.H.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Oshita, F.; Kato, Y.; Yamada, K.; Nomura, I.; Noda, K. Early monocytopenia after chemotherapy as a risk factor for neutropenia. Am. J. Clin. Oncol. 1999, 22, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Jacquelin, S.; Licata, F.; Dorgham, K.; Hermand, P.; Poupel, L.; Guyon, E.; Deterre, P.; Hume, D.A.; Combadiere, C.; Boissonnas, A. CX3CR1 reduces Ly6Chigh-monocyte motility within and release from the bone marrow after chemotherapy in mice. Blood 2013, 122, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Swamydas, M.; Fischer, B.G.; Plantinga, T.S.; Johnson, M.D.; Jaeger, M.; Green, N.M.; Masedunskas, A.; Weigert, R.; Mikelis, C.; et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J. Clin. Investig. 2013, 123, 5035–5051. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.; Hunniger, K.; Bouzani, M.; Jacobsen, I.D.; Barz, D.; Hube, B.; Loffler, J.; Kurzai, O. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J. Infect. Dis. 2014, 209, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Voigt, J.; van der Voort, R.; Jacobsen, I.D.; Verschueren, I.; Hube, B.; Giamarellos-Bourboulis, E.J.; van der Meer, J.W.; Joosten, L.A.; Kurzai, O.; et al. Differential role of NK cells against Candida albicans infection in immunocompetent or immunocompromised mice. Eur. J. Immunol. 2014, 44, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Bar, E.; Whitney, P.G.; Moor, K.; Reis e Sousa, C.; LeibundGut-Landmann, S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 2014, 40, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Fregni, G.; Perier, A.; Pittari, G.; Jacobelli, S.; Sastre, X.; Gervois, N.; Allard, M.; Bercovici, N.; Avril, M.F.; Caignard, A. Unique functional status of natural killer cells in metastatic stage IV melanoma patients and its modulation by chemotherapy. Clin. Cancer Res. 2011, 17, 2628–2637. [Google Scholar] [CrossRef] [PubMed]
- Swidergall, M.; Ernst, J.F. Interplay between Candida albicans and the antimicrobial peptide armory. Eukaryot. Cell 2014, 13, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Porter, E.; Shen, B.; Lee, S.K.; Wilk, D.; Drazba, J.; Yadav, S.P.; Crabb, J.W.; Ganz, T.; Bevins, C.L. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat. Immunol. 2002, 3, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.E.; Bevins, C.L. Defensin-6 mRNA in human Paneth cells: Implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993, 315, 187–192. [Google Scholar] [CrossRef]
- Porter, E.M.; van Dam, E.; Valore, E.V.; Ganz, T. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect. Immun. 1997, 65, 2396–2401. [Google Scholar] [PubMed]
- Edgerton, M.; Koshlukova, S.E. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv. Dent. Res. 2000, 14, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Li, X.S.; Berner, J.C.; Edgerton, M. Distinct antifungal mechanisms: Beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob. Agents Chemother. 2006, 50, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Nayyar, N.; Li, W.; Edgerton, M. Human beta-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob. Agents Chemother. 2007, 51, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.E.; Harder, J.; Gorogh, T.; Weise, J.B.; Schubert, S.; Janssen, D.; Maune, S. Human beta-defensin-2 in oral cancer with opportunistic Candida infection. Anticancer Res. 2004, 24, 1025–1030. [Google Scholar] [PubMed]
- Grunberg, S.M. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: Dosing, efficacy, and tolerability analysis. Ann. Oncol. 2007, 18, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Gianos, M.; Klaustermeyer, W.B. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann. Allergy Asthma Immunol. 2009, 102, 179–187. [Google Scholar] [CrossRef]
- Mackall, C.L.; Fleisher, T.A.; Brown, M.R.; Magrath, I.T.; Shad, A.T.; Horowitz, M.E.; Wexler, L.H.; Adde, M.A.; McClure, L.L.; Gress, R.E. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994, 84, 2221–2228. [Google Scholar] [PubMed]
- Laurent, J.; Speiser, D.E.; Appay, V.; Touvrey, C.; Vicari, M.; Papaioannou, A.; Canellini, G.; Rimoldi, D.; Rufer, N.; Romero, P.; et al. Impact of 3 different short-term chemotherapy regimens on lymphocyte-depletion and reconstitution in melanoma patients. J. Immunother. 2010, 33, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Fidel, P.L., Jr. Candida-host interactions in HIV disease: Implications for oropharyngeal candidiasis. Adv. Dent. Res. 2011, 23, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Marodi, L.; Schreiber, S.; Anderson, D.C.; MacDermott, R.P.; Korchak, H.M.; Johnston, R.B., Jr. Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J. Clin. Investig. 1993, 91, 2596–2601. [Google Scholar] [CrossRef] [PubMed]
- Sechler, J.M.; Malech, H.L.; White, C.J.; Gallin, J.I. Recombinant human interferon-gamma reconstitutes defective phagocyte function in patients with chronic granulomatous disease of childhood. Proc. Natl. Acad. Sci. USA 1988, 85, 4874–4878. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Mencacci, A.; Cenci, E.; Spaccapelo, R.; Mosci, P.; Puccetti, P.; Bistoni, F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J. Immunol. 1993, 150, 925–931. [Google Scholar] [PubMed]
- Romani, L.; Mocci, S.; Bietta, C.; Lanfaloni, L.; Puccetti, P.; Bistoni, F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: Association of Th1 responses with acquired resistance. Infect. Immun. 1991, 59, 4647–4654. [Google Scholar] [PubMed]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2010, 115, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Laan, M.; Cui, Z.H.; Hoshino, H.; Lotvall, J.; Sjostrand, M.; Gruenert, D.C.; Skoogh, B.E.; Linden, A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999, 162, 2347–2352. [Google Scholar] [PubMed]
- Conti, H.R.; Shen, F.; Nayyar, N.; Stocum, E.; Sun, J.N.; Lindemann, M.J.; Ho, A.W.; Hai, J.H.; Yu, J.J.; Jung, J.W.; et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009, 206, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodriguez, E.V.; Rivino, L.; Geginat, J.; Jarrossay, D.; Gattorno, M.; Lanzavecchia, A.; Sallusto, F.; Napolitani, G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007, 8, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Eyerich, S.; Wagener, J.; Wenzel, V.; Scarponi, C.; Pennino, D.; Albanesi, C.; Schaller, M.; Behrendt, H.; Ring, J.; Schmidt-Weber, C.B.; et al. IL-22 and TNF-alpha represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur. J. Immunol. 2011, 41, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Maloy, K.J.; Salaun, L.; Cahill, R.; Dougan, G.; Saunders, N.J.; Powrie, F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 2003, 197, 111–119. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Montagnoli, C.; Zelante, T.; Bonifazi, P.; Bozza, S.; Moretti, S.; D'Angelo, C.; Vacca, C.; Boon, L.; Bistoni, F.; et al. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J. Immunol. 2007, 179, 5999–6008. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Daley, J.; Ghalie, R.; Kaizer, H. Clonal analysis of T-cell deficiencies in autotransplant recipients. Blood 1991, 77, 1845–1850. [Google Scholar] [PubMed]
- Ercolini, A.M.; Ladle, B.H.; Manning, E.A.; Pfannenstiel, L.W.; Armstrong, T.D.; Machiels, J.P.; Bieler, J.G.; Emens, L.A.; Reilly, R.T.; Jaffee, E.M. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J. Exp. Med. 2005, 201, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Bistoni, F.; Baccarini, M.; Blasi, E.; Marconi, P.; Puccetti, P.; Garaci, E. Correlation between in vivo and in vitro studies of modulation of resistance to experimental Candida albicans infection by cyclophosphamide in mice. Infect. Immun. 1983, 40, 46–55. [Google Scholar] [PubMed]
- Litterman, A.J.; Zellmer, D.M.; Grinnen, K.L.; Hunt, M.A.; Dudek, A.Z.; Salazar, A.M.; Ohlfest, J.R. Profound impairment of adaptive immune responses by alkylating chemotherapy. J. Immunol. 2013, 190, 6259–6268. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Spellberg, B.J.; Avenissian, V.; Fu, Y.; Filler, S.G.; Edwards, J.E., Jr. Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infect. Immun. 2005, 73, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13526–13531. [Google Scholar] [CrossRef] [PubMed]
- Torosantucci, A.; Bromuro, C.; Chiani, P.; De Bernardis, F.; Berti, F.; Galli, C.; Norelli, F.; Bellucci, C.; Polonelli, L.; Costantino, P.; et al. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 2005, 202, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A.; Casadevall, A. Recent progress in vaccines against fungal diseases. Curr. Opin. Microbiol. 2012, 15, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.S.; White, C.J.; Ibrahim, A.S.; Filler, S.G.; Fu, Y.; Yeaman, M.R.; Edwards, J.E., Jr.; Hennessey, J.P., Jr. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine 2012, 30, 7594–7600. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Amacker, M.; Arancia, S.; Sandini, S.; Gremion, C.; Zurbriggen, R.; Moser, C.; Cassone, A. A virosomal vaccine against candidal vaginitis: Immunogenicity, efficacy and safety profile in animal models. Vaccine 2012, 30, 4490–4498. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ibrahim, A.S.; Xu, X.; Farber, J.M.; Avanesian, V.; Baquir, B.; Fu, Y.; French, S.W.; Edwards, J.E., Jr.; Spellberg, B. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009, 5, e1000703. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.; Burnie, J.; Smith, D.; Clark, I.; Midgley, J.; Conolly, M.; Gazzard, B. Candida and AIDS: evidence for protective antibody. Lancet 1988, 2, 263–266. [Google Scholar] [CrossRef]
- Lipinski, T.; Wu, X.; Sadowska, J.; Kreiter, E.; Yasui, Y.; Cheriaparambil, S.; Rennie, R.; Bundle, D.R. A beta-mannan trisaccharide conjugate vaccine aids clearance of Candida albicans in immunocompromised rabbits. Vaccine 2012, 30, 6263–6269. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; De Milito, A.; Engstrom, P.; Nordin, M.; Narita, M.; Grillner, L.; Chiodi, F.; Bjork, O. Current chemotherapy protocols for childhood acute lymphoblastic leukemia induce loss of humoral immunity to viral vaccination antigens. Pediatrics 2002, 109, e91. [Google Scholar] [CrossRef] [PubMed]
- Zignol, M.; Peracchi, M.; Tridello, G.; Pillon, M.; Fregonese, F.; D'Elia, R.; Zanesco, L.; Cesaro, S. Assessment of humoral immunity to poliomyelitis, tetanus, hepatitis B, measles, rubella, and mumps in children after chemotherapy. Cancer 2004, 101, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Borella, L.; Webster, R.G. The immunosuppressive effects of long-term combination chemotherapy in children with acute leukemia in remission. Cancer Res. 1971, 31, 420–426. [Google Scholar] [PubMed]
- Paus, R.; Haslam, I.S.; Sharov, A.A.; Botchkarev, V.A. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013, 14, e50–e59. [Google Scholar] [CrossRef]
- Heidary, N.; Naik, H.; Burgin, S. Chemotherapeutic agents and the skin: An update. J. Am. Acad. Dermatol. 2008, 58, 545–570. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, K.; Potten, C.S. Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br. J. Cancer 1983, 47, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Sonis, S.T. Alterations in the oral mucosa caused by chemotherapeutic agents. A histologic study. J. Dermatol. Surg. Oncol. 1981, 7, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.M.; Brealey, J.; Goland, G.J.; Cummins, A.G. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 2000, 47, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Wardill, H.R.; Bowen, J.M. Chemotherapy-induced mucosal barrier dysfunction: An updated review on the role of intestinal tight junctions. Curr. Opin. Support Palliat. Care 2013, 7, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Beutheu Youmba, S.; Belmonte, L.; Galas, L.; Boukhettala, N.; Bole-Feysot, C.; Dechelotte, P.; Coeffier, M. Methotrexate modulates tight junctions through NF-kappaB, MEK, and JNK pathways. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Shitara, Y.; Sekine, S.; Horie, T. Zonula Occludens-1 alterations and enhanced intestinal permeability in methotrexate-treated rats. Cancer Chemother. Pharmacol. 2010, 66, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetiere, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Cottier, F.; Pavelka, N. Complexity and dynamics of host-fungal interactions. Immunol. Res. 2012, 53, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Noverr, M.C.; Huffnagle, G.B. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 2004, 72, 6206–6210. [Google Scholar] [CrossRef] [PubMed]
- Cottier, F.; Tan, A.S.; Chen, J.; Lum, J.; Zolezzi, F.; Poidinger, M.; Pavelka, N. The transcriptional stress response of Candida albicans to weak organic acids. G3 (Bethesda) 2015, 5, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Coughlin, L.A.; Neubauer, M.M.; Kim, J.; Kim, M.S.; Zhan, X.; Simms-Waldrip, T.R.; Xie, Y.; Hooper, L.V.; Koh, A.Y. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 2015, 21, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar]
- Samonis, G.; Anastassiadou, H.; Dassiou, M.; Tselentis, Y.; Bodey, G.P. Effects of broad-spectrum antibiotics on colonization of gastrointestinal tracts of mice by Candida albicans. Antimicrob. Agents Chemother. 1994, 38, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Zhanel, G.G.; Klym, K.A.; Hoban, D.J.; Kabani, A.M. Candidemia in a Canadian tertiary care hospital from 1976 to 1996. Diagn. Microbiol. Infect. Dis. 1997, 29, 5–9. [Google Scholar] [CrossRef]
- van Vliet, M.J.; Harmsen, H.J.; de Bont, E.S.; Tissing, W.J. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010, 6, e1000879. [Google Scholar] [CrossRef] [PubMed]
- Zwielehner, J.; Lassl, C.; Hippe, B.; Pointner, A.; Switzeny, O.J.; Remely, M.; Kitzweger, E.; Ruckser, R.; Haslberger, A.G. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS ONE 2011, 6, e28654. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.B.; Dieleman, L.A.; Ketabi, A.; Bibova, I.; Sawyer, M.B.; Xue, H.; Field, C.J.; Baracos, V.E.; Ganzle, M.G. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS ONE 2012, 7, e39764. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, M.J.; Tissing, W.J.; Dun, C.A.; Meessen, N.E.; Kamps, W.A.; de Bont, E.S.; Harmsen, H.J. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin. Infect. Dis. 2009, 49, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Bristol-Myers Squibb. Hydrea (Hydroxyurea Capsules, USP) Prescribing Information; Bristol-Myers Squibb: Princeton, NJ, USA, 2011. [Google Scholar]
- Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and fungi of the human gut microbiome: Correlations with diet and bacterial residents. PLoS ONE 2013, 8, e66019. [Google Scholar] [CrossRef] [PubMed]
- Calderone, R.A.; Fonzi, W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001, 9, 327–335. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ueta, E.; Tanida, T.; Yoneda, K.; Yamamoto, T.; Osaki, T. Increase of Candida cell virulence by anticancer drugs and irradiation. Oral Microbiol. Immunol. 2001, 16, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.M.; Wang, Y.M.; Zheng, X.D.; Lee, R.T.; Wang, Y. Critical role of DNA checkpoints in mediating genotoxic-stress-induced filamentous growth in Candida albicans. Mol. Biol. Cell 2007, 18, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Alby, K.; Bennett, R.J. Stress-induced phenotypic switching in Candida albicans. Mol. Biol. Cell 2009, 20, 3178–3191. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Pujol, C.; Daniels, K.J.; Miller, M.G.; Johnson, A.D.; Pfaller, M.A.; Soll, D.R. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 2002, 162, 737–745. [Google Scholar] [PubMed]
- Daniels, K.J.; Srikantha, T.; Lockhart, S.R.; Pujol, C.; Soll, D.R. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 2006, 25, 2240–2252. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.Y.; Newport, G.; Murillo, L.A.; Jones, T.; Scherer, S.; Davis, R.W.; Agabian, N. Metabolic specialization associated with phenotypic switching in Candida albicans. Proc. Natl. Acad. Sci. USA 2002, 99, 14907–14912. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.; Wessels, D.; Lockhart, S.R.; Soll, D.R. Release of a potent polymorphonuclear leukocyte chemoattractant is regulated by white-opaque switching in Candida albicans. Infect. Immun. 2004, 72, 667–677. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teoh, F.; Pavelka, N. How Chemotherapy Increases the Risk of Systemic Candidiasis in Cancer Patients: Current Paradigm and Future Directions. Pathogens 2016, 5, 6. https://doi.org/10.3390/pathogens5010006
Teoh F, Pavelka N. How Chemotherapy Increases the Risk of Systemic Candidiasis in Cancer Patients: Current Paradigm and Future Directions. Pathogens. 2016; 5(1):6. https://doi.org/10.3390/pathogens5010006
Chicago/Turabian StyleTeoh, Flora, and Norman Pavelka. 2016. "How Chemotherapy Increases the Risk of Systemic Candidiasis in Cancer Patients: Current Paradigm and Future Directions" Pathogens 5, no. 1: 6. https://doi.org/10.3390/pathogens5010006





