Comparison of Surface Proteomes of Adherence Variants of Listeria Monocytogenes Using LC-MS/MS for Identification of Potential Surface Adhesins
Abstract
:1. Introduction
2. Results and Discussion
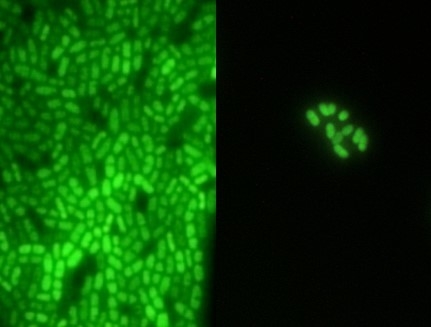
2.1. Adherence Analysis and Molecular Typing of L. Monocytogenes CW35 and 99-38
2.2. Total Surface Protein Identification
2.2.1. Planktonic Cells
2.2.2. Sessile Cells
2.3. Envelope Protein Identification
2.4. Protein Subcellular Localization Prediction
3. Experimental Section
3.1. Bacterial Cultures and Growth Conditions
3.2. Strain Characterization: Adherence Assays, Electron Microscopy, Molecular Typing, Serotyping, and Cellular Hydrophobicity
3.3. Extraction of Surface Proteins from Listeria Monocytogenes
3.4. Protein Concentration Measurement
3.5. SDS-PAGE Analysis
3.6. Orbitrap Mass Spectrometry (LC-MS/MS)
3.7. MS Data Analysis, Protein Identification, and Proteomic Analysis
3.8. Statistical Test for Determining Significant Differential Expression
4. Concluding Remarks
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hancock, I.C. Bacterial cell surface carbohydrates: Structure and assembly. Biochem. Soc. Trans. 1997, 25, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Calvo, E.; Pucciarelli, M.G.; Bierne, H.; Cossart, P.; Pablo Albar, J.; García-del Portillo, F. Analysis of the Listeria cell wall proteome by two-dimensional nanoliquid chromatography coupled to mass spectrometry. Proteomics 2005, 5, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Bierne, H.; Cossart, P. Listeria monocytogenes surface proteins: From genome predictions to function. Microbiol. Mol. Biol. Rev. 2007, 71, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Navarre, W.W.; Schneewind, O. Surface proteins of Gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229. [Google Scholar] [PubMed]
- Mazmanian, S.K.; Ton-That, H.; Schneewind, O. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 2001, 40, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, D.; Dehoux, P.; Dussurget, O.; Frangeul, L.; Cossart, P. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol. 2002, 10, 238–245. [Google Scholar] [CrossRef]
- Solis, N.; Larsen, M.R.; Cordwell, S.J. Improved accuracy of cell surface shaving proteomics in Staphylococcus aureus using a false-positive control. Proteomics 2010, 10, 2037–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portnoy, D.A.; Jacks, P.S.; Hinrichs, D.J. Role of hemolysin for the intracellular growth of listeria monocytogenes. J. Exp. Med. 1988, 167, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Barsig, J.; Kaufmann, S.H. The mechanism of cell death in Listeria monocytogenes-infected murine macrophages is distinct from apoptosis. Infect. Immun. 1997, 65, 4075–4081. [Google Scholar] [PubMed]
- Shaughnessy, L.M.; Hoppe, A.D.; Christensen, K.A.; Swanson, J.A. Membrane perforations inhibit lysosome fusion by altering ph and calcium in Listeria monocytogenes vacuoles. Cell. Microbiol. 2006, 8, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Hussain, M.; Peters, G.; Götz, F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 1997, 24, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Yates, J.R.; Ruse, C.I.; Nakorchevsky, A. Proteomics by mass spectrometry: Approaches, advances, and applications. Annu. Rev. Biomed. Eng. 2009, 11, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Shockman, G.D. Autolytic formation of protoplasts (autoplasts) of Streptococcus faecalis 9790: Release of cell wall, autolysin, and formation of stable autoplasts. J. Bacteriol. 1974, 118, 735–746. [Google Scholar] [PubMed]
- Cornett, J.B.; Johnson, C.A.; Shockman, G.D. Release of autolytic enzyme from Streptococcus faecium cell walls by treatment with dilute alkali. J. Bacteriol. 1979, 138, 699–704. [Google Scholar] [PubMed]
- Hussain, M.; Peters, G.; Chhatwal, G.S.; Herrmann, M. A lithium chloride-extracted, broad-spectrum-adhesive 42-kilodalton protein of Staphylococcus epidermidis is ornithine carbamoyltransferase. Infect. Immun. 1999, 67, 6688–6690. [Google Scholar] [PubMed]
- Tiong, H.K.; Hartson, S.; Muriana, P.M. Comparison of five methods for direct extraction of surface proteins from Listeria monocytogenes for proteomic analysis by orbitrap mass spectrometry. J. Microbiol. Methods 2015, 110, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Solis, N.; Cordwell, S.J. Current methodologies for proteomics of bacterial surface-exposed and cell envelope proteins. Proteomics 2011, 11, 3169–3189. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Doumith, M.; Cazalet, C.; Simoes, N.; Frangeul, L.; Jacquet, C.; Kunst, F.; Martin, P.; Cossart, P.; Glaser, P.; Buchrieser, C. New aspects regarding evolution and virulence of Listeria monocytogenes revealed by comparative genomics and DNA arrays. Infect. Immun. 2004, 72, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Pallen, M.J.; Chaudhuri, R.R.; Henderson, I.R. Genomic analysis of secretion systems. Curr. Opin. Microbiol. 2003, 6, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Rigden, D.J.; Galperin, M.Y.; Jedrzejas, M.J. Analysis of structure and function of putative surface-exposed proteins encoded in the Streptococcus pneumoniae genome: A bioinformatics-based approach to vaccine and drug design. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 143–168. [Google Scholar] [CrossRef] [PubMed]
- Roche, F.M.; Massey, R.; Peacock, S.J.; Day, N.P.J.; Visai, L.; Speziale, P.; Lam, A.; Pallen, M.; Foster, T.J. Characterization of novel lpxtg-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology 2003, 149, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.; De Martinis, E. Current knowledge and perspectives on biofilm formation: The case of Listeria monocytogenes. Appl. Microbiol. Biotechnol. 2013, 97, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Renier, S.; Hébraud, M.; Desvaux, M. Molecular biology of surface colonization by Listeria monocytogenes: An additional facet of an opportunistic Gram-positive foodborne pathogen. Environ. Microbiol. 2011, 13, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, K.; Muriana, P.M. Adherence characteristics of Listeria strains isolated from three ready-to-eat meat processing plants. J. Food Prot. 2009, 72, 2125–2131. [Google Scholar] [PubMed]
- Dieterich, G.; Kärst, U.; Fischer, E.; Wehland, J.; Jänsch, L. Leger: Knowledge database and visualization tool for comparative genomics of pathogenic and non-pathogenic Listeria species. Nucleic Acids Res. 2006, 34, D402–D406. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Kim, T.J.; Jung, Y.S.; Silva, J.L. Attachment strength of Listeria monocytogenes and its internalin-negative mutants. Food Biophys. 2008, 3, 329–332. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Kim, T.-J.; Silva, J.; Jung, Y.-S. Positive correlation between the expression of inla and inlb genes of Listeria monocytogenes and its attachment strength on glass surface. Food Biophys. 2009, 4, 304–311. [Google Scholar] [CrossRef]
- Jordan, S.J.; Perni, S.; Glenn, S.; Fernandes, I.; Barbosa, M.; Sol, M.; Tenreiro, R.P.; Chambel, L.; Barata, B.; Zilhao, I.; et al. Listeria monocytogenes biofilm-associated protein (bapl) may contribute to surface attachment of L. monocytogenes but is absent from many field isolates. Appl. Environ. Microbiol. 2008, 74, 5451–5456. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Steele, C.; Donaldson, J.R.; Banes, M.M.; Kumar, R.; Bridges, S.M.; Arick Ii, M.; Lawrence, M.L. Genome comparison of Listeria monocytogenes serotype 4a strain hcc23 with selected lineage i and lineage ii L. monocytogenes strains and other Listeria strains. Genom. Data 2014, 2, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Glaser, P.; Frangeul, L.; Buchrieser, C.; Rusniok, C.; Amend, A.; Baquero, F.; Berche, P.; Bloecker, H.; Brandt, P.; Chakraborty, T.; et al. Comparative genomics of Listeria species. Science 2001, 294, 849–852. [Google Scholar] [PubMed]
- Moszer, I.; Glaser, P.; Danchin, A. Subtilist: A relational database for the Bacillus subtilis genome. Microbiology 1995, 141, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Boekhorst, J.; Francke, C.; Siezen, R.J. Locatep: Genome-scale subcellular-location predictor for bacterial proteins. BMC Bioinform. 2008, 9, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. Psortb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Fimereli, D.K.; Tsirigos, K.D.; Litou, Z.I.; Liakopoulos, T.D.; Bagos, P.G.; Hamodrakas, S.J. Cw-pred: A hmm-based method for the classification of cell wall-anchored proteins of Gram-positive bacteria. In Artificial intelligence: Theories and applications; Maglogiannis, I., Plagianakos, V., Vlahavas, I., Eds.; Springer Berlin Heidelberg: New York, NY, USA; Philadelphia, PA, USA, 2012; Volume 7297, pp. 285–290. [Google Scholar]
- Bagos, P.G.; Tsirigos, K.D.; Liakopoulos, T.D.; Hamodrakas, S.J. Prediction of lipoprotein signal peptides in Gram-positive bacteria with a hidden markov model. J. Proteom. Res. 2008, 7, 5082–5093. [Google Scholar] [CrossRef] [PubMed]
- Möller, S.; Croning, M.D.R.; Apweiler, R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Sonnhammer, E.L.; von Heijne, G.; Krogh, A. A hidden markov model for predicting transmembrane helices in protein sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1998, 6, 175–182. [Google Scholar] [PubMed]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes1. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.; Engelbrecht, J.; Brunak, S.; von Heijne, G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.; Krogh, A. Prediction of signal peptides and signal anchors by a hidden markov model. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1998, 6, 122–130. [Google Scholar] [PubMed]
- Dyrløv Bendtsen, J.; Nielsen, H.; von Heijne, G.; Brunak, S. Improved prediction of signal peptides: Signalp 3.0. J. Mol. Biol. 2004, 340, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. Signalp 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Bagos, P.G.; Nikolaou, E.P.; Liakopoulos, T.D.; Tsirigos, K.D. Combined prediction of tat and sec signal peptides with hidden markov models. Bioinformatics 2010, 26, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Natale, P.; Brüser, T.; Driessen, A.J.M. Sec- and tat-mediated protein secretion across the bacterial cytoplasmic membrane—Distinct translocases and mechanisms. Biochim. Biophys. Acta (BBA)—Biomembr. 2008, 1778, 1735–1756. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Dorscht, J.; Klumpp, J.; Bielmann, R.; Schmelcher, M.; Born, Y.; Zimmer, M.; Calendar, R.; Loessner, M.J. Comparative genome analysis of Listeria bacteriophages reveals extensive mosaicism, programmed translational frameshifting, and a novel prophage insertion site. J. Bacteriol. 2009, 191, 7206–7215. [Google Scholar] [CrossRef] [PubMed]
- Håvarstein, L.S.; Coomaraswamy, G.; Morrison, D.A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 1995, 92, 11140–11144. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.; Cline, R.T.; Tettelin, H.; Sharov, V.; Morrison, D.A. Gene expression analysis of the Streptococcus pneumoniae competence regulons by use of DNA microarrays. J. Bacteriol. 2000, 182, 6192–6202. [Google Scholar] [CrossRef] [PubMed]
- Camejo, A.; Carvalho, F.; Reis, O.; Leitao, E.; Sousa, S.; Cabanes, D. The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence 2011, 2, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Piercey, M.J.; Hingston, P.A.; Truelstrup Hansen, L. Genes involved in Listeria monocytogenes biofilm formation at a simulated food processing plant temperature of 15 °C. Int. J. Food Microbiol. 2016, 223, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.; Seo, K.S.; Zhang, T.; Wang, C. Characterization of a potential Listeria monocytogenes virulence factor associated with attachment to fresh produce. Appl. Environ. Microbiol. 2013, 79, 6855–6861. [Google Scholar] [CrossRef] [PubMed]
- Yarwood, J.M.; Schlievert, P.M. Quorum sensing in Staphylococcus infections. J. Clin. Investig. 2003, 112, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.S.; Hossain, H.; Otten, S.; Kuenne, C.; Kuchmina, K.; Machata, S.; Domann, E.; Chakraborty, T.; Hain, T. Intracellular gene expression profile of Listeria monocytogenes. Infect. Immun. 2006, 74, 1323–1338. [Google Scholar] [CrossRef] [PubMed]
- Karlowski, W.M.; Zielezinski, A.; Carrère, J.; Pontier, D.; Lagrange, T.; Cooke, R. Genome-wide computational identification of wg/gw argonaute-binding proteins in arabidopsis. Nucleic Acids Res. 2010, 38, 4231–4245. [Google Scholar] [CrossRef] [PubMed]
- Zielezinski, A.; Karlowski, W.M. Agos—A universal web tool for gw argonaute-binding domain prediction. Bioinformatics 2011, 27, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.M.; Scanes, C.G. Endocrine peptides ‘moonlighting’ as immune modulators: Roles for somatostatin and gh-releasing factor. J. Endocrinol. 1995, 147, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Martin, A. Bacterial moonlighting proteins and bacterial virulence. In Between Pathogenicity and Commensalism; Dobrindt, U., Hacker, J.H., Svanborg, C., Eds.; Springer Berlin Heidelberg: Berlin, Germany, 2013; Volume 358, pp. 155–213. [Google Scholar]
- Jeffery, C.J. Moonlighting proteins. Trends Biochem. Sci. 1999, 24, 8–11. [Google Scholar] [CrossRef]
- Pancholi, V.; Fischetti, V.A. A major surface protein on group a streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 1992, 176, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.J.G.; Moore, J.C.; Lane, J.D.; Wilson, R.; Pribul, P.K.; Younes, Z.N.; Dobson, R.J.; Everest, P.; Reason, A.J.; et al. Identification of major outer surface proteins of Streptococcus agalactiae. Infect. Immun. 2002, 70, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Bohle, L.A.; Riaz, T.; Egge-Jacobsen, W.; Skaugen, M.; Busk, O.L.; Eijsink, V.G.; Mathiesen, G. Identification of surface proteins in Enterococcus faecalis v583. BMC Genom. 2011, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, W.; Lu, C. Immunoproteomic assay of surface proteins of streptococcus suis serotype 9. FEMS Immun. Med. Microbiol. 2008, 53, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, J.C.; Beighton, D.; Homer, K.A. Effect of acidic pH on expression of surface-associated proteins of streptococcus oralis. Appl. Environ. Microbiol. 2003, 69, 5290–5296. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Rohde, M.; Chhatwal, G.S.; Hammerschmidt, S. A-enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 2001, 40, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, B.; Koo, O.K.; Kim, K.-P.; Burkholder, K.M.; Mishra, K.K.; Aroonnual, A.; Bhunia, A.K. Lap, an alcohol acetaldehyde dehydrogenase enzyme in Listeria, promotes bacterial adhesion to enterocyte-like caco-2 cells only in pathogenic species. Microbiology 2010, 156, 2782–2795. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, K.M.; Kim, K.-P.; Mishra, K.K.; Medina, S.; Hahm, B.-K.; Kim, H.; Bhunia, A.K. Expression of lap, a seca2-dependent secretory protein, is induced under anaerobic environment. Microbes Infect. 2009, 11, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Mani, M.; Chen, C.; Amblee, V.; Liu, H.; Mathur, T.; Zwicke, G.; Zabad, S.; Patel, B.; Thakkar, J.; Jeffery, C.J. Moonprot: A database for proteins that are known to moonlight. Nucleic Acids Res. 2015, 43, D277–D282. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.M.; Clark, V.L. Role of ribosomal protein l12 in gonococcal invasion of hec1b cells. Infect. Immun. 2000, 68, 5002–5010. [Google Scholar] [CrossRef] [PubMed]
- Gorski, L.; Palumbo, J.D.; Mandrell, R.E. Attachment of Listeria monocytogenes to radish tissue is dependent upon temperature and flagellar motility. Appl. Environ. Microbiol. 2003, 69, 258–266. [Google Scholar] [CrossRef] [PubMed]
- McGann, P.; Ivanek, R.; Wiedmann, M.; Boor, K.J. Temperature-dependent expression of Listeria monocytogenes internalin and internalin-like genes suggests functional diversity of these proteins among the listeriae. Appl. Environ. Microbiol. 2007, 73, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Peel, M.; Donachie, W.; Shaw, A. Temperature-dependent expression of flagella of Listeria monocytogenes studied by electron microscopy, SDS-PAGE and western blotting. J. Gen. Microbiol. 1988, 134, 2171–2178. [Google Scholar] [PubMed]
- Wang, C.H.; Muriana, P.M. Incidence of Listeria monocytogenes in packages of retail franks. J. Food Prot. 1994, 57, 382–386. [Google Scholar]
- Gamble, R.; Muriana, P.A. Microplate fluorescence assay for measurement of the ability of strains of Listeria monocytogenes from meat and meat-processing plants to adhere to abiotic surfaces. Appl. Environ. Microbiol. 2007, 73, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, K.; Muriana, P.M. Comparison of invasiveness among surface-adherent variants of Listeria monocytogenes in caco-2 cell culture assays. Int. J. Food Microbiol. 2010, 138, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, K.; Muriana, P.M. Analysis of tissue invasiveness of adherent strains of Listeria monocytogenes by in vivo mouse assay. Int. J. Food Microbiol. 2010, 141, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the major Listeria monocytogenes serovars by multiplex pcr. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef] [PubMed]
- Coton, E.; Coton, M. Multiplex pcr for colony direct detection of Gram-positive histamine- and tyramine-producing bacteria. J. Microbiol. Methods 2005, 63, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Briandet, R.; Meylheuc, T.; Maher, C.; Bellon-Fontaine, M.N. Listeria monocytogenes Scott A: Cell surface charge, hydrophobicity, and electron donor and acceptor characteristics under different environmental growth conditions. Appl. Environ. Microbiol. 1999, 65, 5328–5333. [Google Scholar] [PubMed]
- Boone, C.W.; Ford, L.E.; Bond, H.E.; Stuart, D.C.; Lorenz, D. Isolation of plasma membrane fragments from hela cells. J. Cell Biol. 1969, 41, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Cordwell, S.J. Sequential extraction of proteins by chemical reagents. In 2d Page: Sample Preparation and Fractionation; Posch, A., Ed.; Humana Press: Totowa, NJ, USA, 2008; Volume 424, pp. 139–146. [Google Scholar]
- Wessel, D.; Flügge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Voruganti, S.; Lacroix, J.C.; Rogers, C.N.; Rogers, J.; Matts, R.L.; Hartson, S.D. The anticancer drug auy922 generates a proteomics fingerprint that is highly conserved among structurally diverse hsp90 inhibitors. J. Proteom. Res. 2013, 12, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Marakalala, M.J.; Raju, R.M.; Sharma, K.; Zhang, Y.J.; Eugenin, E.A.; Prideaux, B.; Daudelin, I.B.; Chen, P.Y.; Booty, M.G.; Kim, J.H.; et al. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat. Med. 2016, 22, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.M.; Ye, F.; Nager, A.R.; Murphy, J.P.; Lee, J.S.; Aguiar, M.; Breslow, D.K.; Gygi, S.P.; Nachury, M.V. The intraflagellar transport protein ift27 promotes bbsome exit from cilia through the gtpase arl6/bbs3. Dev. Cell 2014, 31, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Mosadeghi, R.; Reichermeier, K.M.; Winkler, M.; Schreiber, A.; Reitsma, J.M.; Zhang, Y.; Stengel, F.; Cao, J.; Kim, M.; Sweredoski, M.J.; et al. Structural and kinetic analysis of the cop9-signalosome activation and the cullin-ring ubiquitin ligase deneddylation cycle. eLife 2016. [Google Scholar] [CrossRef] [PubMed]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; VerBerkmoes, N.C.; Langston, M.A.; Uberbacher, E.; Hettich, R.L.; Samatova, N.F. Detecting differential and correlated protein expression in label-free shotgun proteomics. J. Proteom. Res. 2006, 5, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Smith, J.W.; Huang, C.-M. Mass spectrometry-based label-free quantitative proteomics. BioMed Res. Int. 2009. [Google Scholar] [CrossRef] [PubMed]
- Ramnath, M.; Rechinger, K.B.; Jänsch, L.; Hastings, J.W.; Knøchel, S.; Gravesen, A. Development of a Listeria monocytogenes EDGe partial proteome reference map and comparison with the protein profiles of food isolates. Appl. Environ. Microbiol. 2003, 69, 3368–3376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.Y.; Creskey, M.C.; Cyr, T.D.; Brooks, B.; Huang, H.; Pagotto, F.; Lin, M. Proteomic identification of Listeria monocytogenes surface-associated proteins. Proteomics 2013, 13, 3040–3045. [Google Scholar] [PubMed]





| Gene (ListiList) a | Protein Function Homolog (kDa) | Leger b | LocateP c | Psort d | CW-PRED e | PRED-LIPO f | Trans g | SignalP h | PREP-TAT i | Hydropathy Score j | GRAVY Score k | Total Spectrum Count Detected by LC-MS/MS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 99-38 Attached | 99-38 Plank. | CW35 Plank. | ||||||||||||
| lmo0199 * (2.3) | Phosphoribosyl pyrophosphate synthetase (35) | M | CY | 0.03 (E,C) CY | No | No | 0 | No | No | <1.8 | −0.03 | 4 | 15 | 2 |
| lmo0415 * (2.1.1) | Endo-1,4-beta-xylanase (52) | CY | M | 0.33 (E,C,CM) E | No | Yes (TM) | 1 | No | Yes (TM) | >1.8 | −0.41 | 3 | 17 | 0 |
| lmo0978 * (2.2) | Amino acid aminotransferase (37) | CY | CY | 2.5 (CM,E,C) CY | No | No | 0 | No | No | <1.8 | −0.22 | 4 | 9 | 2 |
| lmo1067 * (3.7.4) | GTP-binding elongation factor (69) | M | CY | 1.22 (CY,E,C) CM | No | No | 0 | No | No | <1.8 | −0.39 | 5 | 16 | 3 |
| lmo1072 * (2.1.2) | Pyruvate carboxylase (128) | M/S | CY | 2.5 (CM,E,C) CY | No | No | 0 | No | No | <1.8 | −0.26 | 4 | 22 | 1 |
| lmo1325 * (3.7.3) | Translation initiation factor IF-2 (85) | CY | CY | 0.03 (E,C) CY | No | No | 0 | No | No | <1.8 | −0.45 | 3 | 15 | 1 |
| lmo1504 * (3.7.2) | Alanyl-tRNA synthetase (98) | S | CY | 0 CY | No | No | 0 | No | No | <1.8 | −0.37 | 7 | 24 | 4 |
| lmo1519* (3.7.2) | Aspartyl-tRNA synthetase (66) | M | CY | 0 CY | No | No | 0 | No | No | <1.8 | −0.29 | 6 | 8 | 0 |
| lmo1663 * (2.2) | Asparagine synthetase (72) | M | CY | 2.5 (CM,E,C) CY | No | No | 0 | No | No | <1.8 | −0.43 | 4 | 6 | 0 |
| lmo2558 * (1.8) | Autolysin amidase (102) | C/M/S | Sec | 0.02 (C) E | No | Yes (Sec) | 1 | Yes | Yes (Sec) | <1.8 | −0.48 | 4 | 70 | 1 |
| Lmo2608 (3.7.1) | 30S ribosomal protein S13 (14) | CY | CY | 0.03 (E,C) CY | No | No | 0 | No | No | <1.8 | −0.75 | 11 | 5 | 1 |
| Gene (ListiList) a | Protein Function (kDa) | Leger b | LocateP c | Psort’s Protein Localization d | CW-PRED e | PRED-LIPO f | Trans g | SignalP h | PREP-TAT i | Hydropathy Score j | GRAVY Score k |
|---|---|---|---|---|---|---|---|---|---|---|---|
| lmo0220 * (1.7) | ATP-dependent zinc metalloprotease (76) | C/M | M | 0.01 (CY) CM | No | Yes (Lipo) | 2 | Yes | Yes (Sec) | >1.8 | −0.37 |
| lmo0392 * (5.2) | Hypothetical protein (34) | M | M | 2.5 (CM,E,C) CY | No | Yes (TM) | 1 | No | No | <1.8 | 0.16 |
| lmo0723 * (1.5) | Methyl-accepting chemotaxis protein (66) | CY | M | 0.04 (CY) CM | No | Yes (TM) | 2 | No | Yes (Sec) | >1.8 | −0.2 |
| lmo1068 * (6) | Hypothetical protein (31) | M/S | Extracellular (Lipid anchored) | (Equal score to all) Unknown | No | Yes (Lipo) | 0 | Yes | Yes (Sec) | >1.8 | −0.67 |
| lmo1076 * (1.1) | Autolysin (64) | S | Sec | 0.02 (C) E | No | Yes (Sec) | 1 | Yes | Yes (Sec) | >1.8 | −0.61 |
| lmo2033 * (1.7) | Cell division protein (46) | M | CY | 2.5 (CM,E,C) CY | No | No | 0 | No | No | <1.8 | −0.06 |
| lmo2157 * (5.2) | Hypothetical protein (71) | CY | CY | 2.5 (CM,E,C) CY | No | No | 0 | No | No | <1.8 | −0.35 |
| lmo2206 * (4.1) | Clp protease subunit B (98) | M | CY | 0.03 (E,C) CY | No | No | 0 | No | No | <1.8 | −0.37 |
| lmo2414 * (2.2) | Aminotransferase (48) | C,M | CY | 2.5 (CM,E,C) CY | No | No | 0 | No | No | <1.8 | −0.23 |
| lmo2510 * (1.6) | Preprotein translocase subunit (95) | M | CY | 0.03 (EC) CY | No | No | 0 | No | No | <1.8 | −0.46 |
| lmo2748 * (5.2) | Hypothetical protein (16) | CY | CY | 2.5 (CM,E,C) CY | No | No | 0 | No | No | <1.8 | −0.40 |
| Gene (ListiList) a | Protein Function (kDa) | Leger b | LocateP c | Psort’s Protein Localization d | CW-PRED e | PRED-LIPO f | Trans g | SignalP h | PREP-TAT i | Hydropathy Score j | GRAVY Score k |
|---|---|---|---|---|---|---|---|---|---|---|---|
| lmo0046 (3.7.1) | 30S ribosomal protein S18 (9) | CY | CY | 0.33 (E,C,CM) CY | No | No | 0 | No | No | <1.8 | −0.63 |
| lmo0055 (2.3) | Hypothetical protein | CY | CY | 0.03 (E,C) CY | No | No | 0 | No | No | <1.8 | −0.25 |
| lmo0186 (5.2) | Hypothetical protein (44) | CY | M | (Equal score to all) Unknown | No | Yes (TM) | 1 | No | Yes (TM) | >1.8 | −0.47 |
| lmo0202 (4.6) | Listeriolysin O precursor (59) | C/M/S | S | 0.28 (C,CM,CY) E | No | Yes (Sec) | 0 | Yes | Yes (Sec) | >1.8 | −0.47 |
| lmo0204 (1.8) | Actin-assembly inducing protein (70) | C/M/S | M | CM | Yes | Yes (Sec) | 1 | Yes | Yes (Sec) | >1.8 | −0.80 |
| lmo0214 (3.2) | Transcription-repair coupling factor (135) | CY | CY | 0.03 (E,C) CY | No | No | 0 | No | No | <1.8 | −0.33 |
| lmo0241 (5.2) | Hypothetical protein (28) | CY | CY | 1.84 (CY,C,E) CM | No | No | 0 | No | No | <1.8 | −0.18 |
| lmo0275 (1.10) | Hypothetical protein (30) | CY | M | (Equal score to all) Unknown | No | Yes (Sec) | 0 | Yes | Yes (Sec) | >1.8 | −0.22 |
| lmo0394 (1.1) | Listeria extracellular P60 protein (25) | CY | S | 0.27 (C,CM) E | No | Yes (Sec) | 1 | Yes | Yes (Sec) | >1.8 | −0.33 |
| lmo0434 (1.8) | Internalin B (71) | S | S | 0.79 (CM, E) C | No | No | 1 | No | Yes (sec) | >1.8 | −0.46 |
| lmo0707 (1.5) | Flagellar hook-associated protein 2 FliD (validated) (46) | CY | CY | 0.28 (C,CM,CY) E | No | No | 0 | No | No | <1.8 | −0.33 |
| lmo0724 (5.2) | 27 Hypothetical protein (27) | CY | M | 0.45 (CY,C,E) CM | No | Yes (Sec) | 1 | No | Yes (Sec) | >1.8 | −0.17 |
| lmo1272 (5.2) | Ribosomal biogenesis GTPase (33) | CY | CY | 1.84 (CY,C,E) CM | No | No | 0 | No | No | <1.8 | −0.44 |
| lmo1480 (3.7.1) | 30S ribosomal protein S20 (9) | CY | CY | 0.33 (E,C,CM) CY | No | No | 0 | No | No | <1.8 | −0.83 |
| lmo1784 (3.7.1) | 50S ribosomal protein L35 (8) | CY | CY | 0.33 (E,C,CM) CY | No | No | 0 | Yes | No | <1.8 | −1.22 |
| lmo2156 (5.1) | Hypothetical protein (13) | S | M | (Equal score to all) Unknown | No | Yes (Sec) | 1 | Yes | Yes (Sec) | >1.8 | −0.44 |
| lmo2505 (1.1) | Peptidoglycan lytic protein P45 (43) | C/M/S | S | 0.27 (C,CM) E | No | Yes (sec) | 1 | Yes | Yes (Sec) | <1.8 | −0.55 |
| lmo2619 (3.7.1) | 30S ribosomal protein S14 (7) | CY | CY | 0.33 (E,C,CM) CY | No | No | 0 | No | No | <1.8 | −0.61 |
| lmo2656 (3.7.1) | 30S ribosomal protein S12 (validated) (15) | C/M | CY | 0.03 (E,C) CY | No | No | 0 | No | No | <1.8 | −0.76 |
| lmo2691 (1.1) | Autolysin, N-acetylmuramidase (64) | C/S | M | 2.18 (C,CM) E | No | Yes (Sec) | 1 | No | Yes (Sec) | <1.8 | −0.74 |
| lmo2713 (1.8) | GW repeat-containing cell wall binding repeat protein (35) | CY | S | 0.13 (C,E) CM | No | Yes (Sec) | 0 | Yes | Yes (Sec) | >1.8 | −0.54 |
| Code–Functional Group a | Attached Listeria Strain 99-38 | Planktonic Listeria Strain 99-38 | Planktonic Listeria Strain CW35 | Listeria Strain EGD-e |
|---|---|---|---|---|
| 1—Cell envelope; cellular processes | 14 (2.3%) b | 109 (17.6%) | 67(13.1%) | 620 |
| 2—Intermediary metabolism | 26 (4.3%) | 152 (24.9%) | 94 (18%) | 611 |
| 3—Information pathways | 59 (13.1%) | 139 (30.8%) | 106 (26.1%) | 452 |
| 4—Other | 2 (1.3%) | 34 (22.8%) | 23 (18.1%) | 149 |
| 5—Similar to unknown proteins | 6 (0.8%) | 136 (18.2%) | 100 (14.3%) | 746 |
| 6—No similarity | 0 | 20 (7.7%) | 18 (8.1%) | 260 |
| Total | 107(3.8%) | 590 (20.8%) | 408 (14.4%) | 2838 |
| # | Gene ID | Gene Name | ListiList Functional Category | ListiList Code | Presence in Sessile Cells | Expression in Planktonic Cells | In Vivo Regulation (literature) a,b |
|---|---|---|---|---|---|---|---|
| 1 | lmo0002 * | dnaN | DNA replication | 3.1 | --- | >10 | --- |
| 2 | lmo0055 | purA | Metabolism of nucleotides and nucleic acids | 2.3 | √ | --- | --- |
| 3 | lmo0186 | lmo0186 | Unknown | 5.2 | √ | --- | --- |
| 4 | lmo0202 | hly | Miscellaneous | 4.5 | √ | --- | ↑ |
| 5 | lmo0204 1 | actA | Cell surface proteins protein precursor | 1.8 | √ | --- | ↑ |
| 6 | lmo0241 | lmo0241 | Unknown | 5.2 | √ | --- | --- |
| 7 | lmo0275 1 | lmo0275 | Transformation/competence | 1.10 | √ | --- | --- |
| 8 | lmo0394 1 | lmo0394 | Cell wall | 1.1 | √ | --- | --- |
| 9 | lmo0434 1 | inlB | Cell surface proteins | 1.8 | √ | --- | ↑ |
| 10 | lmo0705 *,1 | lmo0705 | Mobility and chemotaxis | 1.5 | --- | >10 | --- |
| 11 | lmo0707 1 | lmo0707 | Mobility and chemotaxis | 1.5 | √ | --- | --- |
| 12 | lmo0724 | lmo0724 | Unknown | 5.2 | √ | --- | --- |
| 13 | lmo0898 * | lmo0898 | Unknown | 5.2 | --- | >10 | --- |
| 14 | lmo1072 * | pycA | Main glycolytic pathways | 2.1.2 | √ | >10 | Neut |
| 15 | lmo1272 | lmo1272 | Unknown | 5.2 | √ | --- | --- |
| 16 | lmo1325 * | infB | Initiation (translation) | 3.7.3 | √ | >10 | --- |
| 17 | lmo1360 * | folD | Metabolism of coenzymes and prosthetic groups | 2.5 | --- | >10 | --- |
| 18 | lmo1544 *,1 | minD | Cell division | 1.7 | --- | ≥10 | ↓ |
| 19 | lmo1699 *,1 | lmo1699 | Mobility and chemotaxis | 1.5 | --- | >10 | --- |
| 20 | lmo1784 | rpmI | Ribosomal proteins | 3.7.1 | √ | --- | --- |
| 21 | lmo1860 | msrA | Protein modification reductase A | 3.8 | --- | ≥10x | --- |
| 22 | lmo1953 | pnp | Metabolism of nucleotides and nucleic acids | 2.3 | --- | >10 | --- |
| 23 | lmo2156 | lmo2156 | Unknown | 5.1 | √ | --- | --- |
| 24 | lmo2415 *,1 | lmo2415 | Transport/binding proteins and lipoproteins | 1.2 | --- | >10 | --- |
| 25 | lmo2505 1 | spl | Cell wall | 1.1 | √ | --- | ↓ |
| 26 | lmo2525 *,1 | mbl | Cell wall | 1.1 | --- | ≥10 | --- |
| 27 | lmo2558 *,1 | ami | Cell surface proteins | 1.8 | √ | ≥10 | Neut |
| 28 | lmo2656 | rpsL | Ribosomal proteins | 3.7.1 | √ | --- | --- |
| 29 | lmo2691 1 | lmo2691 | Cell wall | 1.1 | √ | --- | ↓ |
| 30 | lmo2713 1 | lmo2713 | Cell surface proteins | 1.8 | √ | --- | ↑ |
| Surface Extract from: | LPXTG/NXZTN a | GW b | LIPO c | Sec d | TM c,d | GW & Sec | GW, LIPO & Sec | LIPO & Sec | Sec & TM | GW & TM | LPXTG, GW, Sec | LPXTG & Sec | Unknown Signal | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Planktonic cells | 0 | 11 | 0 | 13 | 6 | 8 | 5 | 8 | 3 | 1 | 1 | 2 | 57 | 115 |
| Sessile cells | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 5 | 0 | 0 | 0 | 0 | 5 | 14 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiong, H.K.; Hartson, S.D.; Muriana, P.M. Comparison of Surface Proteomes of Adherence Variants of Listeria Monocytogenes Using LC-MS/MS for Identification of Potential Surface Adhesins. Pathogens 2016, 5, 40. https://doi.org/10.3390/pathogens5020040
Tiong HK, Hartson SD, Muriana PM. Comparison of Surface Proteomes of Adherence Variants of Listeria Monocytogenes Using LC-MS/MS for Identification of Potential Surface Adhesins. Pathogens. 2016; 5(2):40. https://doi.org/10.3390/pathogens5020040
Chicago/Turabian StyleTiong, Hung King, Steven D. Hartson, and Peter M. Muriana. 2016. "Comparison of Surface Proteomes of Adherence Variants of Listeria Monocytogenes Using LC-MS/MS for Identification of Potential Surface Adhesins" Pathogens 5, no. 2: 40. https://doi.org/10.3390/pathogens5020040