In Vivo Characterisation of Five Strains of Bovine Viral Diarrhoea Virus 1 (Subgenotype 1c)
Abstract
:1. Introduction
2. Results
2.1. Clinical Assessments
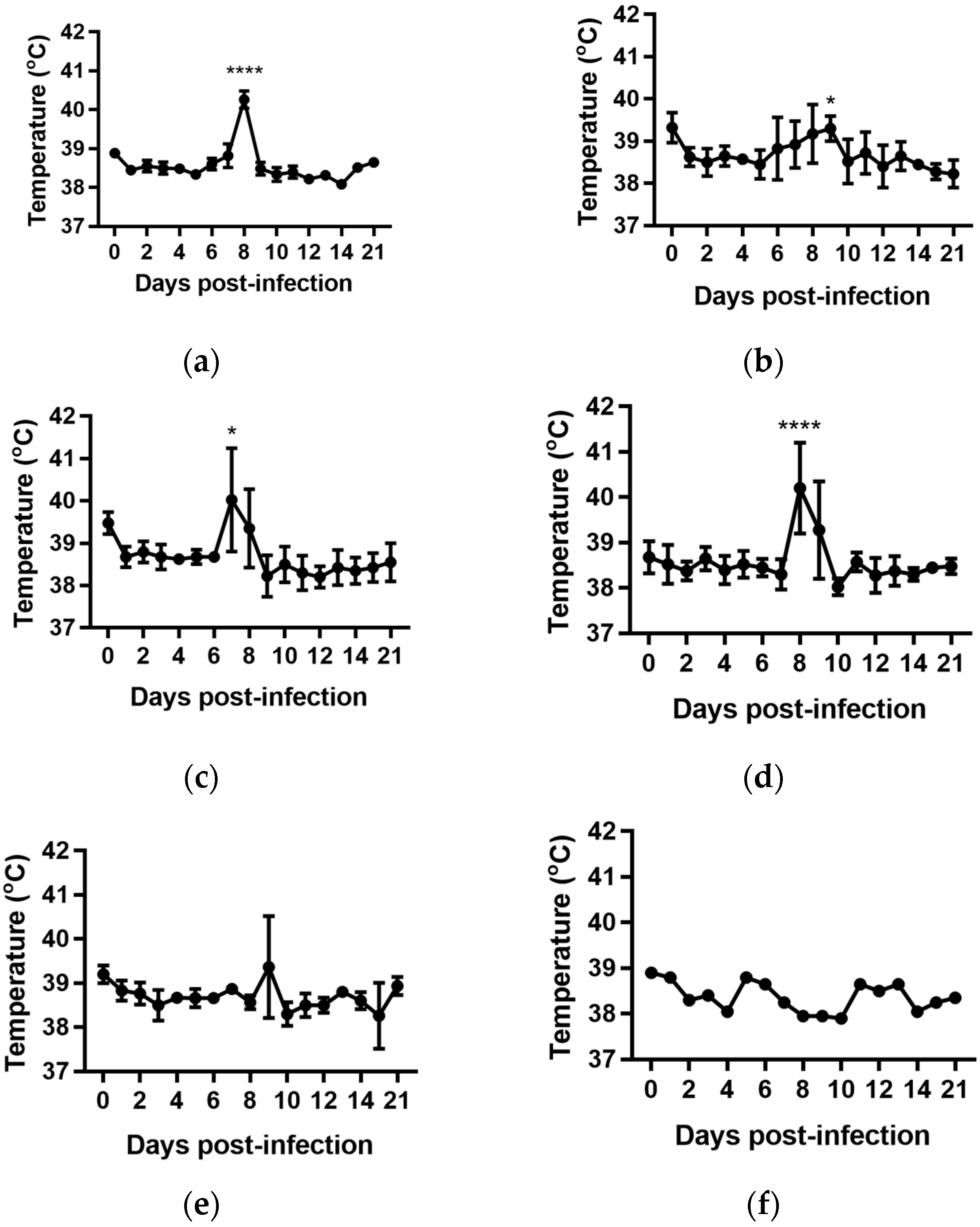
2.2. Rectal Temperatures
2.3. Detection of Virus Post-Infection
2.4. Isolation of BVDV-1c from Nasal Swabs
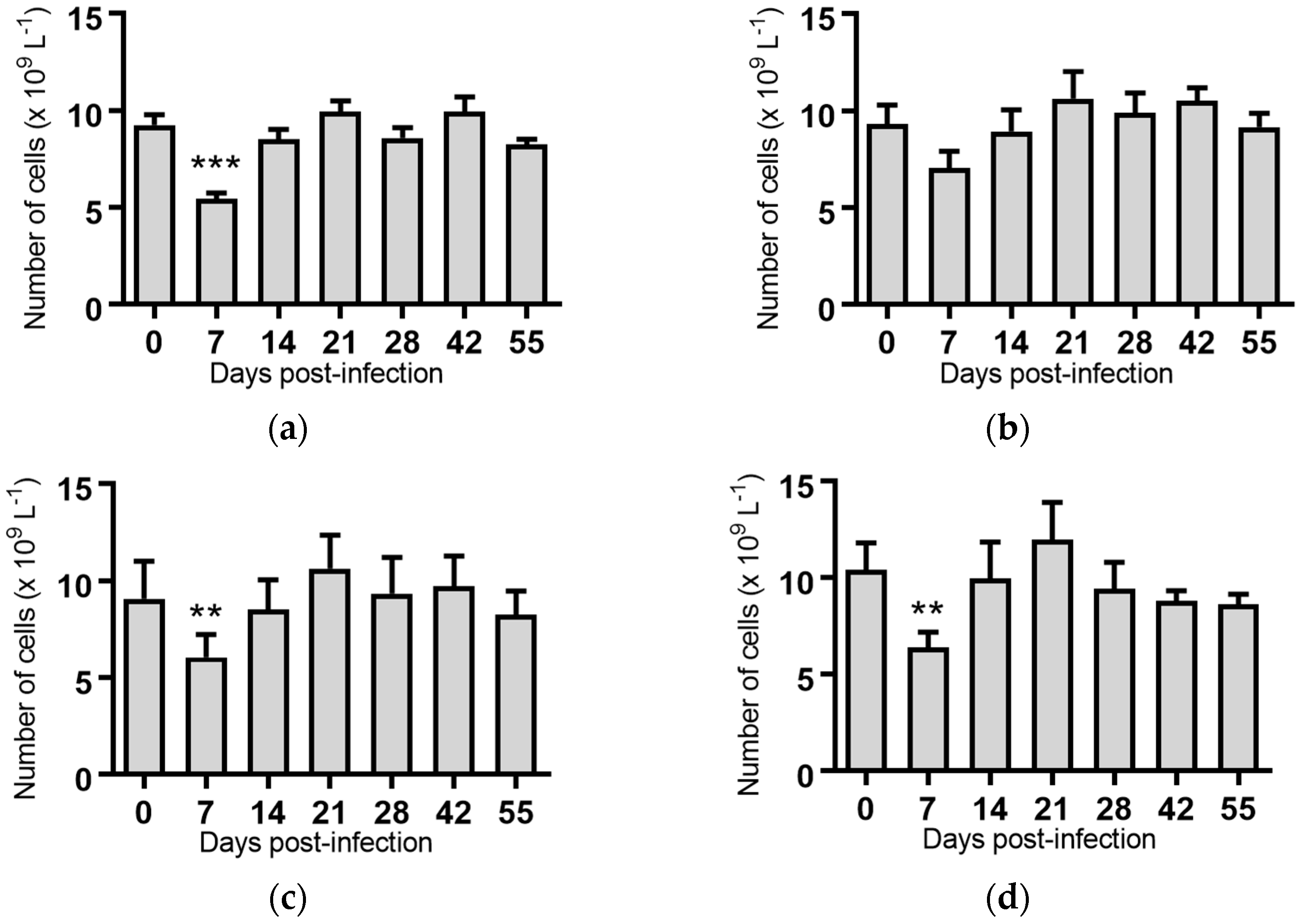
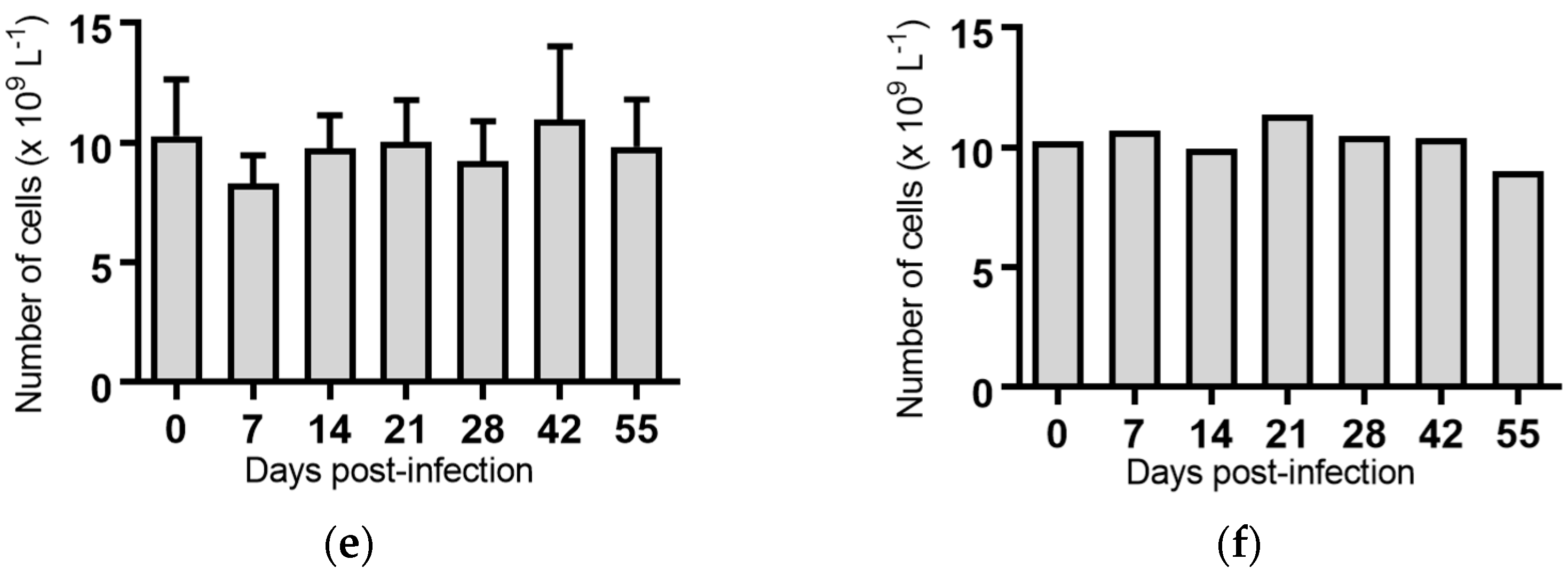
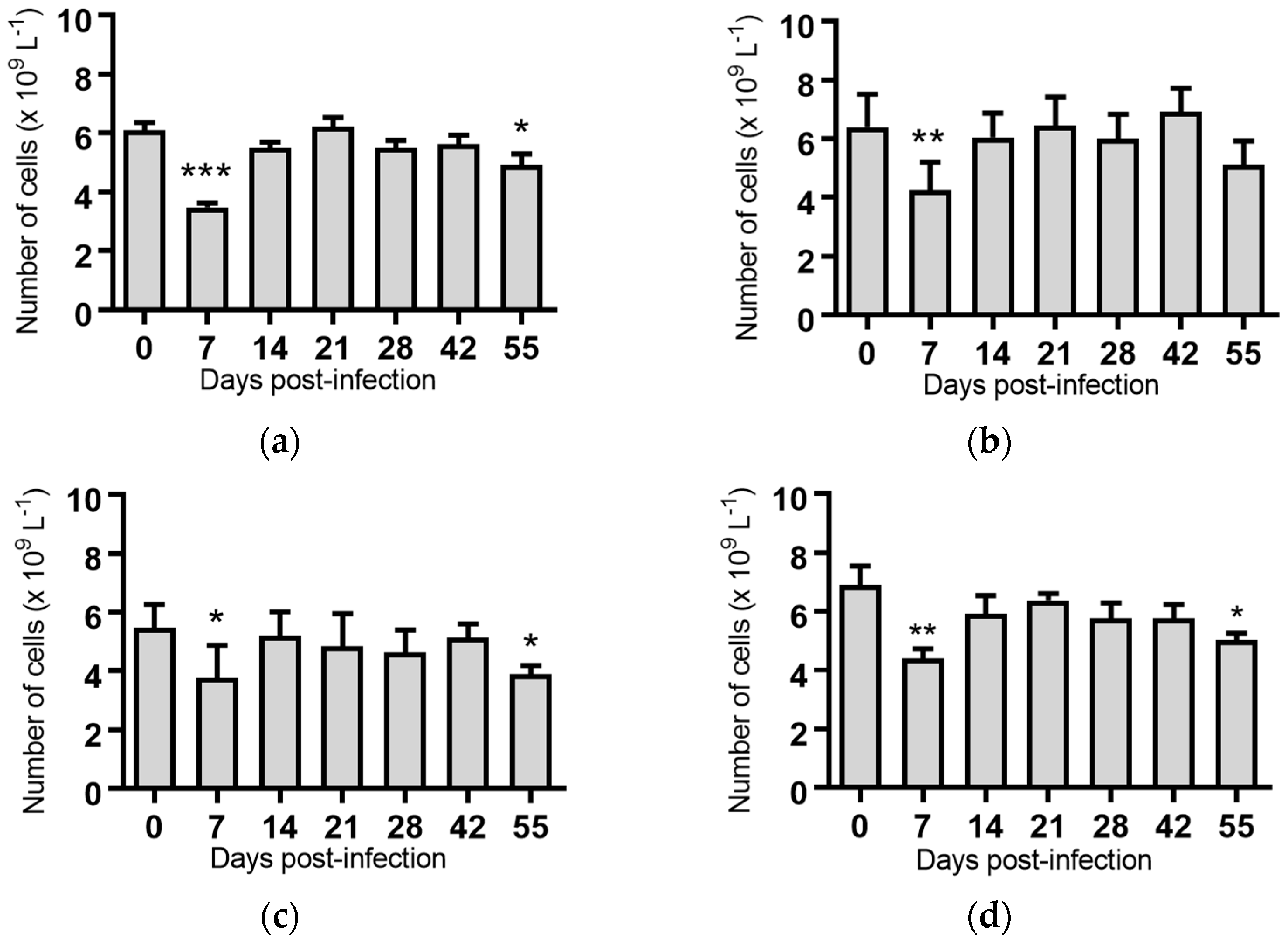
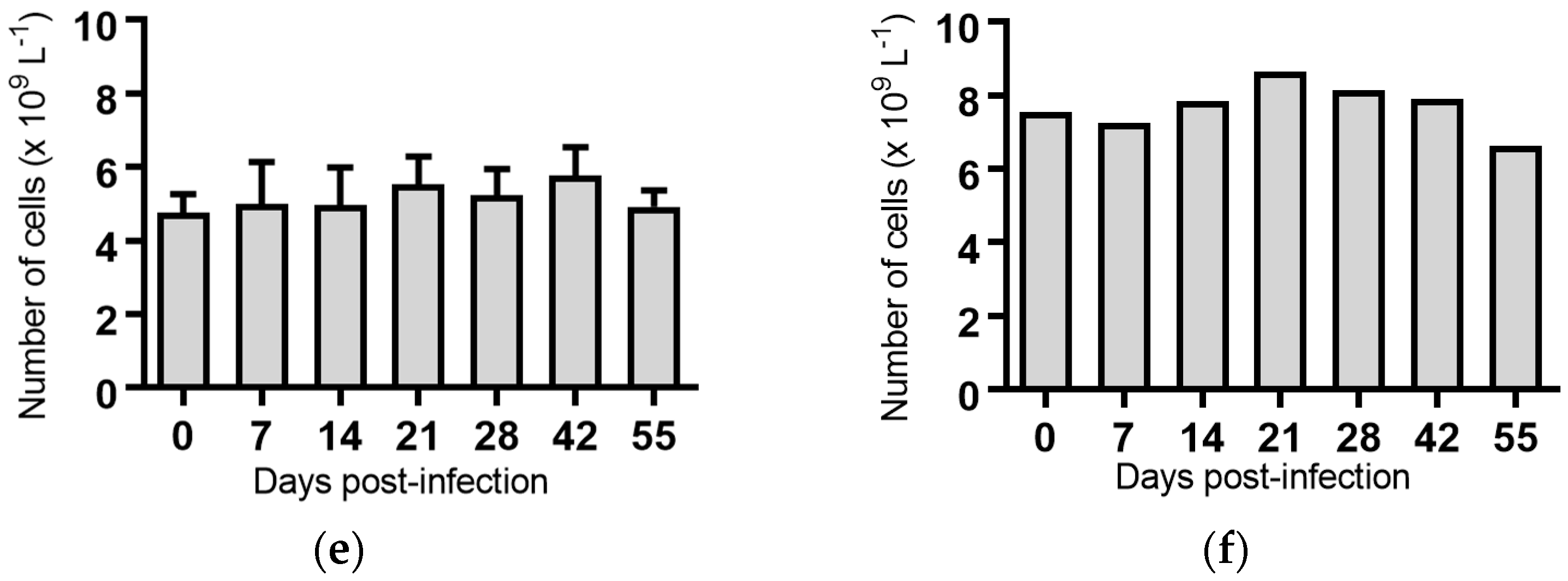
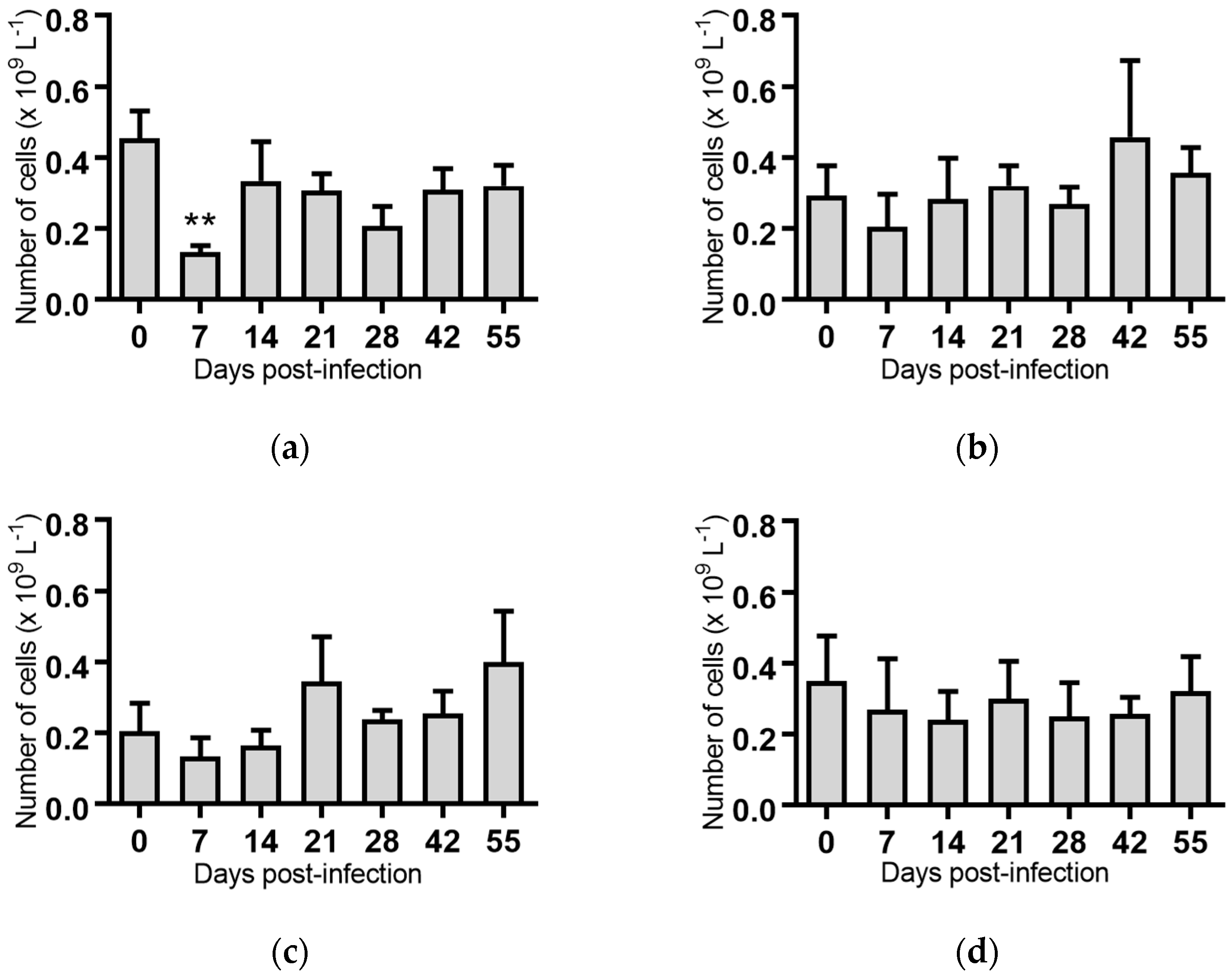
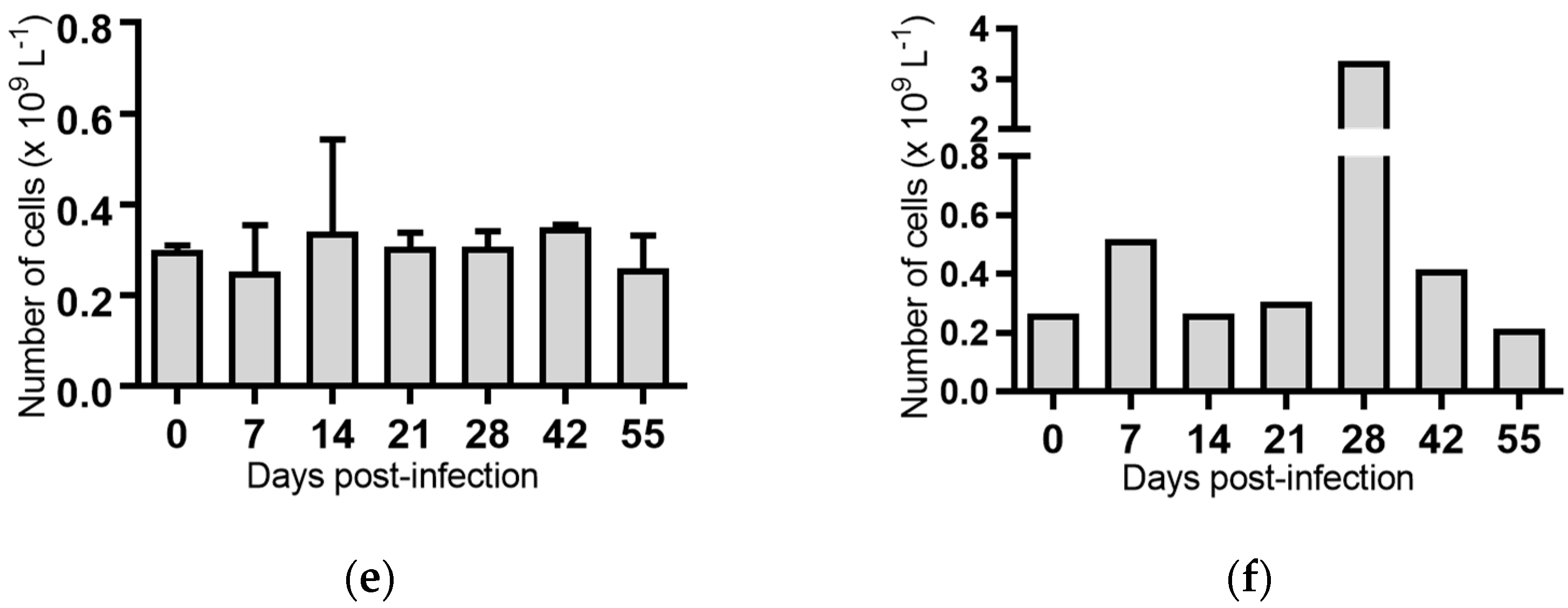
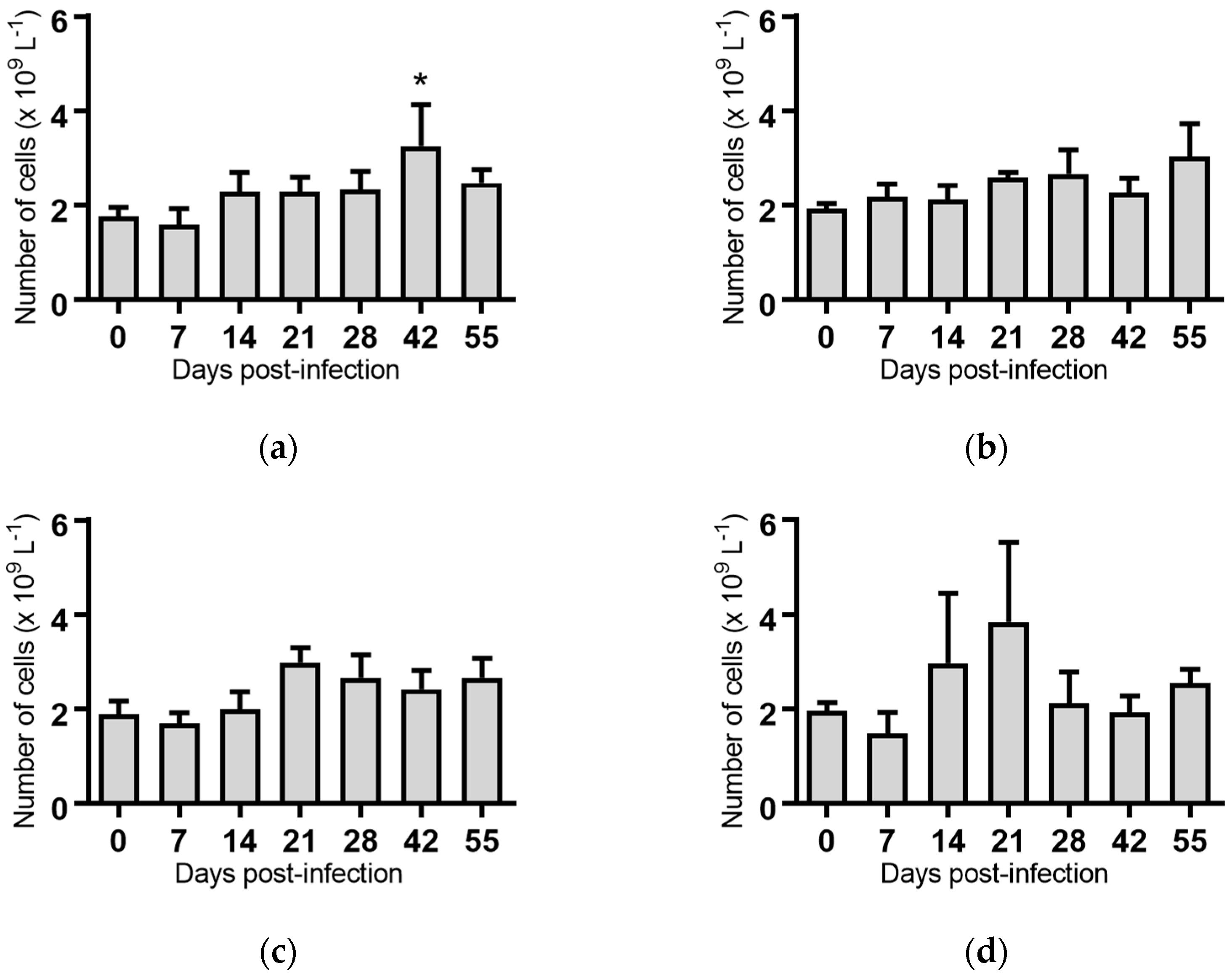
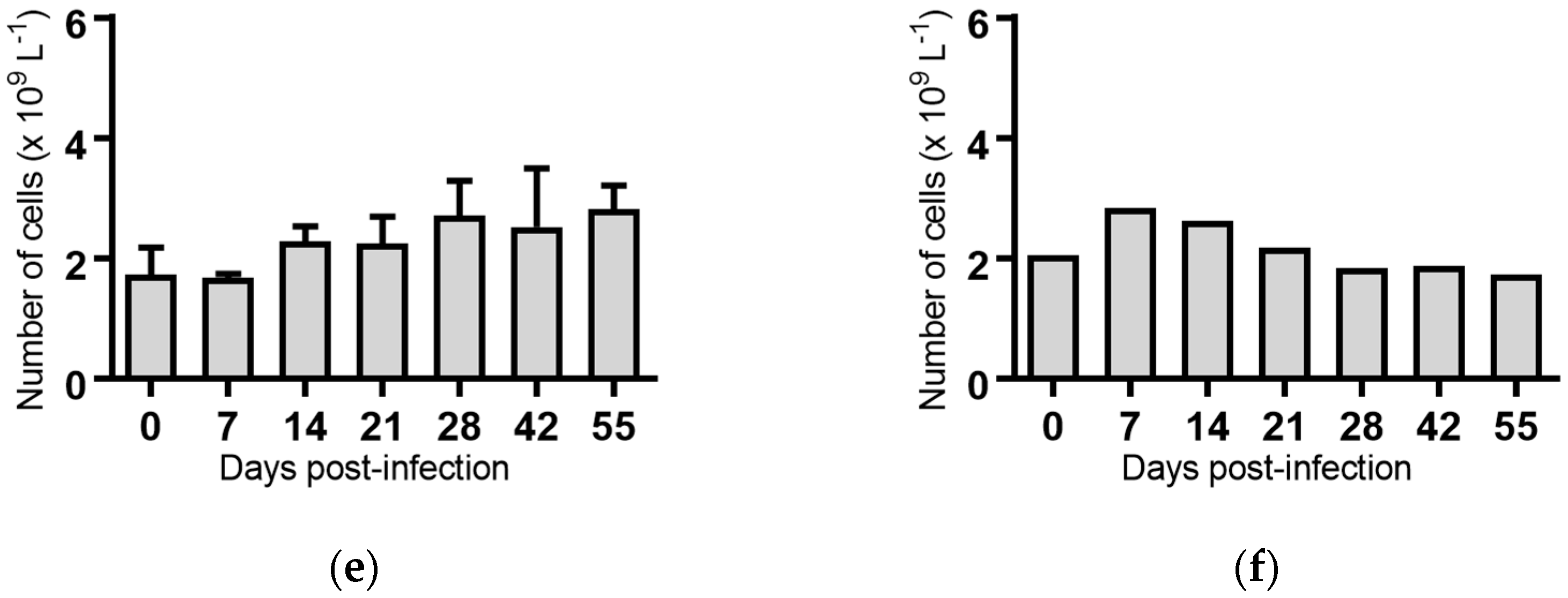
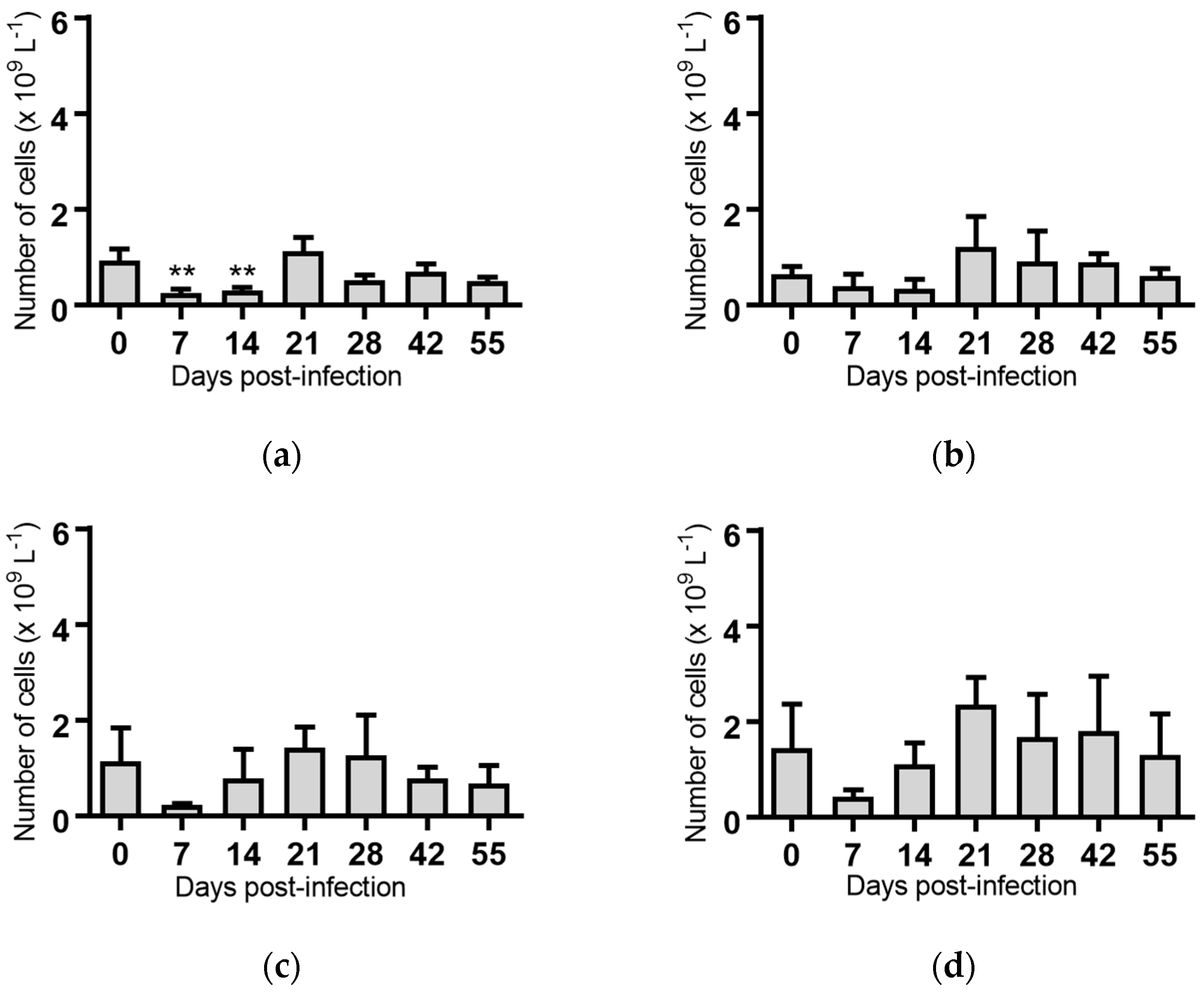
2.5. Haematological Analyses of Cattle Infected with BVDV-1 Strains
2.6. Serological Analyses
3. Discussion
4. Materials and Methods
4.1. Animal Ethics
4.2. Cattle Infection Trial
4.3. Virus Detection in Nasal Swabs and Sera
4.4. Virus Isolation from Nasal Swabs
4.5. Serology and Haematology
4.6. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yesilbag, K.; Alpay, G.; Becher, P. Variability and global distribution of subgenotypes of bovine viral diarrhea virus. Viruses 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Mahony, T.J.; McCarthy, F.M.; Gravel, J.L.; Corney, B.; Young, P.L.; Vilcek, S. Genetic analysis of bovine viral diarrhoea viruses from Australia. Vet. Microbiol. 2005, 106, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F.; Fulton, R.W.; Kirkland, P.D.; Neill, J.D. Prevalence and antigenic differences observed between bovine viral diarrhea virus subgenotypes isolated from cattle in Australia and feedlots in the southwestern United States. J. Vet. Diagn. Investig. 2010, 22, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W.; Ridpath, J.F.; Saliki, J.T.; Briggs, R.E.; Confer, A.W.; Burge, L.J.; Purdy, C.W.; Loan, R.W.; Duff, G.C.; Payton, M.E. Bovine viral diarrhea virus (BVDV) 1b: Predominant BVDV subtype in calves with respiratory disease. Can. J. Vet. Res. 2002, 66, 181–190. [Google Scholar] [PubMed]
- Wernike, K.; Schirrmeier, H.; Strebelow, H.G.; Beer, M. Eradication of bovine viral diarrhea virus in Germany-diversity of subtypes and detection of live-vaccine viruses. Vet. Microbiol. 2017, 208, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F. Bovine viral diarrhea virus: Global status. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Tamura, T.; Torii, S.; Wakamori, S.; Nagai, M.; Mitsuhashi, K.; Mine, J.; Fujimoto, Y.; Nagashima, N.; Yoshino, F.; et al. Genetic and antigenic characterization of bovine viral diarrhea viruses isolated from cattle in Hokkaido, Japan. J. Vet. Med. Sci. 2016, 78, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Ito, T.; Sugita, S.; Genno, A.; Takeuchi, K.; Ozawa, T.; Sakoda, Y.; Nishimori, T.; Takamura, K.; Akashi, H. Genomic and serological diversity of bovine viral diarrhea virus in Japan. Arch. Virol. 2001, 146, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Galav, V.; Mishra, N.; Dubey, R.; Rajukumar, K.; Pitale, S.S.; Shrivastav, A.B.; Pradhan, H.K. Pathogenicity of an Indian isolate of bovine viral diarrhea virus 1b in experimentally infected calves. Res. Vet. Sci. 2007, 83, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Glotov, A.G.; Glotova, T.I.; Koteneva, S.V.; Semenova, O.V.; Sergeev, A.A.; Titova, K.A.; Morozova, A.A.; Sergeev, A.A. Virulent properties of russian bovine viral diarrhea virus strains in experimentally infected calves. Scientifica (Cairo) 2016, 2016, 7034509. [Google Scholar] [CrossRef] [PubMed]
- Larska, M.; Polak, M.P.; Riitho, V.; Strong, R.; Belak, S.; Alenius, S.; Uttenthal, A.; Liu, L. Kinetics of single and dual infection of calves with an Asian atypical bovine pestivirus and a highly virulent strain of bovine viral diarrhoea virus 1. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F.; Neill, J.D.; Peterhans, E. Impact of variation in acute virulence of BVDV1 strains on design of better vaccine efficacy challenge models. Vaccine 2007, 25, 8058–8066. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, X.; Tong, Q.; Wu, Y.; Xia, M.Q.; Ji, Y.; Xue, W.; Wu, H. A bovine viral diarrhea virus type 1a strain in China: Isolation, identification, and experimental infection in calves. Virol. J. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Houe, H. Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Vet. Microbiol. 1999, 64, 89–107. [Google Scholar] [CrossRef]
- Niskanen, R.; Lindberg, A.; Larsson, B.; Alenius, S. Lack of virus transmission from bovine viral diarrhoea virus infected calves to susceptible peers. Acta Vet. Scand. 2000, 41, 93–99. [Google Scholar] [PubMed]
- Niskanen, R.; Lindberg, A.; Traven, M. Failure to spread bovine virus diarrhoea virus infection from primarily infected calves despite concurrent infection with bovine coronavirus. Vet. J. 2002, 163, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.E.; Heaney, J.; Thomas, C.J.; Brownlie, J. Infectivity of pestivirus following persistence of acute infection. Vet. Microbiol. 2009, 138, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Dewulf, J.; Mathijs, E.; Laureyns, J.; Mostin, L.; Cay, A.B. Virulence comparison and quantification of horizontal bovine viral diarrhoea virus transmission following experimental infection in calves. Vet. J. 2014, 202, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Hanon, J.B.; Van der Stede, Y.; Antonissen, A.; Mullender, C.; Tignon, M.; van den Berg, T.; Caij, B. Distinction between persistent and transient infection in a bovine viral diarrhoea (bvd) control programme: Appropriate interpretation of real-time RT-PCR and antigen-ELISA test results. Transbound Emerg. Dis. 2014, 61, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Hay, K.E.; Ambrose, R.C.; Morton, J.M.; Horwood, P.F.; Gravel, J.L.; Waldron, S.; Commins, M.A.; Fowler, E.V.; Clements, A.C.; Barnes, T.S.; et al. Effects of exposure to bovine viral diarrhoea virus 1 on risk of bovine respiratory disease in Australian feedlot cattle. Prev. Vet. Med. 2016, 126, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.A.; Moffat, J.L.; Hemmatzadeh, F.; Cockcroft, P.D. The risk of transmission from sheep experimentally infected with bvdv-1c during the acute phase to bvdv naïve sheep. Small Ruminant Res. 2017, 153, 5–8. [Google Scholar] [CrossRef]
- Strong, R.; La Rocca, S.A.; Paton, D.; Bensaude, E.; Sandvik, T.; Davis, L.; Turner, J.; Drew, T.; Raue, R.; Vangeel, I.; et al. Viral dose and immunosuppression modulate the progression of acute bvdv-1 infection in calves: Evidence of long term persistence after intra-nasal infection. PLoS ONE 2015, 10, e0124689. [Google Scholar] [CrossRef] [PubMed]
- Ridpath, J.F. Practical significance of heterogeneity among BVDV strains: Impact of biotype and genotype on U.S. control programs. Prev. Vet. Med. 2005, 72, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.W.; Ridpath, J.F.; Ore, S.; Confer, A.W.; Saliki, J.T.; Burge, L.J.; Payton, M.E. Bovine viral diarrhoea virus (BVDV) subgenotypes in diagnostic laboratory accessions: Distribution of BVDV1a, 1b, and 2a subgenotypes. Vet. Microbiol. 2005, 111, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, A.; Houe, H. Characteristics in the epidemiology of bovine viral diarrhea virus (BVDV) of relevance to control. Prev. Vet. Med. 2005, 72, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Fredriksen, B.; Sandvik, T.; Loken, T.; Odegaard, S.A. Level and duration of serum antibodies in cattle infected experimentally and naturally with bovine virus diarrhoea virus. Vet. Rec. 1999, 144, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, N.; Gullberg, M.; Lindberg, A.M. Real-time polymerase chain reaction as a rapid and efficient alternative to estimation of picornavirus titers by tissue culture infectious dose 50% or plaque forming units. Microbiol. Immunol. 2009, 53, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kallesh, D.J.; Hosamani, M.; Balamurugan, V.; Bhanuprakash, V.; Yadav, V.; Singh, R.K. Quantitative PCR: A quality control assay for estimation of viable virus content in live attenuated goat pox vaccine. Indian J. Exp. Biol. 2009, 47, 911–915. [Google Scholar] [PubMed]
- Iwami, S.; Holder, B.P.; Beauchemin, C.; Morita, S.; Tada, T.; Sato, K.; Igarashi, T.; Miura, T. Quantification system for the viral dynamics of a highly pathogenic simian/human immunodeficiency virus based on an in vitro experiment and a mathematical model. Retrovirology 2012, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, R.K.L.; Engdahl, E.E.; Fogdell-Hahn, A. Development and validation of a Q-PCR based TCID(50) method for human herpesvirus 6. Virol. J. 2012, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.R.; Kafi, M.; Kirkland, P.D.; Kelly, R.; Bielefeldt-Ohmann, H.; Occhio, M.D.; Jillella, D. Studies of the pathogenesis of bovine pestivirus-induced ovarian dysfunction in superovulated dairy cattle. Theriogenology 2003, 59, 1051–1066. [Google Scholar] [CrossRef]
- McGowan, M.R.; Kirkland, P.D. Early reproductive loss due to bovine pestivirus infection. Br. Vet. J. 1995, 151, 263–270. [Google Scholar] [CrossRef]
- McGowan, M.R.; Kirkland, P.D.; Richards, S.G.; Littlejohns, I.R. Increased reproductive losses in cattle infected with bovine pestivirus around the time of insemination. Vet. Rec. 1993, 133, 39–43. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.R.; Kirkland, P.D.; Rodwell, B.J.; Kerr, D.R.; Carroll, C.L. A field investigation of the effects of bovine viral diarrhea virus infection around the time of insemination on the reproductive performance of cattle. Theriogenology 1993, 39, 443–449. [Google Scholar] [CrossRef]
- Brown, L.M.; Papa, R.A.; Frost, M.J.; Mackintosh, S.G.; Gu, X.; Dixon, R.J.; Shannon, A.D. A single amino acid is critical for the expression of B-cell epitopes on the helicase domain of the pestivirus NS3 protein. Virus. Res. 2002, 84, 111–124. [Google Scholar] [CrossRef]
- Ali, T.M. A Manual for the Primary Animal Health Care Worker; Food and Agriculture Organization (FAO): Rome, Italy, 1994; Available online: http://www.fao.org/docrep/t0690e/t0690e00.htm#Contents (accessed on 27 December 2017).
- Horwood, P.F.; Mahony, T.J. Multiplex real-time RT-PCR detection of three viruses associated with the bovine respiratory disease complex. J. Virol. Methods 2011, 171, 360–363. [Google Scholar] [CrossRef] [PubMed]



| Days Post Infection 1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 14 | ||
| Virus | Animal ID | N | N | N | N | N | N | S | N | N | N | N | N | N | N | S |
| PI506 2 | 2661 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2664 | − | 35.6 | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2665 | − | 35.4 | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2676 | − | − | − | 37.8 | 36.6 | 36.7 | 36.0 | 37.1 | − | − | − | − | − | − | − | |
| 2682 | − | − | − | − | − | − | − | 37.1 | − | − | − | − | − | − | − | |
| 2684 4 | 31.7 | 32.8 | 36.7 | 36.5 | 34.8 | 32.8 | 34.7 | − | − | − | − | − | 36.5 | 36.5 | − | |
| Trangie 2 | 2675 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2677 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2685 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2686 4 | 39.0 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| AO554 3 | 2666 4 | 35.3 | 34.3 | − | − | 33.6 | 32.0 | 32.0 | 36.3 | − | − | − | − | − | − | − |
| 2668 | − | − | 34.8 | 39.5 | 33.8 | 32.4 | 35.7 | − | − | 37.3 | − | − | − | − | − | |
| 2669 4 | − | 36.6 | 37.1 | − | 32.0 | 31.6 | 33.7 | 32.6 | 37.5 | − | − | − | − | − | − | |
| 2673 | 37.0 | − | − | − | 37.3 | 39.8 | 35.9 | 37.4 | − | − | − | − | − | − | − | |
| NS155 3 | 2667 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2672 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2674 | − | − | − | − | 38.4 | − | − | − | − | − | − | − | − | − | − | |
| 2680 | − | − | − | − | − | − | 34.3 | 36.5 | − | − | − | − | 34.6 | − | 39.0 | |
| VR1112 3 | 2662 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2678 | − | 36.8 | 38.8 | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2683 | − | − | − | − | − | − | − | − | − | − | − | − | 39.5 | − | − | |
| Contact 2 | 2663 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2681 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Strain | Animal ID | Days Post-Infection | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | 55 | ||
| PI506 | 2661 | − | − | − | + | ++ | +++ | ++ |
| 2664 | − | − | + | +++ | ++++ | +++++ | ++++ | |
| 2665 | − | − | + | +++ | ++++ | ++++ | +++ | |
| 2676 | − | − | − | ++ | +++ | ++++ | ++++ | |
| 2682 | − | − | − | ++ | ++ | ++++ | ++++ | |
| 2684 | − | − | − | +++ | +++ | ++++ | ++++ | |
| Trangie | 2675 | − | − | − | +++ | ++++ | ++++ | +++ |
| 2677 | − | − | − | ++ | +++ | +++ | +++ | |
| 2685 | − | − | − | +++ | +++ | ++++ | +++++ | |
| 2686 | − | − | ++ | +++ | +++++ | +++++ | +++++ | |
| AO554 | 2666 | − | − | ++ | +++ | ++++ | +++++ | ++++ |
| 2668 | − | − | +++ | − | +++++ | +++++ | +++++ | |
| 2669 | − | − | +++ | ++++ | +++++ | +++++ | +++++ | |
| 2673 | − | − | − | ++ | ++++ | +++++ | ++++ | |
| NS155 | 2667 | − | − | +++ | +++ | +++++ | +++++ | ++++ |
| 2672 | − | − | + | + | +++++ | +++++ | ++++ | |
| 2674 | − | − | − | − | ++++ | ++++ | ++++ | |
| 2680 | − | − | + | ++++ | +++++ | +++++ | +++++ | |
| VR1112 | 2662 | − | − | − | − | ++ | ++ | ++ |
| 2678 | − | − | +++ | +++ | +++++ | +++++ | +++++ | |
| 2683 | − | − | − | + | + | ++ | ++ | |
| Contact | 2663 | − | − | − | − | − | − | − |
| 2681 | − | − | − | − | − | − | − | |
| Isolate | Biotype | Passage | qPCR 1 | Source |
|---|---|---|---|---|
| PI506 | Non-cytopathic | P3 | 19 | Isolated from a persistently infected animal |
| Trangie 2 | Non-cytopathic | P3 | 17 | Dr Peter Kirkland (DPI) [35] |
| AO554 | Non-cytopathic | P3 | 18 | Isolated from a persistently infected animal |
| VR1112 2 | Cytopathic | P4 | 17, (1 × 104.6 TCID50 mL−1) | Dr Jan Smith (JCU) |
| NS155 | Non-cytopathic | P3 | 22 | Isolated from a feedlot animal, treated for bovine respiratory disease |
| Room | Pen Number | Strain | Number of Cattle |
|---|---|---|---|
| 1 | 1.1 | AO554 | 4 |
| 1 | 1.1 | Trangie | 2 |
| 1 | 1.2 | Trangie | 2 |
| 1 | 1.2 | VR1112 | 3 1 |
| 2 | 2.3 | PI506 | 6 |
| 2 | 2.4 | NS155 | 4 |
| 2 | 2.4 | No virus | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrose, R.K.; Gravel, J.L.; Commins, M.A.; Fowler, E.V.; Mahony, T.J. In Vivo Characterisation of Five Strains of Bovine Viral Diarrhoea Virus 1 (Subgenotype 1c). Pathogens 2018, 7, 12. https://doi.org/10.3390/pathogens7010012
Ambrose RK, Gravel JL, Commins MA, Fowler EV, Mahony TJ. In Vivo Characterisation of Five Strains of Bovine Viral Diarrhoea Virus 1 (Subgenotype 1c). Pathogens. 2018; 7(1):12. https://doi.org/10.3390/pathogens7010012
Chicago/Turabian StyleAmbrose, Rebecca K., Jennifer L. Gravel, Margaret A. Commins, Elizabeth V. Fowler, and Timothy J. Mahony. 2018. "In Vivo Characterisation of Five Strains of Bovine Viral Diarrhoea Virus 1 (Subgenotype 1c)" Pathogens 7, no. 1: 12. https://doi.org/10.3390/pathogens7010012





