Property Investigation of Replaceable PDMS Membrane as an Actuator in Microfluidic Device
Abstract
:1. Introduction
2. Materials and Methods
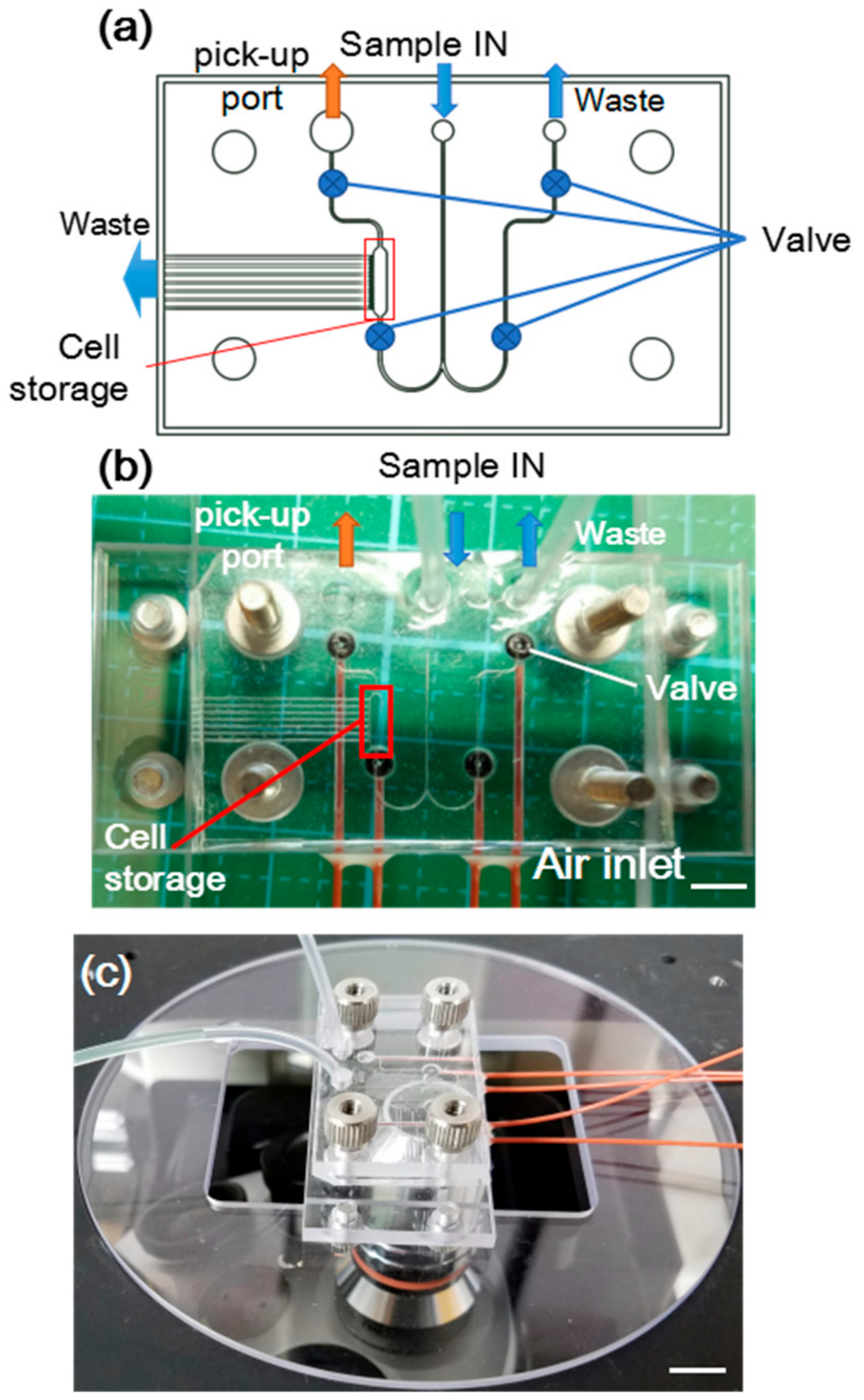
2.1. Design and Fabrication of PDMS Membrane Integrated Microfluidic Device
2.2. Experiment Setting and Method
2.2.1. Simulation Conditions
2.2.2. Applied Pressure versus Deflection of Valve Structure
2.2.3. Applied Pressure and Device Performance
2.2.4. Demonstration of Cell Capturing by Using the PDMS-Membrane Actuator
3. Results and Discussion
3.1. Simulation Results and Calculations
3.2. Prototype Devices
3.3. Applied Pressure and Deflection of Valve Structure
3.4. Applied Pressure and Device Performance
3.5. Demonstration of Capturing Cells Using the PDMS-Membrane Actuator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Becker, H.; Locascio, L.E. Polymer microfluidic devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Madadi, H.; Mohammadi, M.; Casals-Terré, J.; López, R.C. A novel fabrication technique to minimize poly(dimethylsiloxane)-microchannels deformation under high-pressure operation. Electrophoresis 2013, 34, 3126–3132. [Google Scholar] [CrossRef] [PubMed]
- Khanafer, K.; Duprey, A.; Schlicht, M.; Berguer, R. Effects of strain rate, mixing ratio, and stress-strain definition on the mechanical behavior of the polydimethylsiloxane (PDMS) material as related to its biological applications. Biomed. Microdevices 2009, 11, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Ren, D.H.; Xia, Y.; Wang, J.; You, Z. Micropatterning of single cell arrays using the PEG-Silane and Biotin-(Strept)Avidin System with photolithography and chemical vapor deposition. Sens. Actuators B Chem. 2013, 188, 340–346. [Google Scholar] [CrossRef]
- Mcdonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T. Review General Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Harrison, D.J.; Fluri, K.; Seiler, K.; Fan, Z.; Effenhauser, C.S.; Manz, A. Micromachining a Miniaturized Capillary Electrophoresis-Based Chemical Analysis System on a Chip. Science 2016, 261, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Williams, S.E.; Zhou, A. Microfabricated three-electrode on-chip PDMS device with a vibration motor for stripping voltammetric detection of heavy metal ions. Talanta 2015, 132, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kunstmann-Olsen, C.; Hanczyc, M.M.; Hoyland, J.; Rasmussen, S.; Rubahn, H.G. Uniform droplet splitting and detection using Lab-on-Chip flow cytometry on a microfluidic PDMS device. Sens. Actuators B Chem. 2016, 229, 7–13. [Google Scholar] [CrossRef]
- Tian, Z.; Ren, D.; You, Z. Self-oscillation-based frequency tracking for the drive and detection of resonance magnetometers. Sensors 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Stroock, A.D.; Whitesides, G.M. Components for integrated poly(dimethylsiloxane) microfluidic systems. Electrophoresis 2002, 23, 3461–3473. [Google Scholar]
- Yoon, Y.; Lee, D.W.; Lee, J.B. Fabrication of optically transparent PDMS artificial lotus leaf film using underexposed and underbaked photoresist mold. J. Microelectromech. Syst. 2013, 22, 1073–1080. [Google Scholar] [CrossRef]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas sorption, diffusion, and permeation in poly(dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef] [Green Version]
- Delamarche, E.; Schmid, H.; Michel, B.; Biebuyck, H. Patterned Delivery of Immunoglobulins to Surfaces Using Microfluidic Networks Patterned Delivery of Immunoglobulins to Surfaces Using Microfluidic Networks. Science 2013, 779, 779–782. [Google Scholar]
- Whitesides, G.M.; Ostuni, E.; Jiang, X.; Ingber, D.E. Soft L Ithography in Biology and Biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.S.; Takayama, S.; Ostuni, E.; Ingber, D.E.; Whitesides, G.M. Patterning proteins and cells using soft lithography. Biomaterials 1999, 20, 2363–2376. [Google Scholar] [CrossRef]
- Hua, Z.; Xia, Y.; Srivannavit, O.; Rouillard, J.-M.; Zhou, X.; Gao, X.; Gulari, E. A versatile microreactor platform featuring a chemical-resistant microvalve array for addressable multiplex syntheses and assays. J. Micromech. Microeng. 2006, 16, 1433–1443. [Google Scholar] [CrossRef]
- Li, M.; Li, W.H. 1999 Duffy Whitesides Rapid prototyping of microfluidic switches in poly(dimethyl siloxane) and their actuation by electro-osmotic flow. J. Micromech. Microeng. 1999, 9, 211–217. [Google Scholar]
- Ismagilov, R.F.; Rosmarin, D.; Kenis, P.J.A.; Chiu, D.T.; Zhang, W.; Stone, H.A.; Whitesides, G.M. Pressure-driven laminar flow in tangential microchannels: An elastomeric microfluidic switch. Anal. Chem. 2001, 73, 4682–4687. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.A.; Unger, M.A.; Chou, H.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic Microfabricated Valves and Pumps by Multilayer Soft Lithography. Science 2013, 113, 113–117. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, S.; Wang, C.; Hu, J.; Li, C.; Zhuang, Z.; Zhou, Y.; Mathies, R.A.; Yang, C.J. PMMA/PDMS valves and pumps for disposable microfluidics. Lab Chip 2009, 9, 3088–3094. [Google Scholar] [CrossRef] [PubMed]
- Grover, W.H.; Ivester, R.H.C.; Jensen, E.C.; Mathies, R.A. Development and multiplexed control of latching pneumatic valves using microfluidic logical structures. Lab Chip 2006, 6, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Grover, W.H.; Skelley, A.M.; Liu, C.N.; Lagally, E.T.; Mathies, R.A. Monolithic membrane valves and diaphragm pumps for practical large-scale integration into glass microfluidic devices. Sens. Actuators B Chem. 2003, 89, 315–323. [Google Scholar] [CrossRef]
- Lagelly, E.T.; Scherer, J.R.; Blazej, R.G.; Toriello, N.M.; Diep, B.A.; Ramchandani, M.; Sensabaugh, G.F.; Riley, L.W.; Mathies, R.A. Integrated portable genetic analysis microsystem for pathogen/infectious disease detection. Anal. Chem. 2004, 76, 3162–3170. [Google Scholar] [CrossRef] [PubMed]
- Karlinsey, J.M.; Monahan, J.; Marchiarullo, D.J.; Ferrance, J.P.; Landers, J.P. Pressure injection on a valved microdevice for electrophoretic analysis of submicroliter samples. Anal. Chem. 2005, 77, 3637–3643. [Google Scholar] [CrossRef] [PubMed]
- Goldowsky, J.; Knapp, H.F. Gas penetration through pneumatically driven PDMS micro valves. RSC Adv. 2013, 3, 17968–17976. [Google Scholar] [CrossRef]
- Hosokawa, K.; Maeda, R. A pneumatically-actuated three-way microvalve fabricated with polydimethylsiloxane using the membrane transfer technique. J. Micromech. Microeng. 2000, 10, 415–420. [Google Scholar] [CrossRef]
- Amin, A.M.; Wereley, S.T.; Thottethodi, M.; Vijaykumar, T.N.; Jacobson, S.C. Polydimethylsiloxane (pdms) peristaltic pump characterization for programmable lab-on-a-chip applications. In Proceedings of the Twelfth International Conference on Miniaturized Systems for Chemistry and Life Sciences, San Diego, CA, USA, 12–16 October 2008; pp. 1681–1683. [Google Scholar]
- Cheng, Z.; Wu, X.; Cheng, J.; Liu, P. Microfluidic fluorescence-activated cell sorting (μFACS) chip with integrated piezoelectric actuators for low-cost mammalian cell enrichment. Microfluid. Nanofluid. 2017, 21, 1–11. [Google Scholar] [CrossRef]
- Wang, C.H.; Lee, G. Bin Pneumatically driven peristaltic micropumps utilizing serpentine-shape channels. J. Micromech. Microeng. 2006, 16, 341–348. [Google Scholar] [CrossRef]
- Chiou, C.H.; Yeh, T.Y.; Lin, J.L. Deformation analysis of a pneumatically-activated polydimethylsiloxane (PDMS) membrane and potential micro-pump applications. Micromachines 2015, 6, 216–229. [Google Scholar] [CrossRef]
- Tseng, H.Y.; Wang, C.H.; Lin, W.Y.; Lee, G. Bin Membrane-activated microfluidic rotary devices for pumping and mixing. Biomed. Microdevices 2007, 9, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Eddings, M.A.; Gale, B.K. A PDMS-based gas permeation pump for on-chip fluid handling in microfluidic devices. J. Micromech. Microeng. 2006, 16, 2396–2402. [Google Scholar] [CrossRef]
- Li, G.; Luo, Y.; Chen, Q.; Liao, L.; Zhao, J. A “place n play” modular pump for portable microfluidic applications. Biomicrofluidics 2012, 6, 14118–1411816. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Kang, J.H.; Park, S.-J.; Yoon, E.-S.; Park, J.-K. Compressive Cell Stimulation using PDMS Membrane Deflection in a Microfluidic Device. In Proceedings of the TRANSDUCERS 2007—2007 International Solid-State Sensors, Actuators and Microsystems Conference, Lyon, France, 10–14 June 2007; pp. 1873–1876. [Google Scholar]
- Murphy, C.T.; Hu, P.J. Microfluidics as a tool for C. elegans research. WormBook 2013, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Irimia, D.; Toner, M. Cell handling using microstructured membranes. Lab Chip 2006, 6, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Sakai, Y.; Fujii, T. Cell culture in 3-dimensional microfluidic structure of PDMS (polydimenthylsiloxane). Biomed. Microdevices 2003, 5, 109–114. [Google Scholar] [CrossRef]
- Ho, K.K.Y.; Lee, L.M.; Liu, A.P. Mechanically activated artificial cell by using microfluidics. Sci. Rep. 2016, 6, 32912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.C.; Tsai, Y.C.; Shih, W.P. Flexible PDMS micro-lens array with programmable focus gradient fabricated by dielectrophoresis force. Microelectron. Eng. 2011, 88, 2748–2750. [Google Scholar] [CrossRef]
- Schneider, F.; Draheim, J.; Müller, C.; Wallrabe, U. Optimization of an adaptive PDMS-membrane lens with an integrated actuator. Sens. Actuators A Phys. 2009, 154, 316–321. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, G.; Leung, H.M.; Chau, F.S. Tunable liquid-filled lens integrated with aspherical surface for spherical aberration compensation. Opt. Express 2010, 18, 9945–9954. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Han, D.S.; Won, Y.H. Fluidic lens of PDMS membrane driven by voice-coil and magnet. IEEE Photonics Technol. Lett. 2012, 24, 1683–1685. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, A.; Koh, D.; Schneider, P.; Oh, K.W. A robust, portable and backflow-free micromixing device based on both capillary- and vacuum-driven flows. Lab Chip 2018, 2, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.H.; Zhu, W.M.; Zhang, W.; Wu, P.C.; Shen, Z.X.; Yang, Z.C.; Jin, Y.F.; Hao, Y.L.; Bourouina, T.; Leprince-Wang, Y.; et al. Tunable metamaterial lens array via metadroplets. In Proceedings of the 2015 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; pp. 960–963. [Google Scholar]
- Gervais, T.; El-Ali, J.; Günther, A.; Jensen, K.F. Flow-induced deformation of shallow microfluidic channels. Lab Chip 2006, 6, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Hardy, B.S.; Uechi, K.; Zhen, J.; Pirouz Kavehpour, H. The deformation of flexible PDMS microchannels under a pressure driven flow. Lab Chip 2009, 9, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.K.; Overfelt, R.A.; Roh, C. Deformation properties between fluid and periodic circular obstacles in polydimethylsiloxane microchannels: Experimental and numerical investigations under various conditions. Biomicrofluidics 2013, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lötters, J.C.; Olthuis, W.; Veltink, P.H.; Bergveld, P. The Mechanical Properties of the Rubber Elastic Polymer Polydimethilsiloxane for Sensor Applications. J. Micromech. Microeng. 1997, 7, 145–147. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J.; Sun, Y.; Bock, C.; Chen, Q. Thickness-dependent mechanical properties of polydimethylsiloxane membranes. J. Micromech. Microeng. 2009, 19, 035028. [Google Scholar] [CrossRef]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Lamberti, A.; Marasso, S.L.; Cocuzza, M. PDMS membranes with tunable gas permeability for microfluidic applications. RSC Adv. 2014, 4, 61415–61419. [Google Scholar] [CrossRef]
- Firpo, G.; Angeli, E.; Repetto, L.; Valbusa, U. Permeability thickness dependence of polydimethylsiloxane (PDMS) membranes. J. Membr. Sci. 2015, 481, 1–8. [Google Scholar] [CrossRef]
- Schmid, G.H.; Thibault, P. Studies on the s-state distribution in euglena gracilis. Z. Naturforsch. Sect. C J. Biosci. 1983, 38, 60–66. [Google Scholar]
- Bound, K.E.; Tollin, G. Phototactic Response of Euglena gracilis to Polarized Light. Nature 1967, 216, 1042–1044. [Google Scholar] [CrossRef]
- Zhou, Y.; Basu, S.; Wohlfahrt, K.J.; Lee, S.F.; Klenerman, D.; Laue, E.D.; Seshia, A.A. A microfluidic platform for trapping, releasing and super-resolution imaging of single cells. Sens. Actuators B Chem. 2016, 232, 680–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadav, G.L.; Aswal, V.K.; Bhatt, H.; Chaudhari, J.C.; Singh, P.S. Influence of film thickness on the structure and properties of PDMS membrane. J. Membr. Sci. 2012, 415–416, 624–634. [Google Scholar] [CrossRef]





| Rotational Speed (rpm) | 30 | 60 | 120 | 300 | 600 | 1200 |
| Average Thickness (μm) | 71.9 | 59.1 | 34.1 | 29.0 | 18.0 | 12.7 |
| Standard Deviation (μm) | 7.8 | 4 | 9.1 | 11.8 | 0.3 | 0.9 |
| Rotational Speed (rpm) | 30 | 60 | 120 | 300 | 600 | 1200 |
| Average Thickness (μm) | 77.8 | 57.3 | 38.6 | 32.2 | 19.3 | 11.1 |
| Standard Deviation (μm) | 5.3 | 9.1 | 4.0 | 5.6 | 2.1 | 1.5 |
| Thickness of the Membrane (μm) | Production Methods of Membrane | Actuating Pressure of Fluid (KPa) | Minimal Pressure to Close Valve (KPa) |
|---|---|---|---|
| 13 | Sylgard 184 | 10 | 40 |
| 29 | 50 | ||
| 34 | 50 | ||
| 59 | 70 | ||
| 100 | NSG-100 | More than 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Yalikun, Y.; Ota, N.; Tanaka, Y. Property Investigation of Replaceable PDMS Membrane as an Actuator in Microfluidic Device. Actuators 2018, 7, 68. https://doi.org/10.3390/act7040068
Yuan Y, Yalikun Y, Ota N, Tanaka Y. Property Investigation of Replaceable PDMS Membrane as an Actuator in Microfluidic Device. Actuators. 2018; 7(4):68. https://doi.org/10.3390/act7040068
Chicago/Turabian StyleYuan, Yapeng, Yaxiaer Yalikun, Nobutoshi Ota, and Yo Tanaka. 2018. "Property Investigation of Replaceable PDMS Membrane as an Actuator in Microfluidic Device" Actuators 7, no. 4: 68. https://doi.org/10.3390/act7040068





