Base Metal Co-Fired Multilayer Piezoelectrics
Abstract
:1. Introduction
2. Applications of Multilayer Piezoelectrics
3. Multilayer Co-Firing
4. Trends of Metal Electrodes
5. Pb(Zr, Ti)O3
5.1. Volatility of Pb
5.2. Low-Temperature Noble Metal Co-Firing
5.3. Base Metal Co-Firing
6. BaTiO3 and Base Metal Co-Firing
7. (Na, K)NbO3
7.1. Volatility of Alkaline Elements
7.2. Material Modification
7.3. Low-Temperature Co-Firing with Noble Metal
7.4. Low-Temperature Co-Firing with Base Metal
8. Bi0.5Na0.5TiO3
9. Binder Materials
10. Recent Progress on Cu Co-Fired NKN
11. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Randall, C.A.; Kelnberger, A.; Yang, G.Y.; Eitel, R.E.; Shrout, T.R. High Strain Piezoelectric Multilayer Actuators—A Material Science and Engineering Challenge. J. Electroceramics 2005, 14, 177–191. [Google Scholar] [CrossRef]
- Uchino, K.; Takahashi, S. Multilayer Ceramic Actuators. Curr. Opin. Solid State Mater. Sci. 1996, 1, 698–705. [Google Scholar] [CrossRef]
- Uchino, K. Ferroelectric Devices, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Abraham, T. Expanding Markets for Piezoelectrics. Available online: http://www.ceramicindustry.com/articles/93845-expanding-markets-for-piezoelectrics (accessed on 22 October 2015).
- Uchino, K. Entrepreneurship for Engineers; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Knowles, G.J.; Bradley, W.M.; Bird, R. Galvanic Isolated Ceramic Based Voltage Sensors. WO2015,153,798 A1, 8 October 2015. [Google Scholar]
- Imanaka, Y. Multilayered Low Temperature Cofired Ceramics (LTCC) Technology; Springer Science & Business Media: New York, NY, USA, 2005. [Google Scholar]
- Gaskell, D.R. Introduction to the Thermodynamics of Materials, 5th ed.; CRC Taylor & Francis Group, LLC: New York, NY, USA, 2008. [Google Scholar]
- Kishi, H.; Mizuno, Y.; Chazono, H. Base-Metal Electrode-Multilayer Ceramic Capacitors: Past, Present and Future Perspectives. Jpn. J. Appl. Phys. 2003, 42, 1–15. [Google Scholar] [CrossRef]
- Karakaya, I.; Thompson, W.T. The Ag-Pd (Silver-Palladium) System. Bull. Alloy Phase Diagrams 1988, 9, 237–243. [Google Scholar] [CrossRef]
- Wang, S.F.; Dougherty, J.P.; Huebner, W.; Pepin, J.G. Silver-Palladium Thick-Film Conductors. J. Am. Ceram. Soc. 1994, 77, 3051. [Google Scholar] [CrossRef]
- Song, T.; Randall, C.A. Copper Cofire X7R Dielectrics and Multilayer Capacitors Based on Zinc Borate Fluxed Barium Titanate Ceramic. J. Electroceramics 2003, 10, 39–46. [Google Scholar] [CrossRef]
- Yonezawa, T.; Takeoka, S.; Kishi, H.; Ida, K.; Tomonari, M. The Preparation of Copper Fine Particle Paste and its Application as the Inner Electrode Material of a Multilayered Ceramic Capacitor. Nanotechnology 2008, 19, 145706. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, S.; Mizuno, Y. Effect of Internal Electrode Materials in Multilayer Ceramic Capacitors on Electrical Properties. Jpn. J. Appl. Phys. 2011, 50, 1–6. [Google Scholar] [CrossRef]
- Shirane, G.; Takeda, A. Phase Transitions in Solid Solutions of PbZrO3 and PbTiO3 (I) Small Concentrations of PbTiO3. J. Phys. Soc. Jpn 1952, 7, 5–11. [Google Scholar] [CrossRef]
- Shirane, G.; Suzuki, K.; Takeda, A. Phase Transitions in Solid Solutions of PbZrO3 and PbTiO3 (II) X-ray Study. J. Phys. Soc. Jpn. 1952, 7, 12–18. [Google Scholar] [CrossRef]
- Jaffe, B.; Cook, W.R. Piezoelectric Ceramic, 1st ed.; Academic Press: London, UK, 1971. [Google Scholar]
- Kingon, A.I.; Clark, J.B. Sintering of PZT Ceramics: II, Effect of PbO Content on Densification Kinetics. J. Am. Ceram. Soc. 1983, 66, 256–260. [Google Scholar] [CrossRef]
- Holman, R.L.; Fulrath, R.M. Intrinsic Nonstoichiometry in Single-Phase Pb(Zr0.5Ti0.5)O3. J. Am. Ceram. Soc. 1972, 55, 192–194. [Google Scholar] [CrossRef]
- Donnelly, N.J.; Shrout, T.R.; Randall, C.A. Properties of (1-x)PZT-xSKN Ceramics Sintered at Low Temperature Using Li2CO3. J. Am. Ceram. Soc. 2008, 91, 2182–2188. [Google Scholar] [CrossRef]
- Donnelly, N.J.; Shrout, T.R.; Randall, C.A. The Role of Li2CO3 and PbO in the Low-Temperature Sintering of Sr, K, Nb (SKN)-Doped PZT. J. Am. Ceram. Soc. 2009, 92, 1203–1207. [Google Scholar] [CrossRef]
- Wang, X.X.; Murakami, K.; Sugiyama, O.; Kaneko, S. Piezoelectric Properties, Densification Behavior and Microstructural Evolution of Low Temperature Sintered PZT Ceramics with Sintering Aids. J. Eur. Ceram. Soc. 2001, 21, 1367–1370. [Google Scholar] [CrossRef]
- Donnelly, N.J.; Shrout, T.R.; Randall, C.A. Addition of a Sr, K, Nb (SKN) Combination to PZT(53/47) for High Strain Applications. J. Am. Ceram. Soc. 2007, 90, 490–495. [Google Scholar] [CrossRef]
- Kaneko, S.; Dong, D.; Murakami, K. Effect of Simultaneous Addition of BiFeO3 and Ba(Cu0.5W0.5)O3 on Lowering of Sintering Temperature of Pb(Zr,Ti)O3 Ceramics. J. Am. Ceram. Soc. 1998, 81, 1013–1018. [Google Scholar] [CrossRef]
- Ahn, C.W.; Nahm, S.; Ryu, J.; Uchino, K.; Yoon, S.J.; Jung, S.J.; Song, J.S. Effects of CuO and ZnO Additives on Sintering Temperature and Piezoelectric Properties of 0.41Pb(Ni1/3Nb2/3)O3-0.36PbTiO3-0.23PbZrO3 Ceramics. Jpn. J. Appl. Phys. 2004, 43, 205–210. [Google Scholar] [CrossRef]
- Hayashi, T.; Hasegawa, T. Piezoelectric Properties of Low-temperature Sintered Pb0.95Ba0.05[(Mg1/3Nb2/3)0.125Zr0.445Ti0.43]O3 Ceramics with Chemically-added LiBiO2 Sintering Aid. J. Eur. Ceram. Soc. 2005, 25, 2437–2441. [Google Scholar] [CrossRef]
- Donnelly, N.J.; Randall, C.A. Impedance Spectroscopy of PZT Ceramics—Measuring Diffusion Coefficients, Mixed Conduction, and Pb Loss. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nagasaka, T.; Hino, M. Effect of Oxygen on the Evaporation Rate of Lead from Liquid Copper under Reduced Pressure. ISIJ Int. 2001, 41, 706–715. [Google Scholar] [CrossRef]
- Helke, G.; Seifert, S.; Cho, S.-J. Phenomenological and Structural Properties of Piezoelectric Ceramics Based on xPb(Zr,Ti)O3-(1-x)Sr(K0·25Nb0·75)O3 (PZT/SKN) Solid Solutions. J. Eur. Ceram. Soc. 1999, 19, 1265–1268. [Google Scholar] [CrossRef]
- Atkin, R.B.; Fulrath, R.M. Point Defects and Sintering of Lead Zirconate-Titanate. J. Am. Ceram. Soc. 1971, 54, 265–270. [Google Scholar] [CrossRef]
- Donnelly, N.J.; Randall, C.A. Pb Loss in Pb(Zr,Ti)O3 Ceramics Observed by in Situ Ionic Conductivity Measurements. J. Appl. Phys. 2011, 109, 104107. [Google Scholar] [CrossRef]
- Uchino, K.; Nomura, S.; Cross, L.E.; Newnham, R.E.; Jang, S.J. Electrostrictive Effect in Perovskites and Its Transducer Applications. J. Mater. Sci. 1981, 16, 569–578. [Google Scholar] [CrossRef]
- Wersing, W.; Wahl, H.; Schnöller, M. PZT-based Multilayer Piezoelectric Ceramics with AgPd-Internal Electrodes. Ferroelectrics 1988, 87, 271–294. [Google Scholar] [CrossRef]
- Caballero, A.C.; Nieto, E.; Duran, P.; Moure, C.; Kosec, M.; Samardzija, Z.; Drazig, G. Ceramic-Electrode Interaction in PZT and PNN-PZT Multilayer Piezoelectric Ceramics with AG/PD 70/30 Inner Electrode. J. Mater. Sci. 1997, 32, 3257–3262. [Google Scholar] [CrossRef]
- Donnelly, N.J.; Randall, C.A. Refined Model of Electromigration of Ag/Pd Electrodes in Multilayer PZT Ceramics Under Extreme Humidity. J. Am. Ceram. Soc. 2009, 92, 405–410. [Google Scholar] [CrossRef]
- Jacob, K.T.; Saji, V.S. Interaction between Ni/NiO and PbTiO3: Phase Reversal with Redox Switching. J. Phase Equilibria Diffus. 2006, 27, 456–461. [Google Scholar] [CrossRef]
- Florian, H.; Reichmann, K.; Kainz, G. Piezoactuator and method for the production thereof. US Patent US7,304,414, 4 December 2007. [Google Scholar]
- Takayama, Y.; Donnelly, N.J.; Randall, C.A. Piezoelectric Actuators with Cu Inner Electrode. In Proceedings of the 13th US-Japan Seminar on Dielectric and Piezoelectric Ceramics, Hyogo, Japan, 4–7 November 2007; pp. 113–116.
- Florian, H.; Ottlinger, M.; Sedlmaier, P. Method for Producing a Multilayer Ceramic Component. US patent US8,776,364 B2, 15 July 2014. [Google Scholar]
- Piezo Actuators for Fuel Injection Systems—Most Reliable and Cost-efficient. Available online: http://en.tdk.eu/tdk-en/373562/tech-library/articles/applications---cases/applications---cases/most-reliable-and-cost-efficient/1039300 (accessed on 21 November 2015).
- Polotai, A.V.; Yang, G.Y.; Dickey, E.C.; Randall, C.A. Utilization of Multiple-Stage Sintering to Control Ni Electrode Continuity in Ultrathin Ni-BaTiO3 Multilayer Capacitors. J. Am. Ceram. Soc. 2007, 90, 3811–3817. [Google Scholar]
- Opitz, M.R.; Albertsen, K.; Beeson, J.J.; Hennings, D.F.; Routbort, J.L.; Randall, C.A. Kinetic Process of Reoxidation of Base Metal Technology BaTiO3-based Multilayer Capacitors. J. Am. Ceram. Soc. 2003, 86, 1879–1884. [Google Scholar] [CrossRef]
- Pithan, C.; Hennings, D.; Waser, R. Progress in the Synthesis of Nanocrystalline BaTiO3 Powders for MLCC. Int. J. Appl. Ceram. Technol. 2005, 2, 1–14. [Google Scholar] [CrossRef]
- Gui, Z.L.; Wang, Y.L.; Li, L.T. Study on the Interdiffusion in Base-Metal-Electrode MLCCs. Ceram. Int. 2004, 30, 1275–1278. [Google Scholar] [CrossRef]
- Wang, S.; Tsai, Y.; Lee, W. Study on (Ba, Ca)(Ti, Zr)O3 Dielectric Cofired with Copper Electrode. Jpn. J. Appl. Phys. 2014, 53, 061501. [Google Scholar] [CrossRef]
- Yamamatsu, J.; Kawano, N.; Arashi, T.; Sato, A.; Nakano, Y.; Nomura, T. Reliability of Multilayer Ceramic Capacitors with Nickel Electrodes. J. Power Sources 1996, 60, 199–203. [Google Scholar] [CrossRef]
- Randall, C.A. Scientific and Engineering Issues of the State-of-the-Art and Future Multilayer Capacitors. J. Ceram. Soc. Jpn. 2001, 109, S2–S6. [Google Scholar] [CrossRef]
- Yang, G.Y.; Lee, S.I.; Liu, Z.J.; Anthony, C.J.; Dickey, E.C.; Liu, Z.K.; Randall, C.A. Effect of Local Oxygen Activity on Ni-BaTiO3 Interfacial Reactions. Acta Mater. 2006, 54, 3513–3523. [Google Scholar] [CrossRef]
- RoHS Compliance Guide: Regulations, 6 Substances, Exemptions, WEEE. Available online: http://www.rohsguide.com/ (accessed on 11 October 2015).
- Directive 2011/65/Eu of the European Parliament and of the Council of 8 June 2011 on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32011L0065&from=EN (accessed on 25 October 2015).
- Egerton, L.; Dillon, D.M. Piezoelectric and Dielectric Properties of Ceramics in the System Potassium-Sodium Niobate. J. Am. Ceram. Soc. 1959, 42, 438–442. [Google Scholar] [CrossRef]
- Saito, Y.; Takao, H.; Tani, T.; Nonoyama, T.; Takatori, K.; Homma, T.; Nagaya, T.; Nakamura, M. Lead-free Piezoceramics. Nature 2004, 432, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kakimoto, K.; Ohsato, H. Dielectric and Piezoelectric Properties of Lead-free (Na0.5K0.5)NbO3-SrTiO3 Ceramics. Solid State Commun. 2004, 129, 279–284. [Google Scholar] [CrossRef]
- Guo, Y.; Kakimoto, K.; Ohsato, H. (Na0.5K0.5)NbO3-LiTaO3 Lead-free Piezoelectric Ceramics. Mater. Lett. 2005, 59, 241–244. [Google Scholar] [CrossRef]
- Hollenstein, E.; Davis, M.; Damjanovic, D.; Setter, N. Piezoelectric Properties of Li- and Ta-modified (K0.5Na0.5)NbO3 Ceramics. Appl. Phys. Lett. 2005, 87, 182905. [Google Scholar] [CrossRef]
- Kim, M.S.; Jeong, S.J.; Song, J.S. Electromechanical Properties of NKN-5LT Multilayer Actuator. Adv. Mater. Res. 2007, 26–28, 263–266. [Google Scholar] [CrossRef]
- Kim, M.S.; Jeon, S.; Lee, D.S.; Jeong, S.J.; Song, J.S. Lead-free NKN-5LT Piezoelectric Materials for Multilayer Ceramic Actuator. J. Electroceramics 2009, 23, 372–375. [Google Scholar] [CrossRef]
- Kobayashi, K.; Doshida, Y.; Mizuno, Y.; Randall, C.A. Possibility of Cofiring a Nickel Inner Electrode in a (Na0.5K0.5)NbO3–LiF Piezoelectric Actuator. Jpn. J. Appl. Phys. 2013, 52, 1–5. [Google Scholar] [CrossRef]
- Kawada, S.; Kimura, M.; Higuchi, Y.; Takagi, H. (K,Na)NbO3-Based Multilayer Piezoelectric Ceramics with Nickel Inner Electrodes. Appl. Phys. Express 2009, 2, 111401. [Google Scholar] [CrossRef]
- Kawada, S.; Kimura, M.; Hayashi, H.; Ogiso, K.; Konoike, T.; Takagi, H. Study of Nickel Inner Electrode Lead-free Piezoelectric Ceramics. In Proceedings of the 2011 International Symposium on Applications of Ferroelectrics and 2011 International Symposium on Piezoresponse Force Microscopy and Nanoscale Phenomena in Polar Materials (ISAF/PFM), Vancouver, BC, Canada, 24–27 July 2011.
- Liu, C.; Liu, P.; Kobayashi, K.; Qu, W.; Randall, C.A. Enhancement of Piezoelectric Performance of Lead-Free NKN-Based Ceramics via A High-Performance Flux-NaF-Nb2O5. J. Am. Ceram. Soc. 2013, 96, 3120–3126. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Xiao, D.; Zhu, J.; Cheng, X.; Zheng, T.; Zhang, B.; Lou, X.; Wang, X. Giant Piezoelectricity in Potassium-Sodium Niobate Lead-free Ceramics. J. Am. Chem. Soc. 2014, 136, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xia, R.; Shrout, T.R.; Zang, G.; Wang, J. Characterization of Lead Free (K0.5Na0.5)NbO3-LiSbO3 Piezoceramic. Solid State Commun. 2007, 141, 675–679. [Google Scholar] [CrossRef]
- Lévêque, G.; Marchet, P.; Levassort, F.; Tran-Huu-Hue, L.P.; Duclere, J.R. Lead Free (Li,Na,K)(Nb,Ta,Sb)O3 Piezoelectric Ceramics: Influence of Sintering Atmosphere and ZrO2 Doping on Densification, Microstructure and Piezoelectric Properties. J. Eur. Ceram. Soc. 2011, 31, 577–588. [Google Scholar] [CrossRef]
- Matsubara, M.; Yamaguchi, T.; Sakamoto, W.; Kikuta, K.; Yogo, T.; Hirano, S. Processing and Piezoelectric Properties of Lead-Free (K,Na) (Nb,Ta)O3 Ceramics. J. Am. Ceram. Soc. 2005, 88, 1190–1196. [Google Scholar] [CrossRef]
- Kobayashi, K.; Doshida, Y.; Mizuno, Y.; Randall, C.A. A Route Forwards to Narrow the Performance Gap between PZT and Lead-free Piezoelectric Ceramic with Low Oxygen Partial Pressure Processed (Na0.5K0.5) NbO3. J. Am. Ceram. Soc. 2012, 95, 2928–2933. [Google Scholar] [CrossRef]
- Shimizu, H.; Kobayashi, K.; Mizuno, Y.; Randall, C.A. Advantages of Low Partial Pressure of Oxygen Processing of Alkali Niobate: NaNbO3. J. Am. Ceram. Soc. 2014, 97, 1791–1796. [Google Scholar] [CrossRef]
- Shigemi, A.; Wada, T. Enthalpy of Formation of Various Phases and Formation Energy of Point Defects in Perovskite-type NaNbO3 by First-principles Calculation. Jpn. J. Appl. Phys. 2004, 43, 6793–6798. [Google Scholar] [CrossRef]
- Shigemi, A.; Wada, T. Evaluations of Phases and Vacancy Formation Energies in KNbO3 by First-Principles Calculation. Jpn. J. Appl. Physics 2005, 44, 8048–8054. [Google Scholar] [CrossRef]
- Gao, R.; Chu, X.; Huan, Y.; Zhong, Z.; Wang, X.; Li, L. Ceramic-Electrode inter-Diffusion of (K, Na)NbO3-Based Multilayer Ceramics with Ag0.7Pd0.3 Electrode. J. Eur. Ceram. Soc. 2015, 35, 389–392. [Google Scholar] [CrossRef]
- Kobayashi, K.; Randall, C.A. New Opportunity in Alkali Niobate Ceramics Processed in Low Oxygen Partial Pressure. In Proceedings of the Applications of Ferroelectrics held jointly with 2012 European Conference on the Applications of Polar Dielectrics and 2012 International Symp Piezoresponse Force Microscopy and Nanoscale Phenomena in Polar Materials (ISAF/ECAPD/PFM), Aveiro, Portugal, 9–13 July 2012.
- Liu, C.; Liu, P.; Kobayashi, K.; Randall, C.A. Base Metal Co-fired (Na, K)NbO3 Structures with Enhanced Piezoelectric Performance. J. Electroceramics 2014, 32, 301–306. [Google Scholar] [CrossRef]
- Sapper, E.; Gassmann, A.; Gjødvad, L.; Jo, W.; Granzow, T.; Rödel, J. Cycling Stability of Lead-free BNT–8BT and BNT–6BT–3KNN Multilayer Actuators and Bulk Ceramics. J. Eur. Ceram. Soc. 2014, 34, 653–661. [Google Scholar] [CrossRef]
- Yesner, G.; Kuciej, M.; Safari, A. Low Temperature Sintering of Bi0.5Na0.5TiO3 Based Ceramics. In Proceedings of the 2015 Joint IEEE International Symposium on the Applications of Ferroelectric, International Symposium on Integrated Functionalities and Piezoelectric Force Microscopy Workshop (ISAF/ISIF/PFM), Singapore, 24–27 May 2015; pp. 21–23.
- Masia, S.; Calvert, P.; Rhine, W.; Bowen, H. Effect of Oxides on Binder Burnout during Ceramics Processing. J. Mater. Sci. 1989, 24, 1907–1912. [Google Scholar] [CrossRef]
- Liau, L.C.K.; Yang, T.C.K.; Viswanath, D.S. Mechanism of Degradation of Poly(Vinyl Butyral) Using Thermogravimetry/Fourier Transform Infrared Spectrometry. Polym. Eng. Sci. 1996, 36, 2589–2600. [Google Scholar] [CrossRef]
- Liau, L.C.K.; Viswanath, D.S. Thermal Degradation of Poly (Vinyl Butyral)/ Ceramic Composites: A Kinetic Approach. Ind. Eng. Chem. Res. 1998, 37, 49–57. [Google Scholar] [CrossRef]
- Salam, L.A.; Matthews, R.D.; Robertson, H. Pyrolysis of Polyvinyl Butyral (PVB) Binder in Thermoelectric Green Tapes. J. Eur. Ceram. Soc. 2000, 20, 1375–1383. [Google Scholar] [CrossRef]
- Yan, H.; Cannon, W.R.; Shanefield, D.J. Poly(Vinyl Butyral) Pyrolysis: Interactions with Plasticizer and AIN Ceramic Powder. MRS Proc. 1991, 249, 377–382. [Google Scholar] [CrossRef]
- Yan, H.; Cannon, W.R.; Shanefield, D.J. Thermal Decomposition Behaviour of Poly(Propylene Carbonate). Ceram. Int. 1998, 24, 433–439. [Google Scholar] [CrossRef]
- Gao, L.; Ko, S.-W.; Guo, H.; Hennig, E.; Randall, C.A. Demonstration of Copper Co-fired (Na, K)NbO3 Multilayer Structures for Piezoelectric Applications. J. Am. Ceram. Soc. 2016, in press. [Google Scholar]
- Scott, J.F. Ferroelectrics Go Bananas. J. Phys. Condens. Matter 2008, 20, 021001. [Google Scholar] [CrossRef]












| Metals | Melting Point (°C) | Resistivity (×10−8 Ω·m) | Young’s Modulus (GPa) | Firing Atmosphere | Price Ratio* |
|---|---|---|---|---|---|
| Pt | 1768 | 10.5 | 168 | Air | 16390 |
| Ag | 961 | 1.6 | 83 | Air | 133 |
| Cu | 1084 | 1.7 | 110 | Reducing | 1 |
| Ni | 1453 | 6.9 | 200 | Reducing | 2 |
| Pd | 1552 | 10.4 | 121 | Air | 5930 |
| Sintering Aids (% Addition) | Ceramic | Sintering Temperature (°C) | Tc (°C) | εr/ε0 (RT) | d33 (pC/N) | (pm/V) | Reference |
|---|---|---|---|---|---|---|---|
| LiBiO2 (1 wt%) + CuO (0.06 wt%) | PZT | 880 | - | ~1200 | - | - | Wang et al. [22] |
| Li2CO3 (0.2 wt%) + PbO (2 wt%) | PZT with Sr, K, Nb | 900 | 360 | 1200 | - | 779 | Donnelly et al. [20,21,23] |
| BiFeO3 (5 wt%) + Ba(Cu0.5W0.5)O3 (3 wt%) | PZT with 0.5 wt% MnO2 | 935 | 290 | 847 | 201 | - | Kaneko et al. [24] |
| 3ZnO-CuO (3 wt%) | PNN-PZT | 900 | 200 | 3900 | 575 | - | Ahn et al. [25] |
| LiBiO2 (0.7 wt%) | PMN-PZT and 5% Ba | 950 | 290 | ~1000 | 467 | Hayashi et al. [26] |
| Ceramics | Sintering Aid (mol%) | Sintering Temperature (°C) | Tc (°C) | εr/ε0 (RT) | d33 (pC/N) | pm/V | Reference |
|---|---|---|---|---|---|---|---|
| 0.99((Na0.5K0.5)NbO3)-0.01(SrTiO3) | - | 1100 | 344 | 649 | 90 | - | Guo et al. [53] |
| 0.95((Na0.5K0.5)NbO3)-0.05LiTaO3 | - | 1110 | 420 | 570 | 200 | - | Guo et al. [54] |
| (Na0.47K0.47Li0.03)(Nb0.8Ta0.2)O3 | - | ~1100 | ~310 | ~1000 | 190 | 310 | E. Hollenstein et al. [55] |
| 0.95[(Na0.5K0.5)NbO3]-0.05LiTaO3 | Li2O 1 mol% | 1000 | - | ~450 | 250 | 267 | Kim et al. [56,57] |
| (Na0.5K0.5)NbO3 | LiF 5 mol% | 1000 | 456 | 415 | 138 | 210 | Kobayashi et al. [58] |
| 0.96(K0.5Na0.5)NbO3-0.04CaZrO3+0.03Zr | MnCO35 mol% | 1080 | 260 | 1180 | 160 | 360 | Kawada et al. [59,60] |
| 0.02(NaF–0.5Nb2O5)-0.98 [(Na0.5K0.5)(Nb0.8Ta0.2)O3] | NaF | 1200 | 296 | 900 | 140 | 149 | Liu et al. [61] |
| 0.96(K0.48Na0.52)(Nb0.96Sb0.05)O3-0.04Bi0.5(Na0.82K0.18)ZrO3 | - | 1060–1120 | 227 | ~2200 | 490 | - | Wang et al. [62] |
| (K0.5Na0.5)NbO3-LiSbO3 | - | 1160 | 368 | 1380 | 265 | - | Zhang et al. [63] |
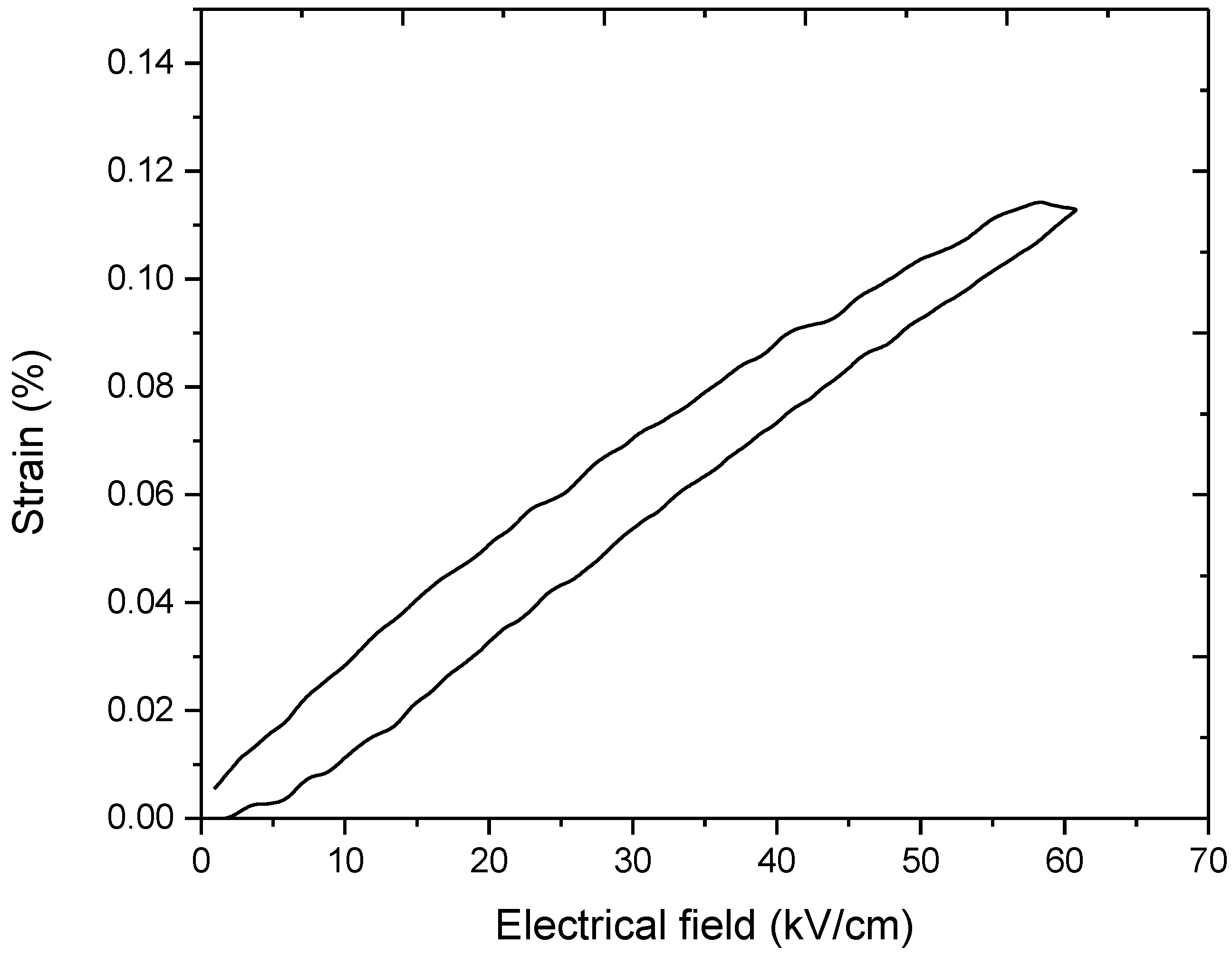
| Tc (°C) | εr (1 kHz) | (pm/V) @ 20 kV/cm | Tanδ (1 kHz) | Ec (kV/cm) | Pr (μC/cm2) |
|---|---|---|---|---|---|
| 330 | 830 | 220 | 0.036 | 10.9 | 22.1 |
| Materials | Incompatible Metal Electrode | Future Trend of Metal Electrodes | Challenges | Progress on Base-Metal Co-Firing |
|---|---|---|---|---|
| PZT | Ni | Cu | Binder, pO2 control | Production in EPCOS/TDK [40] |
| NBT | - | Cu | Binder burnout | Pressed Cu with ceramic pellets [74] |
| NKN | - | Cu | Binder burnout | Cu co-fired prototyped multilayer actuators [81] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Guo, H.; Zhang, S.; Randall, C.A. Base Metal Co-Fired Multilayer Piezoelectrics. Actuators 2016, 5, 8. https://doi.org/10.3390/act5010008
Gao L, Guo H, Zhang S, Randall CA. Base Metal Co-Fired Multilayer Piezoelectrics. Actuators. 2016; 5(1):8. https://doi.org/10.3390/act5010008
Chicago/Turabian StyleGao, Lisheng, Hanzheng Guo, Shujun Zhang, and Clive A. Randall. 2016. "Base Metal Co-Fired Multilayer Piezoelectrics" Actuators 5, no. 1: 8. https://doi.org/10.3390/act5010008






