Detection and Molecular Identification of Salmonella Virulence Genes in Livestock Production Systems in South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Samples and Pre-Enrichment
2.3. Selective Enrichment
2.4. DNA Extraction
2.5. Molecular Confirmation of Salmonella spp. Using PCR
2.6. Gel Electrophoresis and Visualization of PCR Products
2.7. Determination of Virulence Profiles of Salmonella Isolates
3. Statistical Analyses
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bäumler, A.J.; Tsolis, R.M.; Ficht, T.A.; Adams, L.G. Evolution of Host Adaptation in Salmonella enterica. Infect. Immun. 1998, 66, 4579–4587. [Google Scholar] [PubMed]
- Balasubramanian, R.; Im, J.; Lee, J.-S.; Jeon, H.J.; Mogeni, O.D.; Kim, J.H.; Rakotozandrindrainy, R.; Baker, S.; Marks, F. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Hum. Vaccines Immunother. 2019, 15, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.-A.; Herbin, S.; Firmesse, O.; Auvray, F. European food poisoning outbreaks involving meat and meat-based products. Procedia Food Sci. 2015, 5, 93–96. [Google Scholar] [CrossRef]
- Food and Drug Administration. Bad Bug Book, Foodborne Pathogenic Microorganisms and Natural Toxins; Center for Food Safety and Applied Nutrition of the Food and Drug Administration (FDA), US Department of Health and Human Services: Silver Spring, MD, USA, 2012.
- World Health Organization. Salmonella (Non-Typhodial). 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 18 April 2018).
- U.S. Food & Drug Administration. Foodborne Illnesses: What You Need to Know. 2018. Available online: https://www.fda.gov/food/foodborneillnesscontaminants/foodborneillnessesneedtoknow/default.htm (accessed on 30 October 2018).
- Shonhiwa, A.; Ntshoe, G.; Essel, V.; Thomas, J.; McCarthy, K. A review of foodborne diseases outbreaks reported to the outbreak response unit, national institute for communicable diseases, South Africa, 2013–2017. Int. J. Infect. Dis. 2019, 79 (Suppl. 1), 73. [Google Scholar] [CrossRef]
- Marks, F.; Von Kalckreuth, V.; Aaby, P.; Adu-Sarkodie, Y.; El Tayeb, M.A.; Ali, M.; Aseffa, A.; Baker, S.; Biggs, H.M.; Bjerregaard-Andersen, M.; et al. Incidence of invasive Salmonella disease in sub-Saharan Africa: A multicentre population-based surveillance study. Lancet Glob. Health 2017, 5, e310–e323. [Google Scholar] [CrossRef]
- Olobatoke, R.Y.; Mulugeta, S.D. Incidence of non-typhoidal Salmonella in poultry products in the North West Province, South Africa. S. Afr. J. Sci. 2015, 111, 1–7. [Google Scholar] [CrossRef]
- Christison, C.; Lindsay, D.; Von Holy, A. Microbiological survey of ready-to-eat foods and associated preparation surfaces in retail delicatessens, Johannesburg, South Africa. Food Control 2008, 19, 727–733. [Google Scholar] [CrossRef]
- van Nierop, W.; Dusé, A.; Marais, E.; Aithma, N.; Thothobolo, N.; Kassel, M.; Stewart, R.; Potgieter, A.; Fernandes, B.; Galpin, J.; et al. Contamination of chicken carcasses in Gauteng, South. Africa, by Salmonella, Listeria monocytogenes and Campylobacter. Int. J. Food Microbiol. 2005, 99, 1–6. [Google Scholar] [CrossRef] [PubMed]
- International Federation for Animal Health. The Costs of Animal Disease—A Report Produced for the International Federation for Animal Health; IFAH; Oxford Analytica Ltd.: Oxford, UK, 2012; Available online: https://www.bft-online.de/fileadmin/bft/publikationen/IFAH_Oxford-Analytica_The-Costs-of-Animal-Disease_October2012.pdf (accessed on 12 June 2018).
- Muvhali, M.; Smith, A.M.; Rakgantso, A.M.; Keddy, K.H. Investigation of Salmonella Enteritidis outbreaks in South. Africa using multi-locus variable-number tandem-repeats analysis, 2013–2015. BMC Infect. Dis. 2017, 17, 661. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Gouws, A.-M.; Hoyland, G.; Sooka, A.; Keddy, K.H. Outbreaks of food-borne disease: A common occurrence but rarely reported. S. Afr. Med. J. 2007, 97, 1272. [Google Scholar] [PubMed]
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Wong, D.M.L.F.; Jensen, A.B.; Wegener, H.C.; Aarestrup, F.M. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: Results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Ahmer, B.M.; Gunn, J.S. Interaction of Salmonella spp. with the intestinal microbiota. Front. Microbiol. 2011, 2, 101. [Google Scholar] [PubMed]
- Anderson, C.J.; Kendall, M.M. Salmonella enterica serovar Typhimurium strategies for host adaptation. Front. Microbiol. 2017, 8, 1983. [Google Scholar] [CrossRef] [PubMed]
- Porwollik, S.; Boyd, E.F.; Choy, C.; Cheng, P.; Florea, L.; Proctor, E.; McClelland, M. Characterization of Salmonella enterica subspecies I genovars by use of microarrays. J. Bacteriol. 2004, 186, 5883–5898. [Google Scholar] [CrossRef] [PubMed]
- Card, R.; Vaughan, K.; Bagnall, M.; Spiropoulos, J.; Cooley, W.; Strickland, T.; Davies, R.; Anjum, M.F. Virulence characterisation of Salmonella enterica isolates of differing antimicrobial resistance recovered from UK livestock and imported meat samples. Front. Microbiol. 2016, 7, 640. [Google Scholar] [CrossRef]
- Grimont, P.A.; Weill, F.-X. Antigenic Formulae of the Salmonella Serovars; WHO Collaborating Centre for Reference and Research on Salmonella: Paris, France, 2007; Volume 9, pp. 1–166. [Google Scholar]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef]
- Kariuki, S.; Revathi, G.; Kariuki, N.; Kiiru, J.; Mwituria, J.; Muyodi, J.; Githinji, J.W.; Kagendo, D.; Munyalo, A.; Hart, C.A. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: oonotic or anthroponotic transmission? J. Med. Microbiol. 2006, 55, 585–591. [Google Scholar] [CrossRef]
- Smith, A.M. Review of molecular subtyping methodologies used to investigate outbreaks due to multidrug-resistant enteric bacterial pathogens in sub-Saharan Africa. Afr. J. Lab. Med. 2019, 8, 760. [Google Scholar] [CrossRef]
- Foley, S.L.; Johnson, T.J.; Ricke, S.C.; Nayak, R.; Danzeisen, J. Salmonella pathogenicity and host adaptation in chicken-associated serovars. Microbiol. Mol. Biol. Rev. 2013, 77, 582–607. [Google Scholar] [CrossRef]
- Bayoumi, M.A.; Griffiths, M.W. Probiotics down-regulate genes in Salmonella enterica serovar Typhimurium pathogenicity islands 1 and 2. J. Food Prot. 2010, 73, 452–460. [Google Scholar] [CrossRef]
- Galán, J.E. Salmonella interactions with host cells: Type III secretion at work. Annu. Rev. Cell Dev. Biol. 2001, 17, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Morales, F. Impact of Salmonella enterica type III secretion system effectors on the eukaryotic host cell. ISRN Cell Biol. 2012, 2012, 787934. [Google Scholar] [CrossRef]
- Hallstrom, K.N.; McCormick, B.A. Pathogenicity Islands: Origins, Structure, and Roles in Bacterial Pathogenesis, in Molecular Medical Microbiology; Elsevier: Issy-les-Moulineaux, France, 2015; pp. 303–314. [Google Scholar]
- Coburn, B.; Li, Y.; Owen, D.; Vallance, B.A.; Finlay, B.B. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 2005, 73, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.L.; Zhang, S.; Tsolis, R.M.; Kingsley, R.A.; Adams, L.G.; Baumler, A.J. Animal models of Salmonella infections: Enteritis versus typhoid fever. Microbes Infect. 2001, 3, 1335–1344. [Google Scholar] [CrossRef]
- Juhas, M.; Van Der Meer, J.R.; Gaillard, M.; Harding, R.M.; Hood, D.W.; Crook, D.W. Genomic islands: Tools of bacterial horizontal gene transfer and evolution. FEMS Microbiol. Rev. 2009, 33, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Fookes, M.; Schroeder, G.N.; Langridge, G.C.; Blondel, C.J.; Mammina, C.; Connor, T.R.; Seth-Smith, H.; Vernikos, G.S.; Robinson, K.S.; Sanders, M. Salmonella bongori provides insights into the evolution of the Salmonellae. PLoS Pathog. 2011, 7, e1002191. [Google Scholar] [CrossRef]
- Gerlach, R.G.; Hensel, M. Salmonella pathogenicity islands in host specificity, host pathogen-interactions and antibiotics resistance of Salmonella enterica. Berl. Munch. Tierarztl. Wochenschr. 2007, 120, 317–327. [Google Scholar]
- El-Sebay, N.A.; Abu Shady, H.M.; El-Rashed El-Zeedy, S.A.; Samy, A.A. InvA gene sequencing of Salmonella Typhimurium isolated from Egyptian poultry. Asian J. Sci. Res. 2017, 10, 194–202. [Google Scholar] [CrossRef]
- Galán, J.E. Molecular genetic bases of Salmonella entry into host cells. Mol. Microbiol. 1996, 20, 263–271. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Heffron, F.; Reissbrodt, R. Rapid detection of Salmonella enterica with primers specific for iroB. J. Clin. Microbiol. 1997, 35, 1224–1230. [Google Scholar]
- Baumler, A.J.; Tsolis, R.M.; Van Der Velden, A.W.; Stojiljkovic, I.; Anic, S.; Heffron, F. Identification of a new iron regulated locus of Salmonella Typhi. Gene 1996, 183, 207–213. [Google Scholar] [CrossRef]
- Li, X.; Yang, F.; Gao, W.; Song, H.; Tian, H.; Xu, B. Application of pyrosequencing for Salmonella enterica rapid identification. J. Microbiol. Methods 2012, 89, 49–52. [Google Scholar] [CrossRef]
- Shanmugasundaram, M.; Radhika, M.; Murali, H.S.; Batra, H.V. Detection of Salmonella enterica serovar Typhimurium by selective amplification of fliC, fljB, iroB, invA, rfbJ, STM2755, STM4497 genes by polymerase chain reaction in a monoplex and multiplex format. World J. Microbiol. Biotechnol. 2009, 25, 1385–1394. [Google Scholar] [CrossRef]
- Ganesan, V.; Harish, B.N.; Menezes, G.A.; Parija, S.C. Detection of Salmonella in blood by PCR using iroB gene. J. Clin. Diagn. Res. JCDR 2014, 8, DC01–DC03. [Google Scholar] [CrossRef]
- Hantke, K.; Nicholson, G.; Rabsch, W.; Winkelmann, G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. USA 2003, 100, 3677–3682. [Google Scholar] [CrossRef]
- Crouch, M.L.V.; Castor, M.; Karlinsey, J.E.; Kalhorn, T.; Fang, F.C. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2008, 67, 971–983. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Lin, H.; Zhou, L.; Yu, Y.; Abergel, R.J.; Liu, D.R.; Raymond, K.N.; Wanner, B.L.; Strong, R.K.; Walsh, C.T.; et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc. Natl. Acad. Sci. USA 2006, 103, 16502–16507. [Google Scholar] [CrossRef]
- Raffatellu, M.; George, M.D.; Akiyama, Y.; Hornsby, M.J.; Nuccio, S.P.; Paixao, T.A.; Butler, B.P.; Chu, H.; Santos, R.L.; Berger, T.; et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 2009, 5, 476–486. [Google Scholar] [CrossRef]
- Uchiya, K.-I.; Nikai, T. Salmonella virulence factor SpiC is involved in expression of flagellin protein and mediates activation of the signal transduction pathways in macrophages. Microbiology 2008, 154, 3491–3502. [Google Scholar] [CrossRef]
- Ingram, J.P.; Brodsky, I.E.; Balachandran, S. Interferon-γ in Salmonella pathogenesis: New tricks for an old dog. Cytokine 2017, 98, 27–32. [Google Scholar] [CrossRef]
- Figueira, R.; Holden, D.W. Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 2012, 158, 1147–1161. [Google Scholar] [CrossRef]
- Wood, M.W.; Jones, M.A.; Watson, P.R.; Hedges, S.; Wallis, T.S.; Galyov, E.E. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol. Microbiol. 1998, 29, 883–891. [Google Scholar] [CrossRef]
- Khoo, C.H.; Sim, J.H.; Salleh, N.A.; Cheah, Y.K. Pathogenicity and phenotypic analysis of sopB, sopD and pipD virulence factors in Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Agona. Antonie Leeuwenhoek 2015, 107, 23–37. [Google Scholar] [CrossRef]
- Lawley, T.D.; Chan, K.; Thompson, L.J.; Kim, C.C.; Govoni, G.R.; Monack, D.M. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2006, 2, e11. [Google Scholar] [CrossRef]
- Ashbolt, N.J.; Amézquita, A.; Backhaus, T.; Borriello, P.; Brandt, K.K.; Collignon, P.; Coors, A.; Finley, R.; Gaze, W.H.; Heberer, T.; et al. Human health risk assessment (HHRA) for environmental development and transfer of antibiotic resistance. Environ. Health Perspect. 2013, 121, 993–1001. [Google Scholar] [CrossRef]
- Hall, R.M. Integrons and gene cassettes: Hotspots of diversity in bacterial genomes. Ann. N. Y. Acad. Sci. 2012, 1267, 71–78. [Google Scholar] [CrossRef]
- Khan, A.A.; Ponce, E.; Nawaz, M.S.; Cheng, C.M.; Khan, J.A.; West, C.S. Identification and characterization of class 1 integron resistance gene cassettes among Salmonella strains isolated from imported seafood. Appl. Environ. Microbiol. 2009, 75, 1192–1196. [Google Scholar] [CrossRef]
- Ribeiro, V.B.; Lincopan, N.; Landgraf, M.; Franco, B.D.; Destro, M.T. Characterization of class 1 integrons and antibiotic resistance genes in multidrug-resistant Salmonella enterica isolates from foodstuff and related sources. Braz. J. Microbiol. 2011, 42, 685–692. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, L.; Li, L.; Guo, S.; Zhang, X.; Yamasaki, S.; Miyoshi, S.-I.; Shinoda, S. Identification and characterization of class 1 integron resistance gene cassettes among Salmonella strains isolated from healthy humans in China. Microbiol. Immunol. 2004, 48, 639–645. [Google Scholar] [CrossRef]
- Doublet, B.; Boyd, D.; Mulvey, M.R.; Cloeckaert, A. The Salmonella genomic island 1 is an integrative mobilizable element. Mol. Microbiol. 2005, 55, 1911–1924. [Google Scholar] [CrossRef]
- Boucher, Y.; Labbate, M.; Koenig, J.E.; Stokes, H. Integrons: Mobilizable platforms that promote genetic diversity in bacteria. Trends Microbiol. 2007, 15, 301–309. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef]
- Gillings, M.; Boucher, Y.; Labbate, M.; Holmes, A.; Krishnan, S.; Holley, M.; Stokes, H.W. The evolution of class 1 integrons and the rise of antibiotic resistance. J. Bacteriol. 2008, 190, 5095–5100. [Google Scholar] [CrossRef]
- Vo, A.T.T.; Van Duijkeren, E.; Gaastra, W.; Fluit, A.C. Antimicrobial resistance, class 1 integrons, and genomic island 1 in Salmonella isolates from Vietnam. PLoS ONE 2010, 5, e9440. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Reports of Selected Salmonella Outbreak Investigations. US Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/Salmonella/outbreaks.html (accessed on 18 October 2018).
- Mathole, M.; Muchadeyi, F.; Mdladla, K.; Malatji, D.; Dzomba, E.; Madoroba, E. Presence, distribution, serotypes and antimicrobial resistance profiles of Salmonella among pigs, chickens and goats in South Africa. Food Control 2017, 72, 219–224. [Google Scholar] [CrossRef]
- Zishiri, O.T.; Mkhize, N.; Mukaratirwa, S. Prevalence of virulence and antimicrobial resistance genes in Salmonella spp. isolated from commercial chickens and human clinical isolates from South Africa and Brazil. Onderstepoort J. Vet. Res. 2016, 83, 1–11. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, W.; Zhang, D.; Yu, T.; Yin, Y.; Ju, H.; Ding, S. Rapid and sensitive strategy for Salmonella detection using an InvA gene-based electrochemical DNA sensor. Int. J. Electrochem. Sci. 2012, 7, 844–856. [Google Scholar]
- Hughes, A.L.; Shopland, S.; Wigley, P.; Bradon, H.; Leatherbarrow, A.H.; Williams, N.J.; Bennett, M.; De Pinna, E.; Lawson, B.; Cunningham, A.A.; et al. Characterisation of Salmonella enterica serotype Typhimurium isolates from wild birds in northern England from 2005–2006. BMC Vet. Res. 2008, 4, 4. [Google Scholar] [CrossRef]
- Pan, J.-C.; Ye, R.; Meng, D.-M.; Zhang, W.; Wang, H.-Q.; Liu, K.-Z. Molecular characteristics of class 1 and class 2 integrons and their relationships to antibiotic resistance in clinical isolates of Shigella sonnei and Shigella flexneri. J. Antimicrob. Chemother. 2006, 58, 288–296. [Google Scholar] [CrossRef]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef]
- Ahmed, O.B.; Asghar, A.; Abd El-Rahim, I.; Al, H. Detection of Salmonella in food samples by culture and polymerase chain reaction methods. J. Bacteriol. Parasitol. 2014, 5, 187. Available online: https://www.longdom.org/open-access/detection-of-salmonella-in-food-samples-by-culture-and-polymerase-chain-reaction-methods-2155-9597.1000187.pdf (accessed on 6 October 2018).
- Klose, A.; Irsigler, H.; Jaeger, D.; Hafez, H.M.; Langkabel, N.; Bräutigam, L.; Fries, R. Comparison of methods for the detection of Salmonella in poultry. J. Appl. Poult. Res. 2014, 23, 403–408. [Google Scholar]
- Leonard, S.R.; Lacher, D.W.; Lampel, K.A. Acquisition of the lac operon by Salmonella enterica. BMC Microbiol. 2015, 15, 173. [Google Scholar] [CrossRef]
- Eswarappa, S.M.; Karnam, G.; Nagarajan, A.G.; Chakraborty, S.; Chakravortty, D. lac repressor is an antivirulence factor of Salmonella enterica: Its role in the evolution of virulence in Salmonella. PLoS ONE 2009, 4, e5789. [Google Scholar] [CrossRef]
- Hurley, D.; Hoffmann, M.; Muruvanda, T.; Allard, M.W.; Brown, E.W.; Martins, M.; Fanning, S. Atypical Salmonella enterica serovars in murine and human infection models: Is it time to reassess our approach to the study of salmonellosis? BioRxiv 2016, 058610. Available online: https://www.biorxiv.org/content/biorxiv/early/2016/06/13/058610.1.full.pdf (accessed on 10 October 2018).
- McDonough, P.L.; Shin, S.J.; Lein, D.H. Diagnostic and public health dilemma of lactose-fermenting Salmonella enterica serotype Typhimurium in cattle in the northeastern United States. J. Clin. Microbiol. 2000, 38, 1221–1226. [Google Scholar]
- Jamshidi, A.; Kalidari, G.A.; Hedayati, M. Isolation and identification of Salmonella Enteritidis and Salmonella Typhimurium from the eggs of retail stores in Mashhad, Iran. using conventional culture method and multiplex PCR assay. J. Food Saf. 2010, 30, 558–568. [Google Scholar]
- Manning, J.; Gole, V.; Chousalkar, K. Screening for Salmonella in backyard chickens. Prev. Vet. Med. 2015, 120, 241–245. [Google Scholar] [CrossRef]
- Abatcha, M.G.; Effarizah, M.E.; Rusul, G. Prevalence, antimicrobial resistance, resistance genes and class 1 integrons of Salmonella serovars in leafy vegetables, chicken carcasses and related processing environments in Malaysian fresh food markets. Food Control 2018, 91, 170–180. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T. Genetic analysis of multiple antimicrobial resistance in Salmonella isolated from diseased broilers in Egypt. Microbiol. Immunol. 2012, 56, 254–261. [Google Scholar] [CrossRef]
- Magwedere, K.; Rauff, D.; De Klerk, G.; Dziva, F.; Keddy, K.H. Incidence of nontyphoidal Salmonella in food-producing animals, animal feed, and the associated environment in South. Africa, 2012–2014. Clin. Infect. Dis. 2015, 61, S283–S289. [Google Scholar] [CrossRef]
- Dione, M.M.; Ikumapayi, U.; Saha, D.; Mohammed, N.I.; Adegbola, R.A.; Geerts, S.; Ieven, M.; Antonio, M. Antimicrobial resistance and virulence genes of non-typhoidal Salmonella isolates in The Gambia and Senegal. J. Infect. Dev. Ctries. 2011, 5, 765–775. [Google Scholar] [CrossRef]
- Gyles, C.; Boerlin, P. Horizontally transferred genetic elements and their role in pathogenesis of bacterial disease. Vet. Pathol. 2014, 51, 328–340. [Google Scholar] [CrossRef]
- Gillings, M.R. Integrons: Past, present, and future. Microbiol. Mol. Biol. Rev. 2014, 78, 257–277. [Google Scholar] [CrossRef]
- Domingues, S.; da Silva, G.J.; Nielsen, K.M. Integrons: Vehicles and pathways for horizontal dissemination in bacteria. Mob. Genet. Elem. 2012, 2, 211–223. [Google Scholar] [CrossRef]
- Threlfall, E.J. Antimicrobial drug resistance in Salmonella: Problems and perspectives in food-and water-borne infections. FEMS Microbiol. Rev. 2002, 26, 141–148. [Google Scholar] [CrossRef]
- Jiang, C.; Shaw, K.S.; Romeo, C.; Blythe, D.; Mitchell, C.; Murtugudde, R.; Sapkota, A.R.; Sapkota, A. Climate change, extreme events and increased risk of salmonellosis in Maryland, USA: Evidence for coastal vulnerability. Environ. Int. 2015, 83, 58–62. [Google Scholar] [CrossRef] [Green Version]
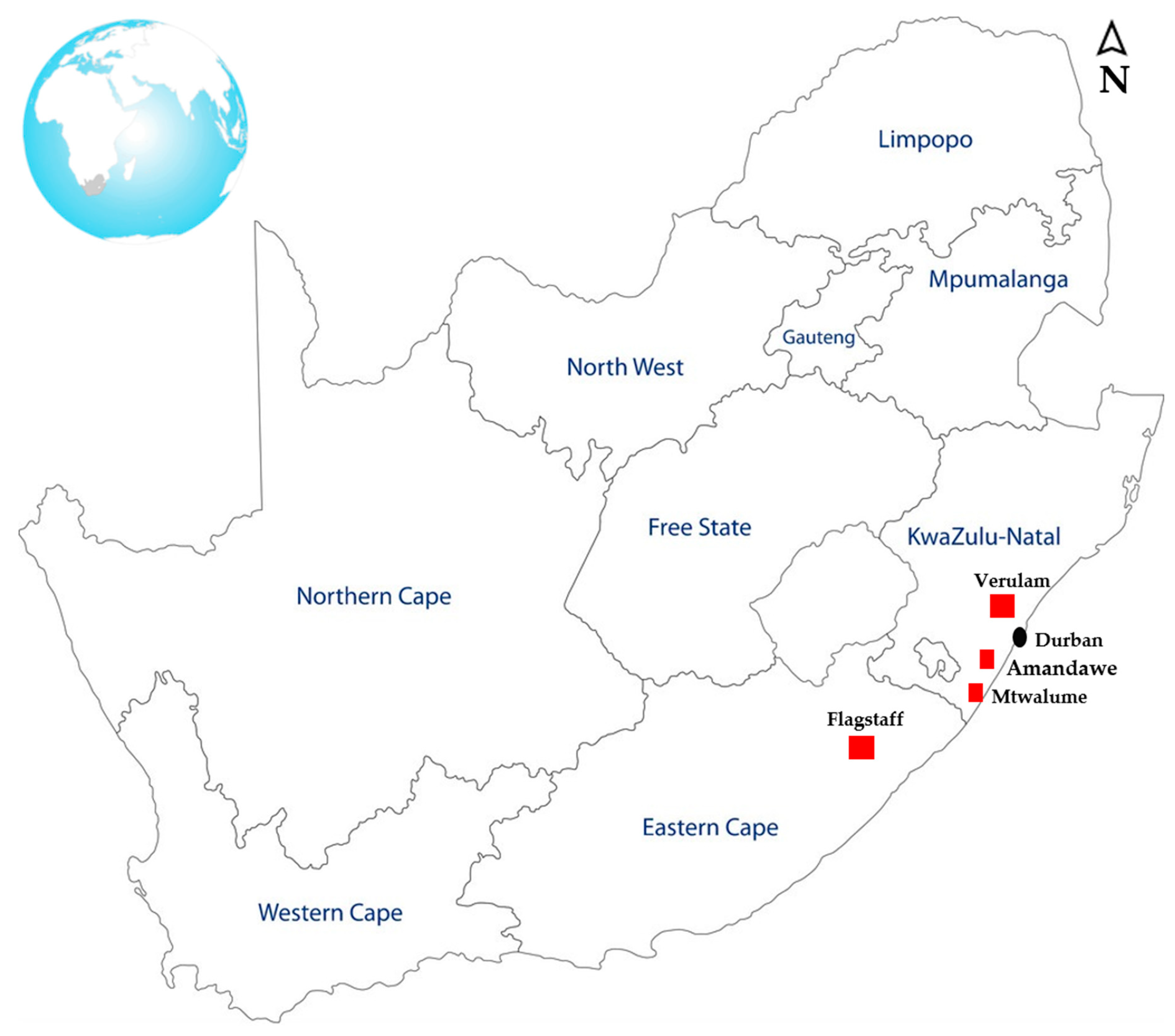
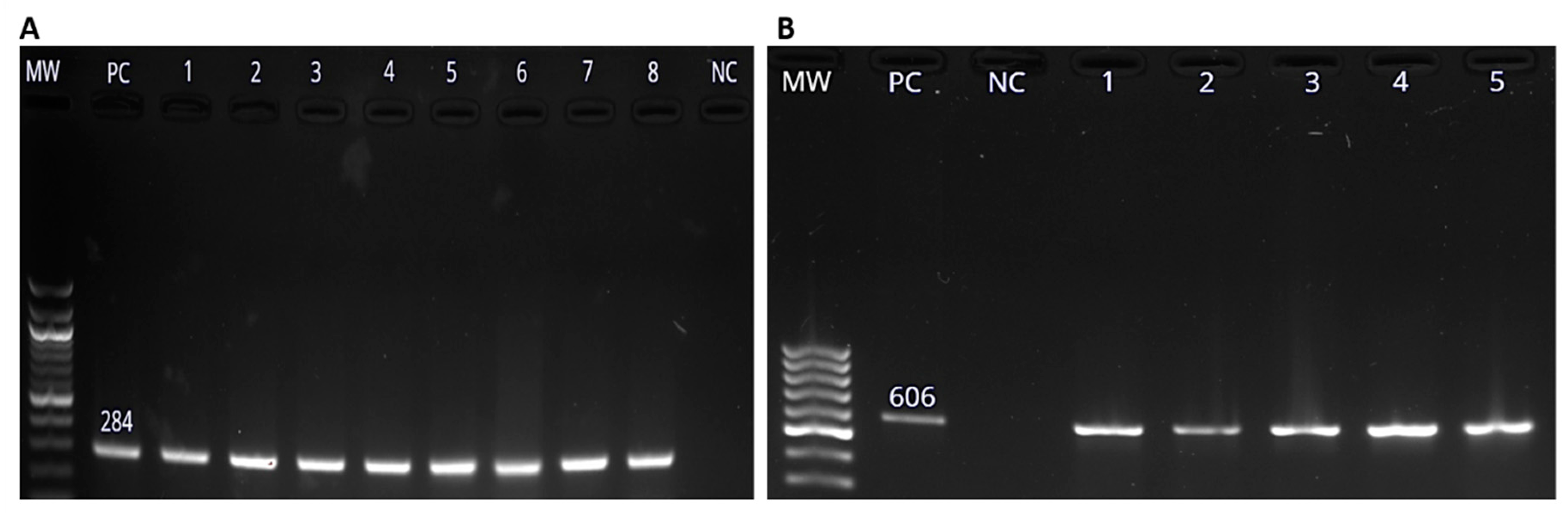
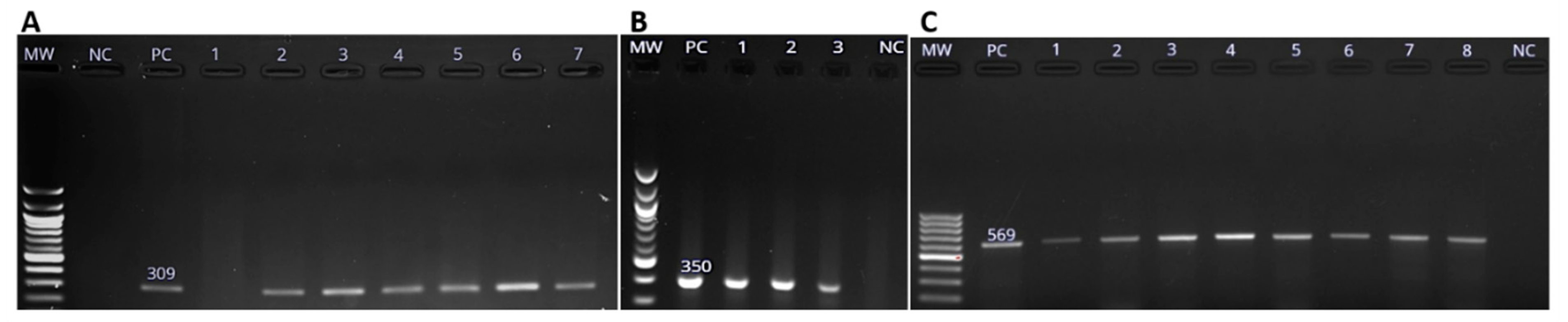
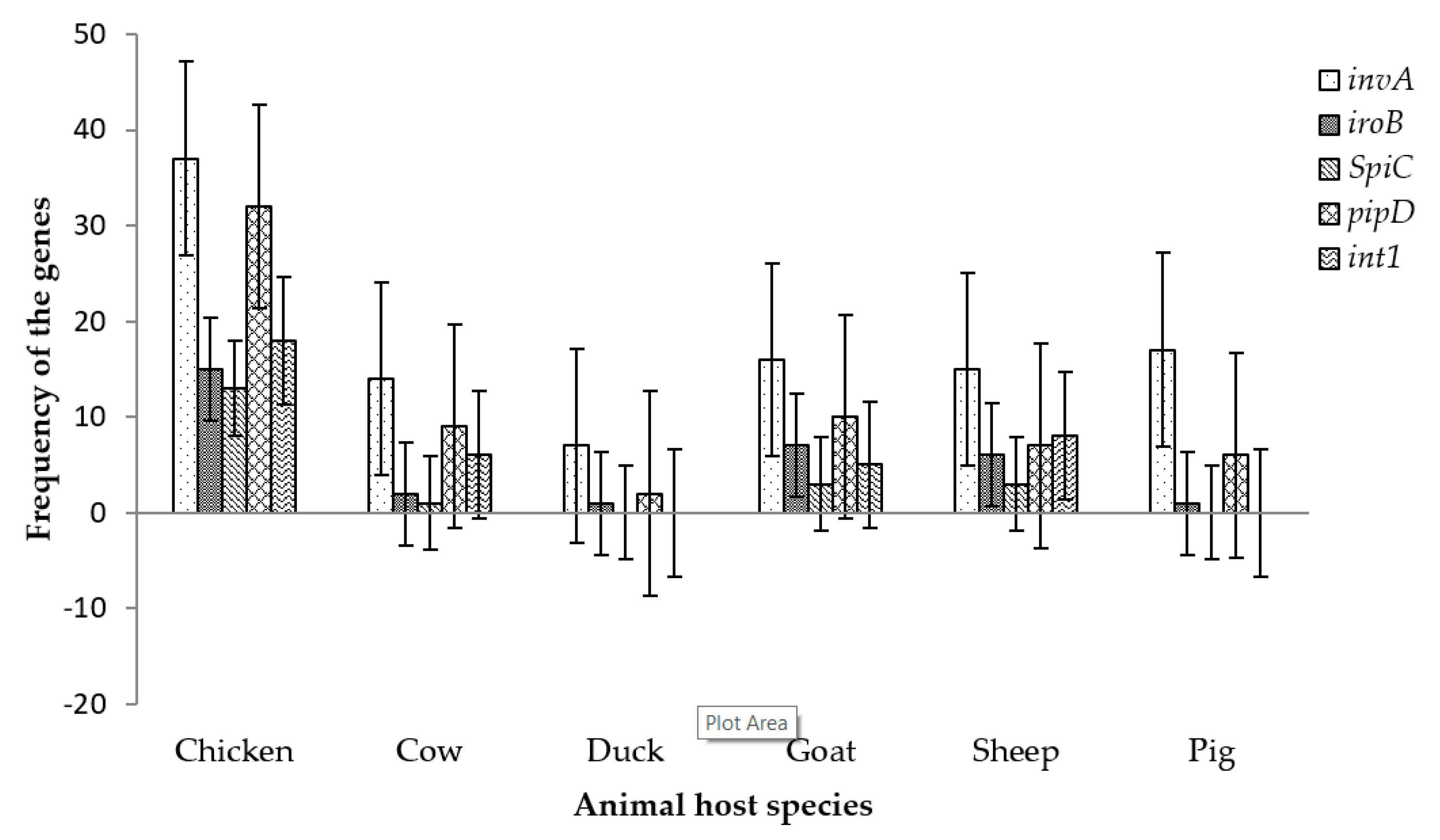

| Animal Host | Flagstaff | Verulam | South Coast | Total | Positive Samples (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oral | Fecal | Feed | Soil | Water | Oral | Fecal | Feed | Soil | Water | Oral | Fecal | Feed | Soil | Water | |||
| Chicken | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | 40 | 40 | 0 | 5 | 5 | 114 | 10.25 |
| Ducks | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 1.94 |
| Cow | 0 | 5 | 0 | 5 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 10 | 0 | 5 | 5 | 50 | 3.88 |
| Goats | 10 | 9 | 0 | 6 | 6 | 0 | 10 | 0 | 0 | 0 | 17 | 16 | 0 | 0 | 5 | 79 | 4.43 |
| Sheep | 12 | 10 | 0 | 6 | 0 | 0 | 10 | 0 | 0 | 0 | 4 | 8 | 0 | 0 | 0 | 50 | 4.15 |
| Pigs | 17 | 17 | 9 | 9 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 58 | 4.71 |
| Total | 39 | 51 | 9 | 26 | 12 | 0 | 64 | 0 | 0 | 0 | 61 | 74 | 0 | 10 | 15 | 361 | 29.36 |
| p-Value | Odds Ratio | 95% C.I. for Odds Ratio | |
|---|---|---|---|
| iroB | |||
| Location | 0.005 | ||
| Verulam | 0.007 | 5.429 | (1.577, 18.686) |
| South coast | 0.881 | 0.924 | (0.330, 2.587) |
| pipD | |||
| Location | 0.007 | ||
| Verulam | 0.006 | 19.991 | (2.330, 171.530) |
| South coast | 0.994 | 0.994 | (0.237, 4.173) |
| Int1 | |||
| Location | 0.022 | ||
| Verulam | 0.042 | 8.053 | (1.801, 59.968) |
| South coast | 0.409 | 0.564 | (0.145, 2.193) |
| Variables | Pearson’s Correlation (p-Value) |
|---|---|
| XLD and invA | 0.402 (0.000) |
| iroB and spiC | 0.407 (0.000) |
| iroB and pipD | 0.258 (0.008) |
| iroB and int1 | 0.294 (0.002) |
| spiC and pipD | 0.357 (0.000) |
| spiC and int1 | 0.102 (0.298) * |
| pipD and int1 | 0.325 (0.001) |
| Variable | iroB | spiC | pipD | Int1 |
|---|---|---|---|---|
| Location | 0.002 | 0.000 | 0.000 | 0.000 |
| Animal host | 0.037 | 0.019 | 0.002 | 0.000 |
| Sampling season | 0.001 | 0.000 | 0.000 | 0.000 |
| Sample material | 0.345 * | 0.467 * | 0.365 * | 0.004 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mthembu, T.P.; Zishiri, O.T.; El Zowalaty, M.E. Detection and Molecular Identification of Salmonella Virulence Genes in Livestock Production Systems in South Africa. Pathogens 2019, 8, 124. https://doi.org/10.3390/pathogens8030124
Mthembu TP, Zishiri OT, El Zowalaty ME. Detection and Molecular Identification of Salmonella Virulence Genes in Livestock Production Systems in South Africa. Pathogens. 2019; 8(3):124. https://doi.org/10.3390/pathogens8030124
Chicago/Turabian StyleMthembu, Thobeka P., Oliver T. Zishiri, and Mohamed E. El Zowalaty. 2019. "Detection and Molecular Identification of Salmonella Virulence Genes in Livestock Production Systems in South Africa" Pathogens 8, no. 3: 124. https://doi.org/10.3390/pathogens8030124





