Potential Probiotic Properties of Exopolysaccharide-Producing Lacticaseibacillus paracasei EPS DA-BACS and Prebiotic Activity of Its Exopolysaccharide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Condition
2.2. Isolation of EPS-Producing LAB
2.2.1. Isolation of LAB Strains
2.2.2. Isolation of Ropy EPS-Producing LAB Strains
2.3. Identification of Lactic Acid Bacteria
2.3.1. Identification of L. paracasei EPS DA-BACS
2.3.2. Biochemical Characteristics of L. paracasei EPS DA-BACS
2.4. Production of EPS from LAB and Removal of EPS
2.5. Visualization of EPS
2.5.1. Crystal Violet Staining
2.5.2. Measuring the Ropiness of EPS
2.5.3. Scanning Electron Microscopy of L. paracasei EPS DA-BACS
2.6. Production and Purification of EPS
2.7. Analysis of EPS Structure
2.7.1. Analysis of Monosaccharide Composition
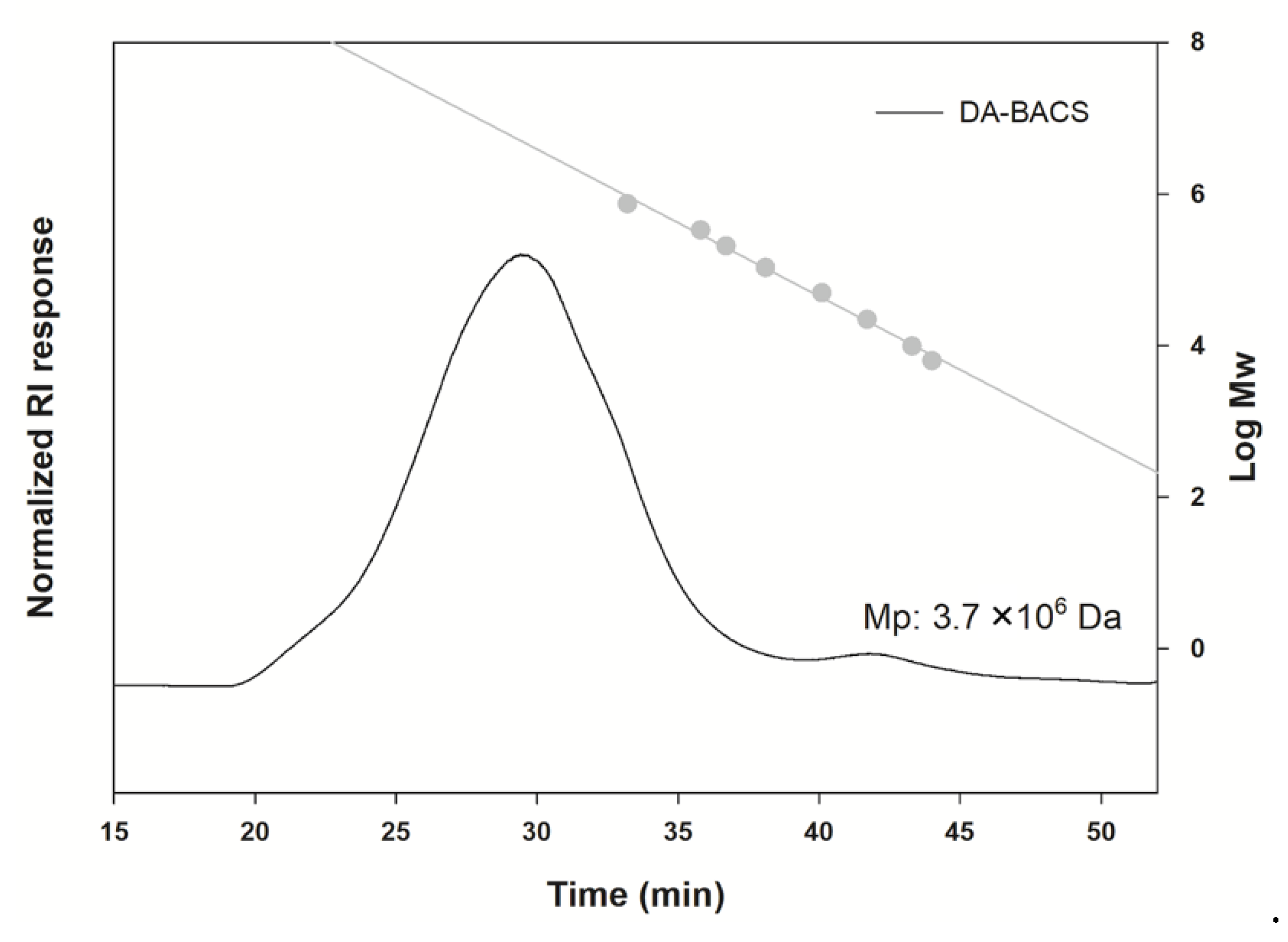
2.7.2. Analysis of Molecular Size
2.7.3. Methylation Analysis for Linkage Pattern
2.8. Gastrointestinal Tract Tolerance
2.9. Antibacterial and Antifungal Activity of LAB
2.10. Growth Inhibitory Activity of EPS and Culture Broth of L. paracasei EPS DA-BACS against Clostridium Difficile
2.11. Gut Adhesion Ability Assay
2.12. Cell Viability Assay
2.13. Anti-Inflammatory Activity Assay
2.14. Prebiotic Activity of Purified-EPS
2.15. Whole Genome Sequencing of L. paracasei EPS DA-BACS
2.16. Antibiotics Susceptibility Test
2.17. Statical Analysis
3. Results and Discussion
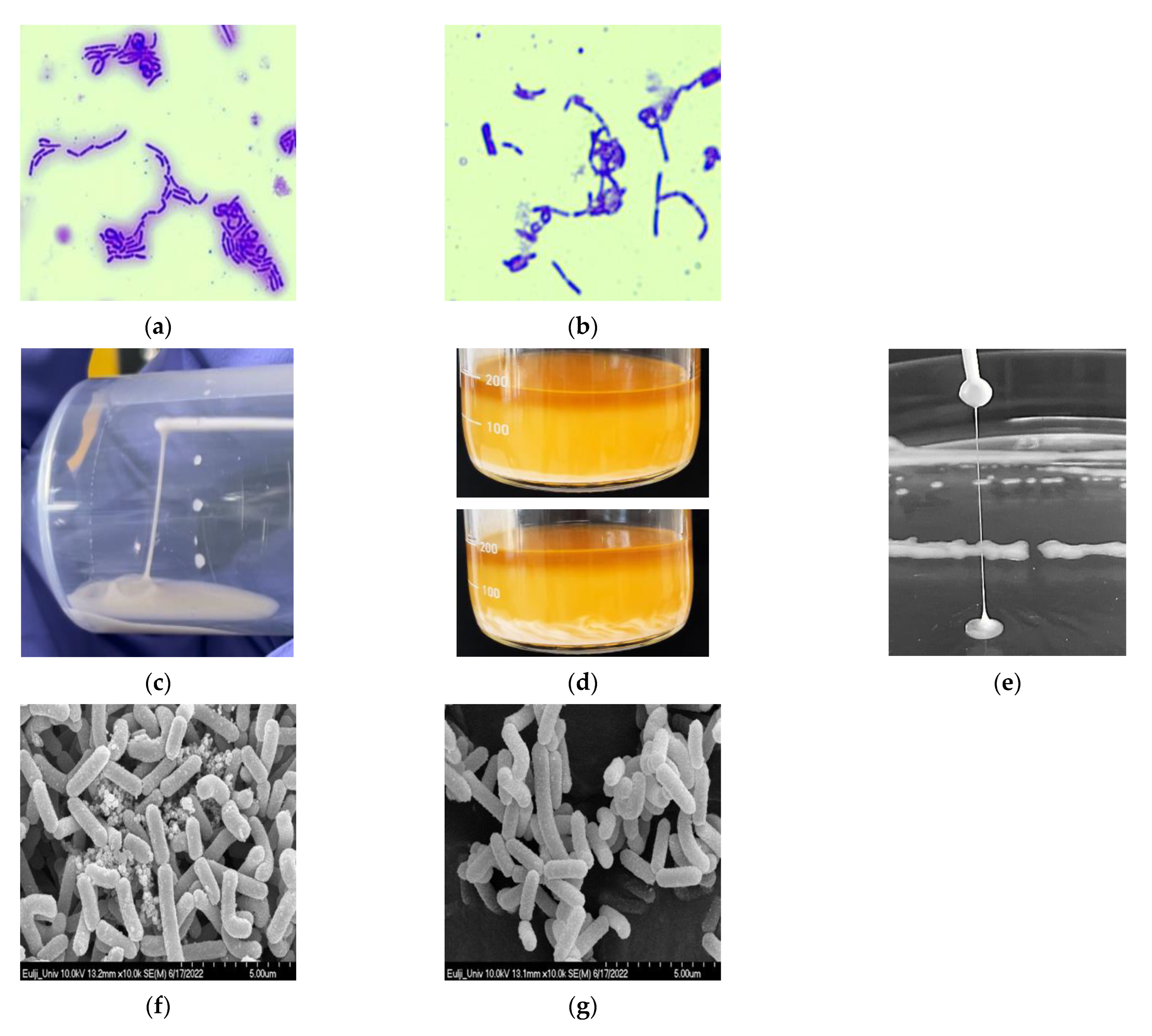
3.1. Isolation and Identification of L. paracasei EPS DA-BACS
3.2. Visualization of EPS
3.3. Proposed Structures of EPS from the L. paracasei EPS DA-BACS
3.4. Tolerance to Gastrointestinal Environment
3.5. Gut Adhesion Ability Assay
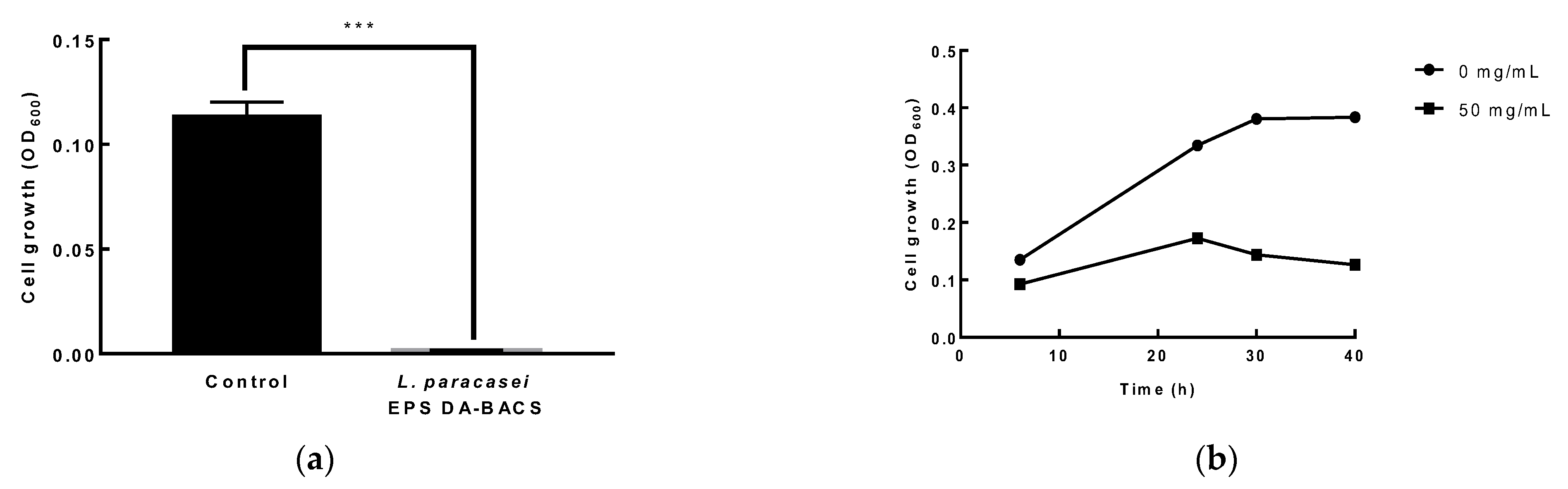
3.6. Antibacterial and Antifungal Activity of LAB
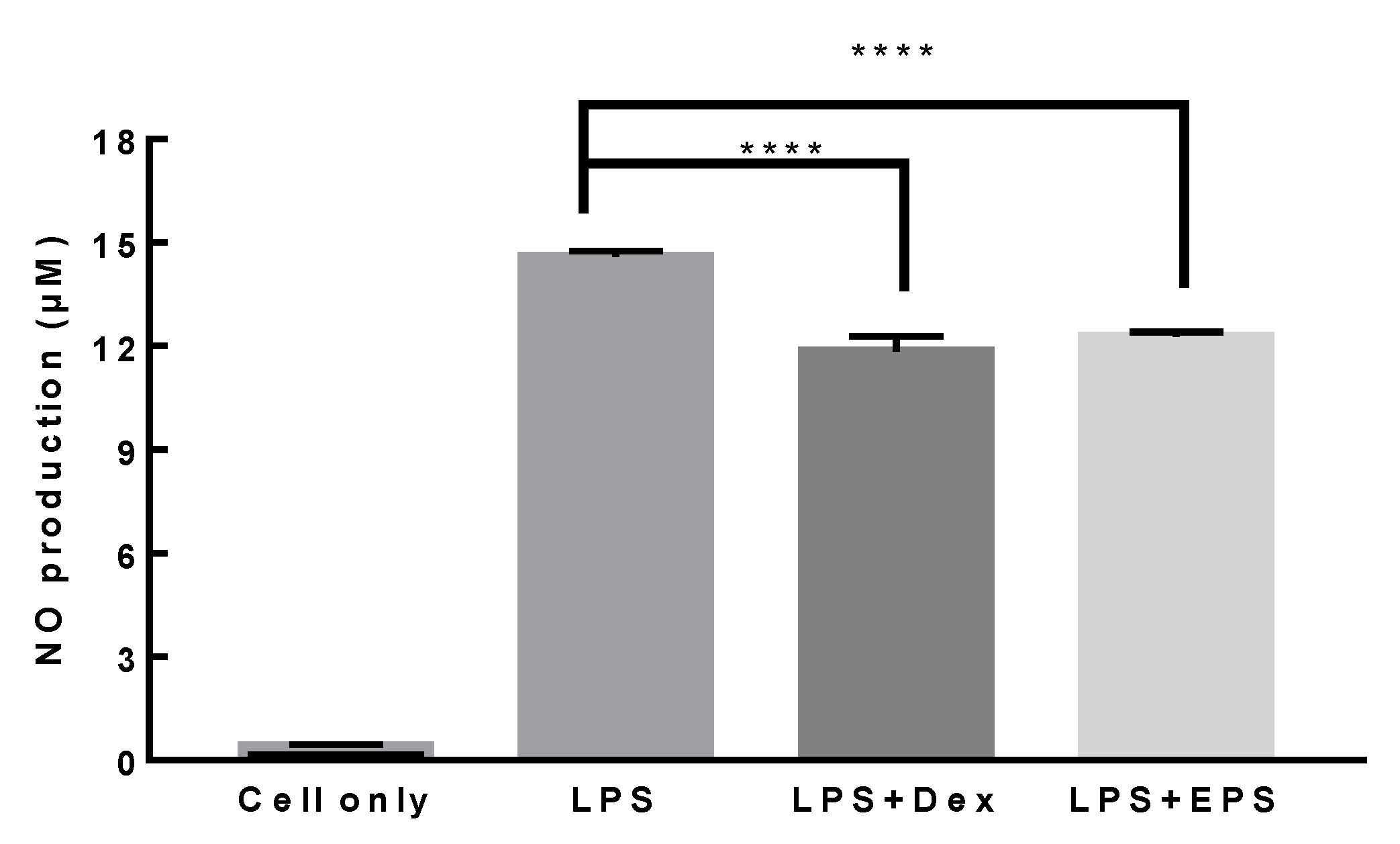
3.7. Anti-Inflammatory Activity of L. paracasei EPS DA-BACS
3.8. Prebiotic Activity
3.9. Analysis of the Whole Genome Sequencing of L. paracasei EPS DA-BACS
3.10. Antibiotics Susceptibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food Agriculture Organization/World Health Organization Working Group. Guidelines for the Evaluation of Probiotics in Food; Report of a Joint FAO/WHO; FAO/WHO: London, ON, Canada, 2002.
- Bengoa, A.A.; Dardis, C.; Garrote, G.L.; Abraham, A.G. Health-promoting properties of Lacticaseibacillus paracasei: A focus on kefir isolates and exopolysaccharide-producing strains. Foods 2021, 10, 2239. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei group: History and health related applications. Front. Microbiol. 2018, 9, 2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R. The use of Lactobacillus casei and Lactobacillus paracasei in clinical trials for the improvement of human health. In The Microbiota in Gastrointestinal Pathophysiology; Floch, M.H., Ringel, Y., Walker, W.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–108. [Google Scholar]
- Oleksy, M.; Klewicka, E. Exopolysaccharides produced by Lactobacillus sp.: Biosynthesis and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 450–462. [Google Scholar] [PubMed]
- Kang, H.-J.; Baick, S.-C.; Yu, J.-H. Studies on the properties of the stirred yogurt manufactured by exopolysaccharide producing lactic acid bacteria. Food Sci. Anim. Resour. 2005, 25, 84–91. [Google Scholar]
- Ryan, P.; Ross, R.; Fitzgerald, G.; Caplice, N.; Stanton, C. Sugar-coated: Exopolysaccharide producing lactic acid bacteria for food and human health applications. Food Funct. 2015, 6, 679–693. [Google Scholar] [CrossRef]
- Oerlemans, M.M.; Akkerman, R.; Ferrari, M.; Walvoort, M.T.; de Vos, P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct. Foods 2021, 76, 104289. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Chou, L.-S.; Weimer, B. Isolation and characterization of acid-and bile-tolerant isolates from strains of Lactobacillus acidophilus. J. Dairy Sci. 1999, 82, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Duboc, P.; Mollet, B. Applications of exopolysaccharides in the dairy industry. Int. Dairy J. 2001, 11, 759–768. [Google Scholar] [CrossRef]
- Kim, D.-J.; Lee, S.-Y. Isolation of the exopolysaccharide producing Enterobacter sp. and physicochemical properties of the polysaccharide produced by this strain. KSBB J. Bioeng. 2001, 16, 370–375. [Google Scholar]
- Cerning, J.; Bouillanne, C.; Desmazeaud, M.; Landon, M. Exocellular polysaccharide production by Streptococcus thermophilus. Biotechnol. Lett. 1988, 10, 255–260. [Google Scholar] [CrossRef]
- Bhat, B.; Bajaj, B.K. Hypocholesterolemic potential and bioactivity spectrum of an exopolysaccharide from a probiotic isolate Lactobacillus paracasei M7. Bioact. Carbohydr. Diet. Fibre 2019, 19, 100191. [Google Scholar] [CrossRef]
- Liu, C.F.; Tseng, K.C.; Chiang, S.S.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Oleksy, M.; Klewicka, E. Capsular polysaccharides of Lactobacillus spp.: Theoretical and practical aspects of simple visualization methods. Probiotics Antimicrob. Proteins 2017, 9, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Jeong, H.; Kim, S.; Shin, J.; Song, Y.; Lee, B.-H.; Park, H.-G.; Lee, T.-H.; Jiang, H.-H.; Han, Y.-S.; et al. Structural analysis and prebiotic activity of exopolysaccharide produced by probiotic strain Bifidobacterium bifidum EPS DA-LAIM. Food Sci. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Alves, E.F.; Bose, S.K.; Francis, R.C.; Colodette, J.L.; Iakovlev, M.; Van Heiningen, A. Carbohydrate composition of eucalyptus, bagasse and bamboo by a combination of methods. Carbohydr. Polym. 2010, 82, 1097–1101. [Google Scholar] [CrossRef]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 2012, 7, 1590–1607. [Google Scholar] [CrossRef]
- Inglin, R.C.; Stevens, M.J.; Meile, L.; Lacroix, C.; Meile, L. High-throughput screening assays for antibacterial and antifungal activities of Lactobacillus species. J. Microbiol. 2015, 114, 26–29. [Google Scholar] [CrossRef]
- Kwon, A.; Park, Y.-S. Immunostimulatory activity of synbiotics using Lactococcus lactis SG-030 and glucooligosaccharides from Weissella cibaria YRK005. Microorganisms 2021, 9, 2437. [Google Scholar] [CrossRef]
- Kim, M.; Jang, J.-K.; Park, Y.-S. Production optimization, structural analysis, and prebiotic- and anti-inflammatory effects of gluco-oligosaccharides produced by Leuconostoc lactis SBC001. Microorganisms 2021, 9, 200. [Google Scholar] [CrossRef]
- Hussein, M.-D.M.; Ghaly, M.F.; Osman, M.Y.; Al Shimaa, G.S.; Helal, M.M. Production and prebiotic activity of exopolysaccharides derived from some probiotics. Egypt. Pharm. J. 2015, 14, 1. [Google Scholar]
- EPo, A. Feed PoSuiA. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Chew, S.C.; Yang, L. Biofilms. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 407–415. [Google Scholar]
- Patel, A.K.; Michaud, P.; Singhania, R.R.; Soccol, C.R.; Pandey, A. Polysaccharides from probiotics: New developments as food additives. Food Technol. Biotechnol. 2010, 48, 451–463. [Google Scholar]
- Werning, M.L.; Hernández-Alcántara, A.M.; Ruiz, M.J.; Soto, L.P.; Dueñas, M.T.; López, P.; Frizzo, L.S. Biological functions of exopolysaccharides from lactic acid acteria and their potential benefits for humans and farmed animals. Foods 2022, 11, 1284. [Google Scholar] [CrossRef]
- Broadbent, J.R.; McMahon, D.J.; Welker, D.L.; Oberg, C.J.; Moineau, S. Biochemistry, genetics, and applications of exopolysaccharide production in Streptococcus thermophilus: A review. J. Dairy Sci. 2003, 86, 407–423. [Google Scholar] [CrossRef]
- Nakajima, H.; Suzuki, Y.; Hirota, T. Cholesterol lowering activity of ropy fermented milk. J. Food Sci. 1992, 57, 1327–1329. [Google Scholar] [CrossRef]
- Coulon, J.; Houlès, A.; Dimopoulou, M.; Maupeu, J.; Dols-Lafargue, M. Lysozyme resistance of the ropy strain Pediococcus parvulus IOEB 8801 is correlated with beta-glucan accumulation around the cell. Int. J. Food Microbiol. 2012, 159, 25–29. [Google Scholar] [CrossRef]
- Noda, M.; Sultana, N.; Hayashi, I.; Fukamachi, M.; Sugiyama, M. Exopolysaccharide produced by Lactobacillus paracasei IJH-SONE68 prevents and improves the picryl chloride-induced contact dermatitis. Molecules 2019, 24, 2970. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, C.; Shetty, P.K.H. Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int. J. Biol. Macromol. 2016, 90, 100–106. [Google Scholar] [CrossRef]
- Cirrincione, S.; Breuer, Y.; Mangiapane, E.; Mazzoli, R.; Pessione, E. ‘Ropy’ phenotype, exopolysaccharides and metabolism: Study on food isolated potential probiotics LAB. Microbiol. Res. 2018, 214, 137–145. [Google Scholar] [CrossRef]
- Ismail, B.; Nampoothiri, K.M. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, V.B.; Neto, J.H.P.L.; Leite, K.S.; de Andrade Vieira, É.; Iacomini, M.; Silva, C.M.; dos Santos, K.M.O.; Cardarelli, H.R. Partial characterization and antioxidant activity of exopolysaccharides produced by Lactobacillus plantarum CNPC003. LWT 2020, 127, 109349. [Google Scholar] [CrossRef]
- Sasikumar, K.; Vaikkath, D.K.; Devendra, L.; Nampoothiri, K.M. An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Bioresour. Technol. 2017, 241, 1152–1156. [Google Scholar] [CrossRef]
- Ruszova, E.; Pavek, S.; Hajkova, V.; Jandova, S.; Velebny, V.; Papezikova, I.; Kubala, L. Photoprotective effects of glucomannan isolated from Candida utilis. Carbohydr. Res. 2008, 343, 501–511. [Google Scholar] [CrossRef]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, X.; Wen, J.; Wu, X.; Ma, W.; Cui, S.W.; Xie, M.; Nie, S. Comprehensive characterization of glucomannans from different sources to trigger moderate macrophages immune activation. Carbohydr. Polym. 2022, 296, 119933. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Muschin, T.; Kanamoto, T.; Nakashima, H.; Yoshida, T. Sulfation and biological activities of konjac glucomannan. Carbohydr. Polym. 2013, 94, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.; Kahyaoglu, T. Gelation and structural characteristics of deacetylated salep glucomannan. Food Hydrocoll. 2017, 69, 255–263. [Google Scholar] [CrossRef]
- Tester, R.; Al-Ghazzewi, F. Glucomannans and nutrition. Food Hydrocoll. 2017, 68, 246–254. [Google Scholar] [CrossRef]
- Tester, R.; Al-Ghazzewi, F.H. Role of glucomannans in immunology. J. Pharm. Pharm. Sci. 2017, 20, 97–114. [Google Scholar] [CrossRef]
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Mulaw, G.; Sisay Tessema, T.; Muleta, D.; Tesfaye, A. In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. Int. J. Microbiol. 2019, 2019, 7179514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sornsenee, P.; Singkhamanan, K.; Sangkhathat, S.; Saengsuwan, P.; Romyasamit, C. Probiotic properties of Lactobacillus species isolated from fermented palm sap in Thailand. Probiotics Antimicrob. Proteins 2021, 13, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Talib, N.; Mohamad, N.E.; Yeap, S.K.; Hussin, Y.; Aziz, M.N.M.; Masarudin, M.J.; Sharifuddin, S.A.; Hui, Y.W.; Ho, C.L.; Alitheen, N.B. Isolation and characterization of Lactobacillus spp. from kefir samples in Malaysia. Molecules 2019, 24, 2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stack, H.M.; Kearney, N.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Association of beta-glucan endogenous production with increased stress tolerance of intestinal lactobacilli. Appl. Environ. Microbiol. 2010, 76, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Anandharaj, M.; Sivasankari, B. Isolation of potential probiotic Lactobacillus oris HMI68 from mother’s milk with cholesterol-reducing property. J. Biosci. Bioeng. 2014, 118, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, C.G.; Reinheimer, J.A. Lactic acid starter and probiotic bacteria: A comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res. Int. 2003, 36, 895–904. [Google Scholar] [CrossRef]
- Korcz, E.; Kerényi, Z.; Varga, L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Llamas, M.G.; Iraporda, C.; Dueñas, M.T.; Abraham, A.G.; Garrote, G.L. Impact of growth temperature on exopolysaccharide production and probiotic properties of Lactobacillus paracasei strains isolated from kefir grains. Food Microbiol. 2018, 69, 212–218. [Google Scholar] [CrossRef]
- Živković, M.; Miljković, M.S.; Ruas-Madiedo, P.; Markelić, M.B.; Veljović, K.; Tolinački, M.; Soković, S.; Korać, A.; Golić, N. EPS-SJ exopolisaccharide produced by the strain Lactobacillus paracasei subsp. paracasei BGSJ2-8 is involved in adhesion to epithelial intestinal cells and decrease on E. coli association to Caco-2 cells. Front. Microbiol. 2016, 7, 286. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, H.C.; de Sousa Melo, D.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Probiotic properties of lactobacilli and their ability to inhibit the adhesion of enteropathogenic bacteria to Caco-2 and HT-29 cells. Probiotics Antimicrob. Proteins 2021, 13, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Palencia, P.; López, P.; Corbí, A.L.; Peláez, C.; Requena, T. Probiotic strains: Survival under simulated gastrointestinal conditions, in vitro adhesion to Caco-2 cells and effect on cytokine secretion. Eur. Food Res. Technol. 2008, 227, 1475–1484. [Google Scholar] [CrossRef] [Green Version]
- Sophatha, B.; Teanpaisan, R. Factors Relating to Adhesion and Aggregation of Lactobacillus paracasei and Lactobacillus rhamnosus Strains. Microbiology 2021, 90, 793–800. [Google Scholar] [CrossRef]
- Dertli, E.; Mayer, M.J.; Narbad, A. Impact of the exopolysaccharide layer on biofilms, adhesion and resistance to stress in Lactobacillus johnsonii FI9785. BMC Microbiol. 2015, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzaretti, S.; Taverniti, V.; Guglielmetti, S.; Fiore, W.; Minuzzo, M.; Ngo, H.N.; Ngere, J.B.; Sadiq, S.; Humphreys, P.N.; Laws, A.P. A novel rhamnose-rich hetero-exopolysaccharide isolated from Lactobacillus paracasei DG activates THP-1 human monocytic cells. Appl. Environ. Microbiol. 2017, 83, e02702–e02716. [Google Scholar] [CrossRef] [PubMed]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef] [Green Version]
- Bengoa, A.A.; Zavala, L.; Carasi, P.; Trejo, S.A.; Bronsoms, S.; de los Ángeles Serradell, M.; Garrote, G.L.; Abraham, A.G. Simulated gastrointestinal conditions increase adhesion ability of Lactobacillus paracasei strains isolated from kefir to Caco-2 cells and mucin. Food Res. Int. 2018, 103, 462–467. [Google Scholar] [CrossRef]
- Lyerly, D.M.; Krivan, H.C.; Wilkins, T.D. Clostridium difficile: Its disease and toxins. Clin. Microbiol. Rev. 1988, 1, 1–18. [Google Scholar] [CrossRef]
- Trejo, F.M.; Minnaard, J.; Perez, P.F.; De Antoni, G.L. Inhibition of Clostridium difficile growth and adhesion to enterocytes by Bifidobacterium supernatants. Anaerobe 2006, 12, 186–193. [Google Scholar] [CrossRef]
- Schoster, A.; Kokotovic, B.; Permin, A.; Pedersen, P.; Dal Bello, F.; Guardabassi, L. In vitro inhibition of Clostridium difficile and Clostridium perfringens by commercial probiotic strains. Anaerobe 2013, 20, 36–41. [Google Scholar] [CrossRef]
- Shahrokhi, M.; Nagalli, S. Probiotics; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naaber, P.; Smidt, I.; Štšepetova, J.; Brilene, T.; Annuk, H.; Mikelsaar, M. Inhibition of Clostridium difficile strains by intestinal Lactobacillus species. J. Med. Microbiol. 2004, 53, 551–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allonsius, C.N.; van den Broek, M.F.; De Boeck, I.; Kiekens, S.; Oerlemans, E.F.; Kiekens, F.; Foubert, K.; Vandenheuvel, D.; Cos, P.; Delputte, P. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb. Biotechnol. 2017, 10, 1753–1763. [Google Scholar] [CrossRef]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as antimicrobial agents: Mechanism and spectrum of activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Fisher, P.B. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006, 236, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Russell, W.M.; Douglas-Escobar, M.; Hauser, N.; Lopez, M.; Neu, J. Live and heat-killed Lactobacillus rhamnosus GG: Effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr. Res. 2009, 66, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Uchinaka, A.; Azuma, N.; Mizumoto, H.; Nakano, S.; Minamiya, M.; Yoneda, M.; Aoyama, K.; Komatsu, Y.; Yamada, Y.; Murohara, T. Anti-inflammatory effects of heat-killed Lactobacillus plantarum L-137 on cardiac and adipose tissue in rats with metabolic syndrome. Sci. Rep. 2018, 8, 1–20. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Sarikaya, H.; Aslim, B.; Yuksekdag, Z. Assessment of anti-biofilm activity and bifidogenic growth stimulator (BGS) effect of lyophilized exopolysaccharides (l-EPSs) from Lactobacilli strains. Int. J. Food Prop. 2017, 20, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Salazar, N.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Exopolysaccharides produced by lactic acid bacteria and bifidobacteria as fermentable substrates by the intestinal microbiota. Crit. Rev. Food Sci. Nutr. 2016, 56, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Júnior, A.I.M.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhang, R.; Tian, X.; Zhou, X.; Pan, X.; Wong, A. Assessing the risk of probiotic dietary supplements in the context of antibiotic resistance. Front. Microbiol. 2017, 8, 908. [Google Scholar] [CrossRef] [PubMed]
- Tokatlı, M.; Gülgör, G.; Bağder Elmacı, S.; Arslankoz İşleyen, N.; Özçelik, F. In vitro properties of potential probiotic indigenous lactic acid bacteria originating from traditional pickles. BioMed Res. Int. 2015, 2015, 315819. [Google Scholar] [CrossRef] [PubMed]






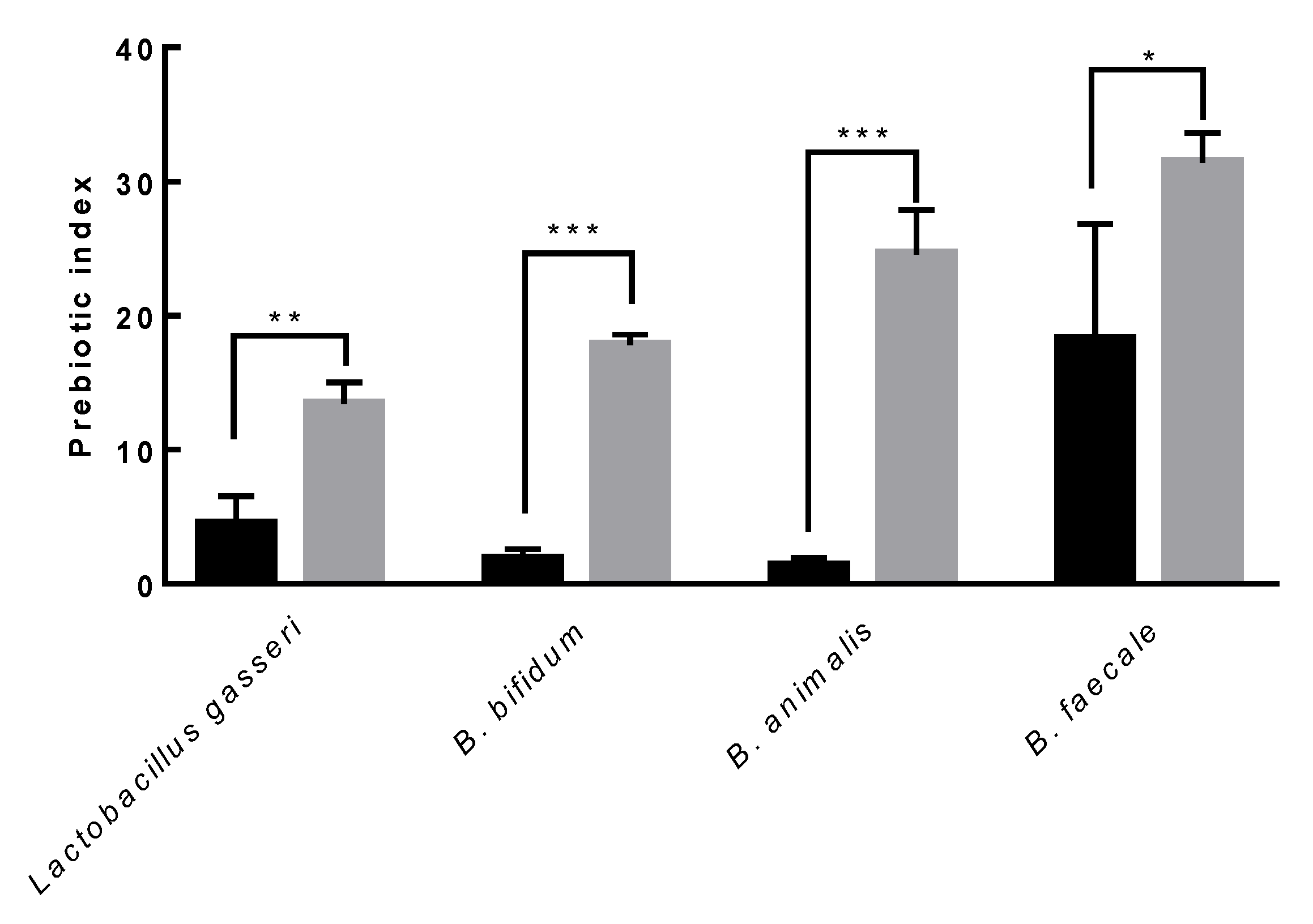
| GIT Tolerance (%) | L. paracasei EPS DA-BACS | |
|---|---|---|
| Culture Broth (In the Presence of EPS) | Pellet * (In the Absence of EPS) | |
| Acid | 101 ± 0.5 a | 98 ± 0.7 b |
| Bile Salt | 102 ± 5.3 a | 97 ± 0.5 a |
| Pancreatin | 101 ± 0.7 a | 99 ± 0.8 a |
| L. paracasei EPS DA-BACS | ||
|---|---|---|
| Culture Broth (In the Presence of EPS) | Pellet * (In the Absence of EPS) | |
| Gut Adhesion Ability (%) | 74.9 ± 1.4 a | 75.5 ± 1.2 a |
| RGI Criteria | AMR Gene Family | Drug Class | % Identity Matching Region |
|---|---|---|---|
| Strict | Small multidrug resistance (SMR) antibiotic efflux pump | Disinfecting agents and antiseptics | 38.24 |
| Loose | Elfamycin resistant EF-Tu | Elfamycin antibiotic | 72.19 |
| Rifamycin-resistant beta-subunit of RNA polymerase (rpoB) | Rifamycin antibiotic | 71.59 | |
| Antibiotic resistant fusA | Fusidic acid | 72.19 |
| Antibiotics | Cut-Off Value (µg/mL) * | MIC | Susceptibility | Assessment |
|---|---|---|---|---|
| (µg/mL) | ||||
| Ampicillin | 4 | 0.25 | S *** | Acceptable |
| Vancomycin | n.r. ** | n.r. | n.r. | Acceptable |
| Gentamycin | 32 | 4 | S | Acceptable |
| Kanamycin | 64 | 48 | S | Acceptable |
| Streptomycin | 64 | 24 | S | Acceptable |
| Erythromycin | 1 | 0.125 | S | Acceptable |
| Clindamycin | 1 | 0.023 | S | Acceptable |
| Tetracycline | 4 | 1 | S | Acceptable |
| Chloramphenicol | 4 | 4 | S | Acceptable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-G.; Joeng, H.; Shin, J.; Kim, S.; Lee, C.; Song, Y.; Lee, B.-H.; Park, H.-G.; Lee, T.-H.; Jiang, H.-H.; et al. Potential Probiotic Properties of Exopolysaccharide-Producing Lacticaseibacillus paracasei EPS DA-BACS and Prebiotic Activity of Its Exopolysaccharide. Microorganisms 2022, 10, 2431. https://doi.org/10.3390/microorganisms10122431
Lee M-G, Joeng H, Shin J, Kim S, Lee C, Song Y, Lee B-H, Park H-G, Lee T-H, Jiang H-H, et al. Potential Probiotic Properties of Exopolysaccharide-Producing Lacticaseibacillus paracasei EPS DA-BACS and Prebiotic Activity of Its Exopolysaccharide. Microorganisms. 2022; 10(12):2431. https://doi.org/10.3390/microorganisms10122431
Chicago/Turabian StyleLee, Min-Gyu, Huijin Joeng, Jaein Shin, Suin Kim, Chaeeun Lee, Youngbo Song, Byung-Hoo Lee, Hyoung-Geun Park, Tae-Ho Lee, Hai-Hua Jiang, and et al. 2022. "Potential Probiotic Properties of Exopolysaccharide-Producing Lacticaseibacillus paracasei EPS DA-BACS and Prebiotic Activity of Its Exopolysaccharide" Microorganisms 10, no. 12: 2431. https://doi.org/10.3390/microorganisms10122431






