Environmental Effects on the Polypyrrole Tri-layer Actuator
Abstract
:1. Introduction
2. Experimental Section
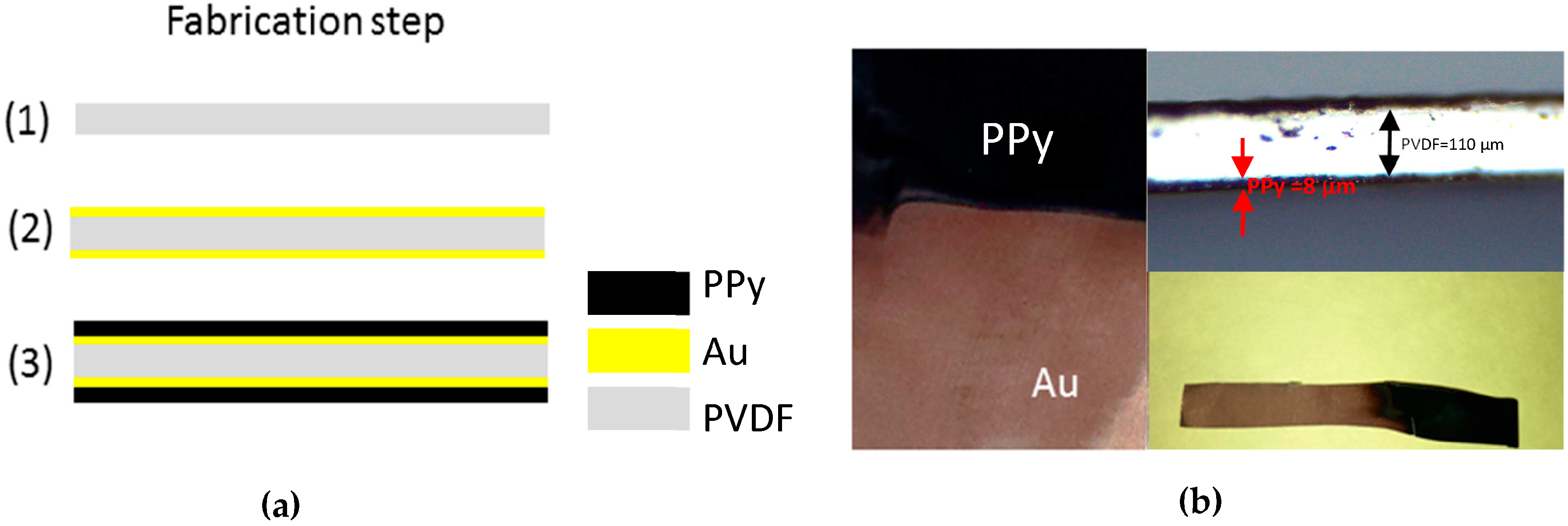
2.1. Materials and Methods
2.2. Electropolymerization of Pyrrole
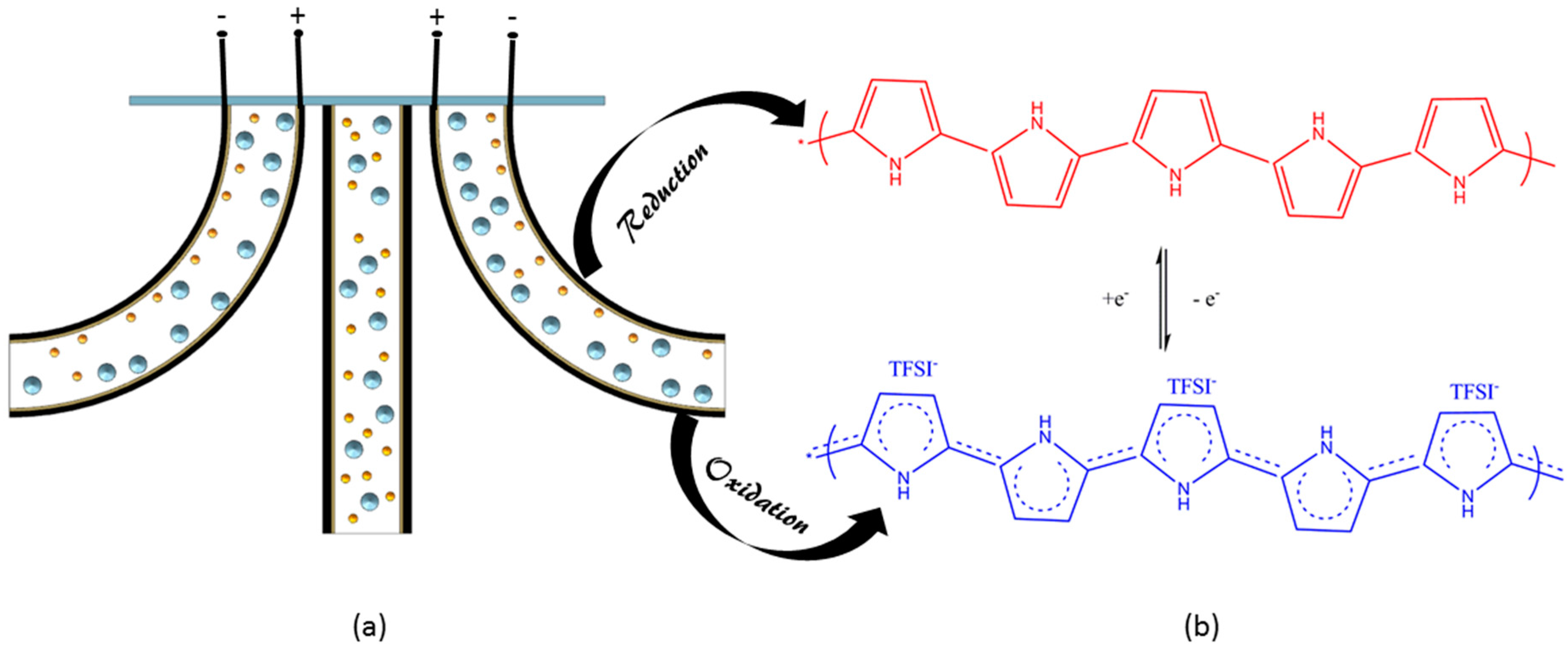
3. Results and Discussion
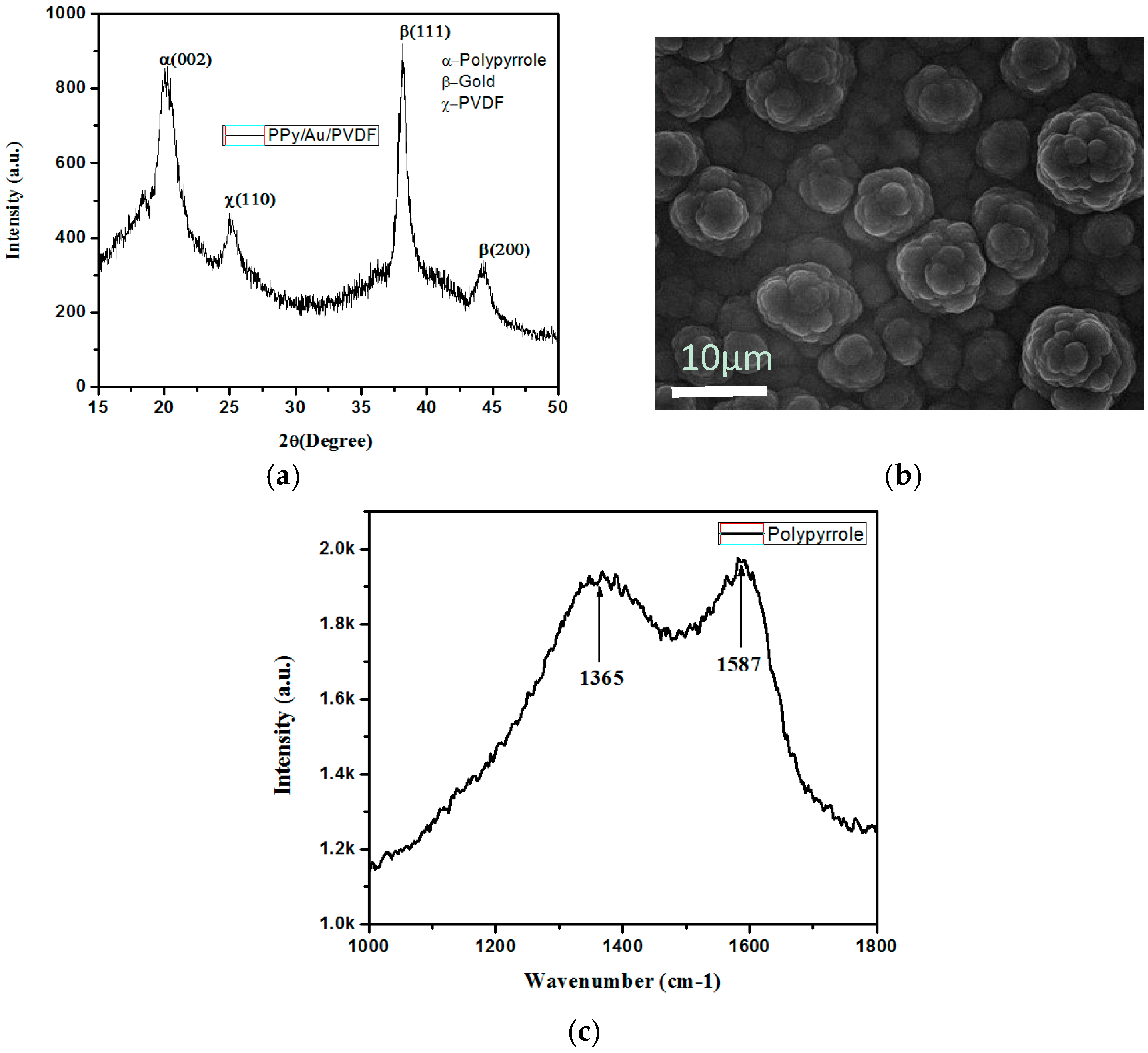
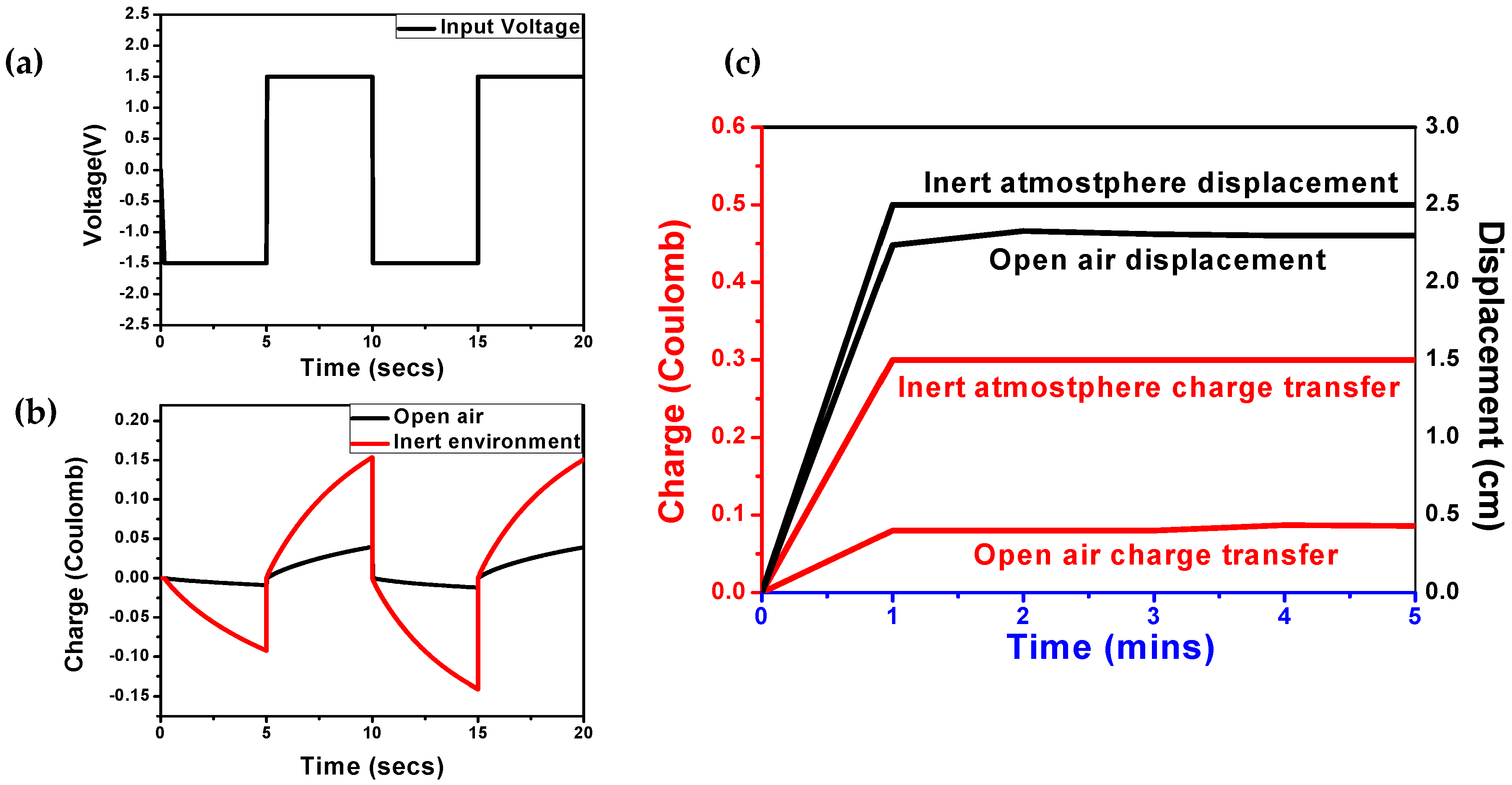
3.1. Characterization of PPy
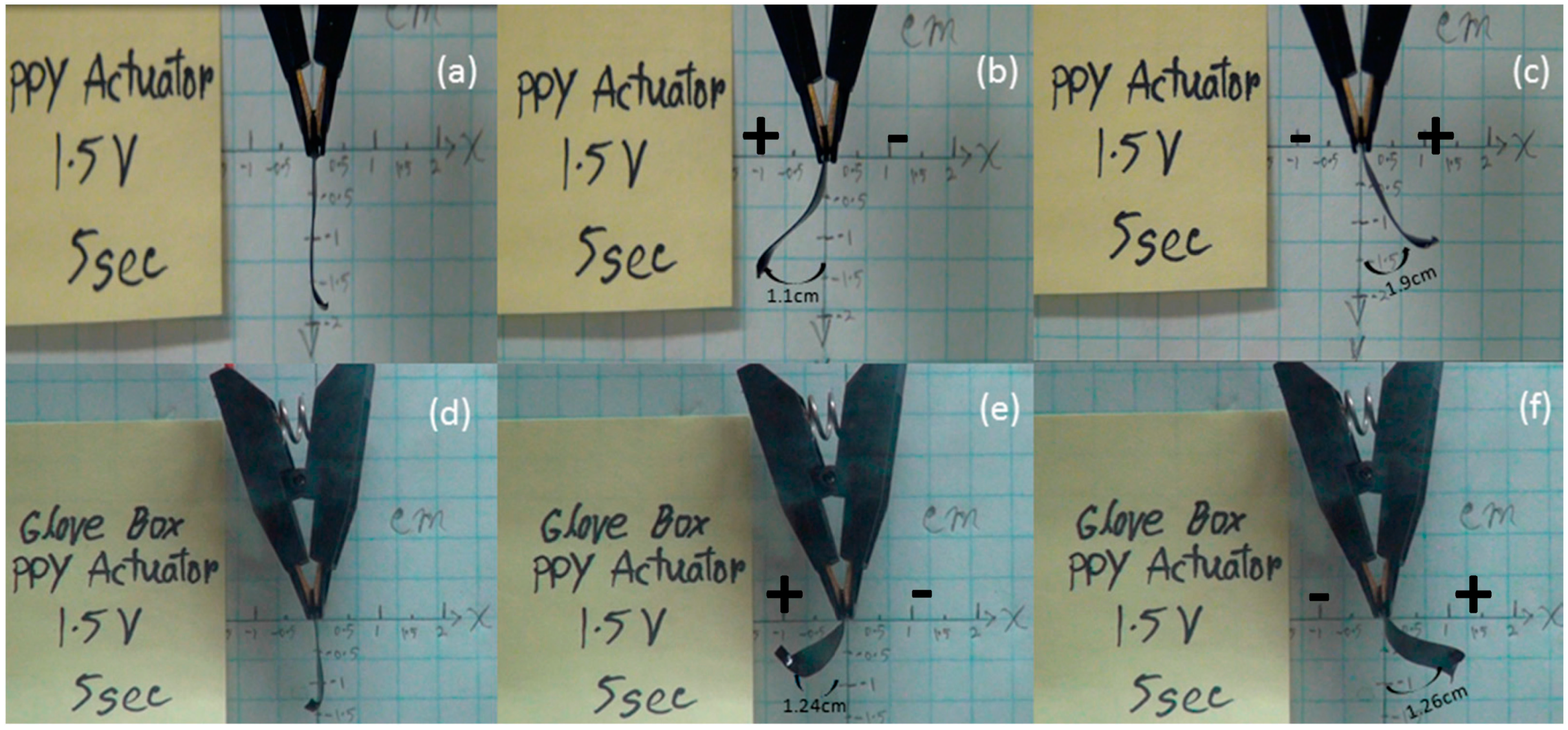
3.2. Mechanical Properties of the Tri-layer Actuator
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spinks, G.M.; Liu, L.; Wallace, G.G.; Zhou, D. Strain response from polypyrrole actuators under load. Adv. Funct. Mater. 2002, 12, 437–440. [Google Scholar] [CrossRef]
- Baughman, R.H.; Cui, C.; Zakhidov, A.A.; Iqbal, Z.; Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Mazzoldi, A.; de Rossi, D.; Rinzler, A.G.; Jaschinski, O.; et al. Carbon nanotube actuators. Science 1999, 284, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R. Conducting polymer artificial muscles. Synth. Metals 1996, 78, 339–353. [Google Scholar] [CrossRef]
- Pei, Q.; Inganäs, O. Electrochemical applications of the bending beam method. 1. Mass transport and volume changes in polypyrrole during redox. J. Phys. Chem. 1992, 96, 10507–10514. [Google Scholar] [CrossRef]
- Lu, W.; Fadeev, A.G.; Qi, A.; Smela, E.; Mattes, B.R.; Ding, J.; Spinks, G.M.; Mazurkiewicz, J.; Zhou, D.; Wallace, G.G.; et al. Use of ionic liquids for π-conjugated polymer electrochemical devices. Science 2002, 297, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.D.; Cush, R.A.; Kanigan, T.S.; Brenan, C.J.; Hunter, I.W. Encapsulated polypyrrole actuators. Synth. Metals 1999, 105, 61–64. [Google Scholar] [CrossRef]
- Liu, A.; Zhao, L.; Bai, H.; Zhao, H.; Xing, X.; Shi, G. Polypyrrole actuator with a bioadhesive surface for accumulating bacteria from physiological media. ACS Appl. Mater. Interfaces 2009, 1, 951–955. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Li, C.; Chen, F.E.; Shi, G.Q. Polypyrrole microtubule actuators for seizing and transferring microparticles. Adv. Funct. Mater. 2007, 17, 2911–2917. [Google Scholar] [CrossRef]
- Jager, E.W.; Smela, E.; Inganäs, O. Microfabricating conjugated polymer actuators. Science 2000, 290, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Jager, E.W.; Masurkar, N.; Nworah, N.F.; Gaihre, B.; Alici, G.; Spinks, G.M. Patterning and electrical interfacing of individually controllable conducting polymer microactuators. Sens. Actuators B Chem. 2013, 183, 283–289. [Google Scholar] [CrossRef]
- Jager, E.; Masurkar, N.; Nworah, N.F.; Gaihre, B.; Alici, G.; Spinks, G.M. Individually controlled conducting polymer tri-layer microactuators. In Proceedings of the 2013 Transducers & Eurosensors XXVII: The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers & Eurosensors XXVII), Barcelona, Spain, 16–20 June 2013. [Google Scholar]
- Alici, G.; Metz, P.; Spinks, G.M. A methodology towards geometry optimization of high performance polypyrrole (PPy) actuators. Smart Mater. Struct. 2006, 15, 243–252. [Google Scholar] [CrossRef]
- Adeloju, S.; Wallace, G. Conducting polymers and the bioanalytical sciences: New tools for biomolecular communications. A review. Analyst 1996, 121, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Snook, G.A.; Chen, G.Z.; Fray, D.J.; Hughes, M.; Shaffer, M. Studies of deposition of and charge storage in polypyrrole–chloride and polypyrrole–carbon nanotube composites with an electrochemical quartz crystal microbalance. J. Electroanal. Chem. 2004, 568, 135–142. [Google Scholar] [CrossRef]
- Kim, J.H.; Lau, K.T.; Shepherd, R.; Wu, Y.; Wallace, G.; Diamond, D. Performance characteristics of a polypyrrole modified polydimethylsiloxane (PDMS) membrane based microfluidic pump. Sens. Actuators A Phys. 2008, 148, 239–244. [Google Scholar] [CrossRef]
- Lopez-Crapez, E.; Livache, T.; Marchand, J.; Grenier, J. K-ras mutation detection by hybridization to a polypyrrole DNA chip. Clin. Chem. 2001, 47, 186–194. [Google Scholar] [PubMed]
- Alici, G.; Devaud, V.; Renaud, P.; Spinks, G. Conducting polymer microactuators operating in air. J. Micromech. Microeng. 2009, 19, 025017. [Google Scholar] [CrossRef]
- Gu, F.; Zhang, L.; Yin, X.; Tong, L. Polymer single-nanowire optical sensors. Nano Lett. 2008, 8, 2757–2761. [Google Scholar] [CrossRef] [PubMed]
- Naficy, S.; Stoboi, N.; Whitten, P.G.; Spinks, G.M.; Wallace, G.G. Evaluation of encapsulating coatings on the performance of polypyrrole actuators. Smart Mater. Struct. 2013, 22, 075005. [Google Scholar] [CrossRef]
- Cot, A.; Chikhaoui, M.T.; Rabenorosoa, K.; Rougeot, P.; Andreff, N. Synthesis, encapsulation, and performance analysis of large deformation tri-layer polypyrrole actuator. In Proceedings of the 2016 IEEE International Conference on Advanced Intelligent Mechatronics (AIM), Banff, AB, Canada, 12–15 July 2016. [Google Scholar]
- Otero, T.F.; Cortes, M.T. A sensing muscle. Sens. Actuators B Chem. 2003, 96, 152–156. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, D.; Spinks, G.; Wallace, G.; Forsyth, S.; Forsyth, M.; MacFarlane, D. Use of ionic liquids as electrolytes in electromechanical actuator systems based on inherently conducting polymers. Chem. Mater. 2003, 15, 2392–2398. [Google Scholar] [CrossRef]
- Bay, L.; Jacobsen, T.; Skaarup, S.; West, K. Mechanism of actuation in conducting polymers: Osmotic expansion. J. Phys. Chem. B 2001, 105, 8492–8497. [Google Scholar] [CrossRef]
- Babu, G.; Masurkar, N.; Al Salem, H.; Arava, L.M.R. Transition metal dichalcogenide atomic layers for lithium polysulfides electrocatalysis. J. Am. Chem. Soc. 2017, 139, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Sansinena, J.; Olazabal, V.; Otero, T.F.; Da Fonseca, C.P.; De Paoli, M.A. A solid state artificial muscle based on polypyrrole and a solid polymeric electrolyte working in air. Chem. Commun. 1997, 22, 2217–2218. [Google Scholar] [CrossRef]
- Wu, Y.; Alici, G.; Madden, J.D.; Spinks, G.M.; Wallace, G.G. Soft mechanical sensors through reverse actuation in polypyrrole. Adv. Funct. Mater. 2007, 17, 3216–3222. [Google Scholar] [CrossRef]
- Gaihre, B.; Alici, G.; Spinks, G.M.; Cairney, J.M. Synthesis and performance evaluation of thin film PPy-PVDF multilayer electroactive polymer actuators. Sens. Actuators A Phys. 2011, 165, 321–328. [Google Scholar] [CrossRef]
- Bay, L.; West, K.; Sommer-Larsen, P.; Skaarup, S.; Benslimane, M. A conducting polymer artificial muscle with 12% linear strain. Adv. Mater. 2003, 15, 310–313. [Google Scholar] [CrossRef]
- Sun, B.; Jones, J.J.; Burford, R.P.; Skyllas-Kazacos, M. Conductivity and anisotropy of electrochemically prepared conducting polypyrrole films. J. Electrochem. Soc. 1989, 136, 698–701. [Google Scholar] [CrossRef]
- Madden, J.D.; Cush, R.A.; Kanigan, T.S.; Hunter, I.W. Fast contracting polypyrrole actuators. Synth. Metals 2000, 113, 185–192. [Google Scholar] [CrossRef]
- Pelrine, R.E.; Kornbluh, R.D.; Joseph, J.P. Electrostriction of polymer dielectrics with compliant electrodes as a means of actuation. Sens. Actuators A Phys. 1998, 64, 77–85. [Google Scholar] [CrossRef]
- Carpi, F.; Kornbluh, R.; Sommer-Larsen, P.; Alici, G. Electroactive polymer actuators as artificial muscles: Are they ready for bioinspired applications? Bioinspiration Biomim. 2011, 6, 045006. [Google Scholar] [CrossRef] [PubMed]
- Alici, G.; Huynh, N.N. Predicting force output of trilayer polymer actuators. Sens. Actuators A Phys. 2006, 132, 616–625. [Google Scholar] [CrossRef]
- Cvetko, B.F.; Brungs, M.P.; Burford, R.P.; Skyllas-Kazacos, M. Structure, strength and electrical performance of conducting polypyrroles. J. Mater. Sci. 1988, 23, 2102–2106. [Google Scholar] [CrossRef]
- Wallace, G.G.; Teasdale, P.R.; Spinks, G.M.; Kane-Maguire, L.A. Conductive Electroactive Polymers: Intelligent Polymer Systems; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Yoon, C.O.; Sung, H.K.; Kim, J.H.; Barsoukov, E.; Kim, J.H.; Lee, H. The effect of low-temperature conditions on the electrochemical polymerization of polypyrrole films with high density, high electrical conductivity and high stability. Synth. Metals 1999, 99, 201–212. [Google Scholar] [CrossRef]
- Deshpande, S.; Kim, J.; Yun, S.-R. Studies on conducting polymer electroactive paper actuators: Effect of humidity and electrode thickness. Smart Mater. Struct. 2005, 14, 876–880. [Google Scholar] [CrossRef]
- Partch, R.; Gangolli, S.G.; Matijević, E.; Cal, W.; Arajs, S. Conducting polymer composites: I. Surface-induced polymerization of pyrrole on iron (III) and cerium (IV) oxide particles. J. Colloid Interface Sci. 1991, 144, 27–35. [Google Scholar] [CrossRef]
- Madakbaş, S.; Çakmakçı, E.; Kahraman, M.; Esmer, K. Preparation, characterisation, and dielectric properties of polypyrrole-clay composites. Chem. Pap. 2013, 67, 1048–1053. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Li, G. In situ ZnO nanowire growth to promote the PVDF piezo phase and the ZnO–PVDF hybrid self-rectified nanogenerator as a touch sensor. Phys. Chem. Chem. Phys. 2014, 16, 5475–5479. [Google Scholar] [CrossRef] [PubMed]
- Manivel, P.; Kanagaraj, S.; Balamurugan, A.; Ponpandian, N.; Mangalaraj, D.; Viswanathan, C. Rheological behavior and electrical properties of polypyrrole/thermally reduced graphene oxide nanocomposite. Colloids Surf. A 2014, 441, 614–622. [Google Scholar] [CrossRef]
- Beck, F.; Barsch, U.; Michaelis, R. Corrosion of conducting polymers in aqueous media. J. Electroanal. Chem. 1993, 351, 169–184. [Google Scholar] [CrossRef]
- Schlenoff, J.B.; Xu, H. Evolution of physical and electrochemical properties of polypyrrole during extended oxidation. J. Electrochem. Soc. 1992, 139, 2397–2401. [Google Scholar] [CrossRef]
- Freire, T.J.; Gonzalez, E.R. Effect of membrane characteristics and humidification conditions on the impedance response of polymer electrolyte fuel cells. J. Electroanal. Chem. 2001, 503, 57–68. [Google Scholar] [CrossRef]
- Otero, T.; Martinez, J.; Arias-Pardilla, J. Biomimetic electrochemistry from conducting polymers. A review: Artificial muscles, smart membranes, smart drug delivery and computer/neuron interfaces. Electrochim. Acta 2012, 84, 112–128. [Google Scholar] [CrossRef]
- Wu, G.; Hu, Y.; Liu, Y.; Zhao, J.; Chen, X.; Whoehling, V.; Plesse, C.; Nguyen, G.T.M.; Vidal, F.; Chen, W. Graphitic carbon nitride nanosheet electrode-based high-performance ionic actuator. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masurkar, N.; Jamil, K.; Arava, L.M.R. Environmental Effects on the Polypyrrole Tri-layer Actuator. Actuators 2017, 6, 17. https://doi.org/10.3390/act6020017
Masurkar N, Jamil K, Arava LMR. Environmental Effects on the Polypyrrole Tri-layer Actuator. Actuators. 2017; 6(2):17. https://doi.org/10.3390/act6020017
Chicago/Turabian StyleMasurkar, Nirul, Kawsar Jamil, and Leela Mohana Reddy Arava. 2017. "Environmental Effects on the Polypyrrole Tri-layer Actuator" Actuators 6, no. 2: 17. https://doi.org/10.3390/act6020017




