Cranberry Arabino-Xyloglucan and Pectic Oligosaccharides Induce Lactobacillus Growth and Short-Chain Fatty Acid Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. High Performance Size Exclusion Chromatography (HPSEC)
2.3. Monosaccharide Analysis
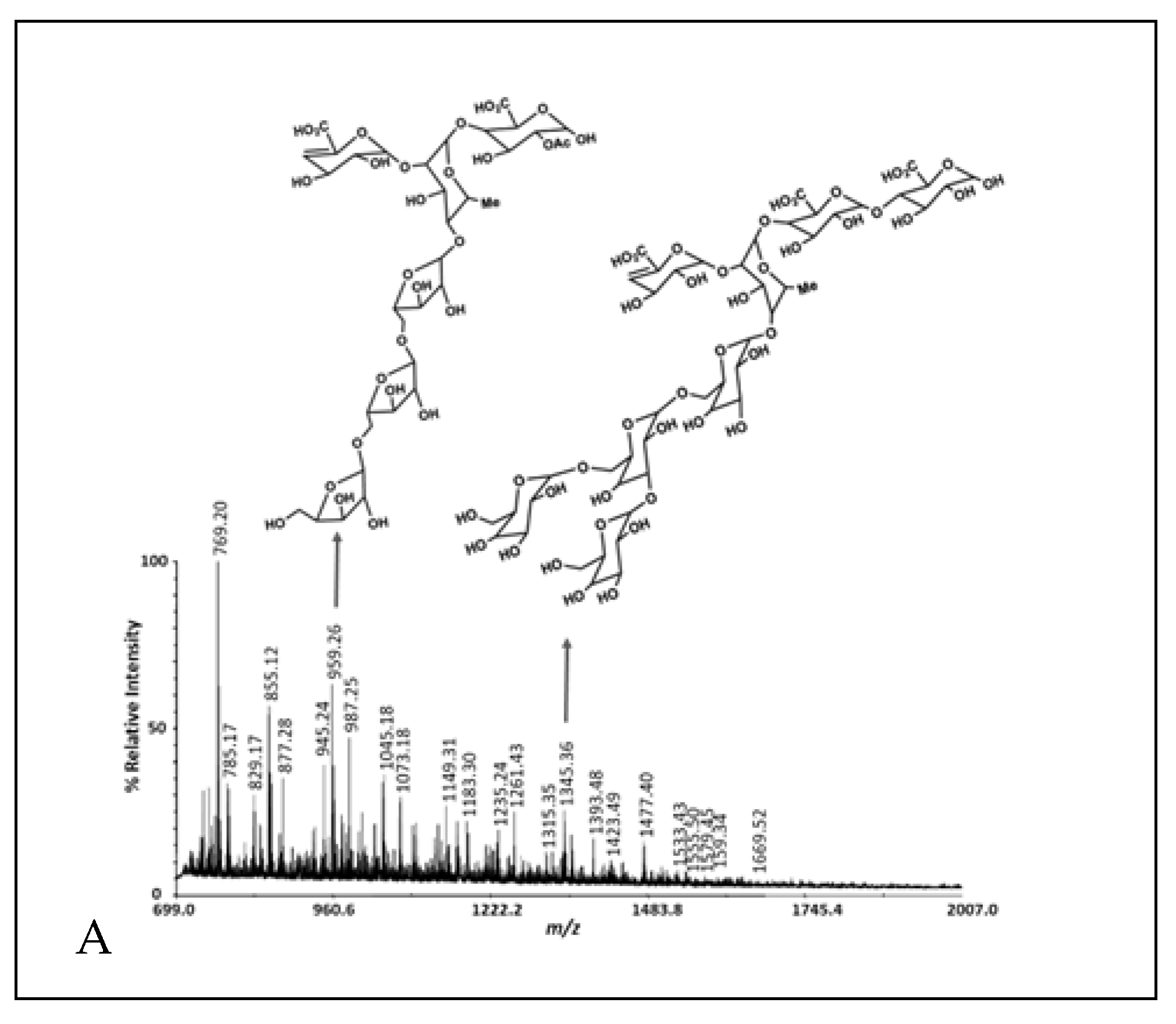
2.4. Oligosaccharide Structure
2.5. Oligosaccharide Glycosyl-Linkage Positions
2.6. Preparative HPLC
2.7. Lactic Acid Bacteria Growth on Cranberry Xyloglucans
2.8. Miniature Fecal Batch Cultures
2.9. Statistical Analysis
3. Results and Discussion
3.1. Composition and Characterization by HPSEC
3.2. Monosaccharide and Oligosaccharide Composition
3.3. Glycosyl-Linkage
3.4. Growth of Lactobacillus Plantarum BAA 793 on Prebiotics
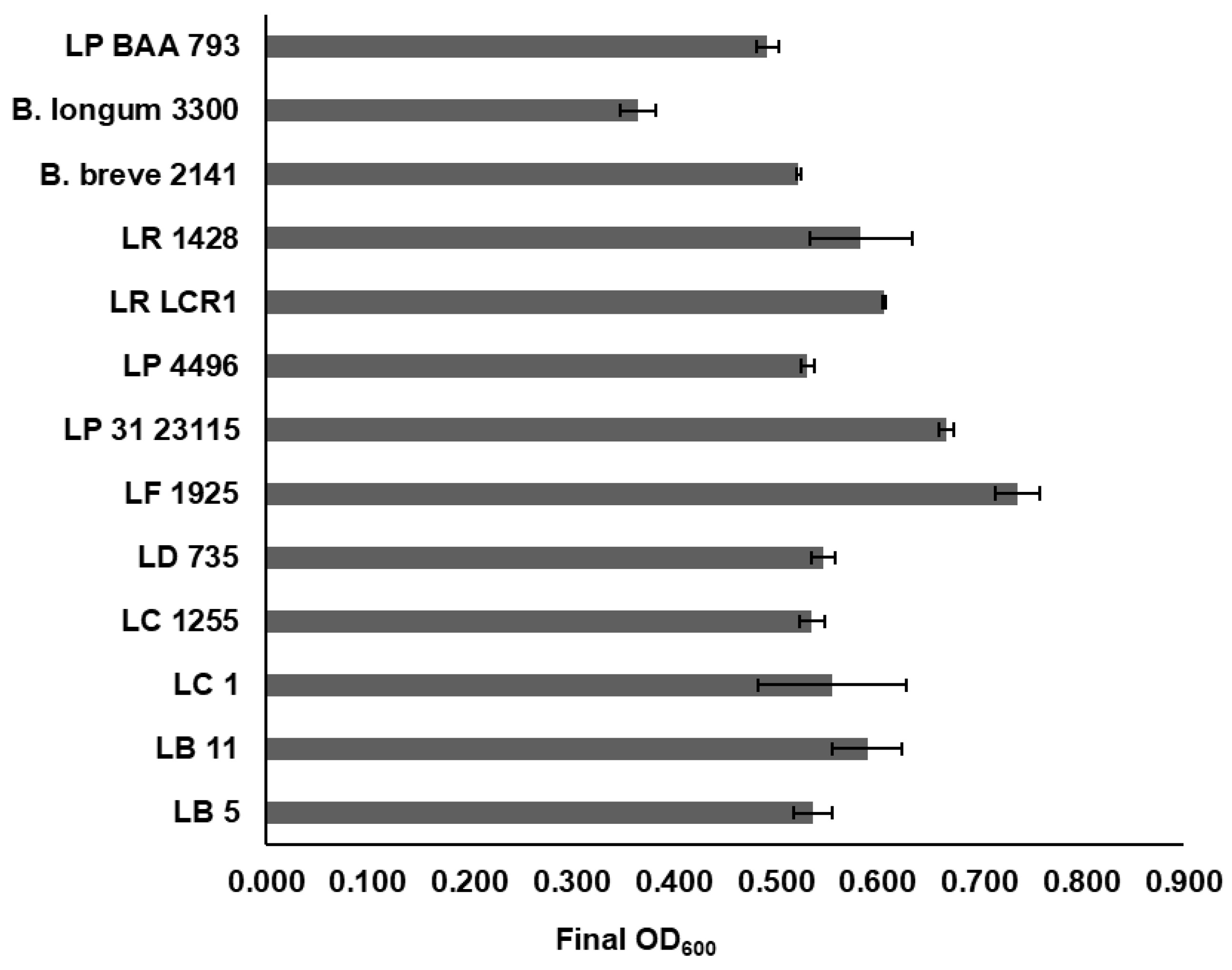
3.5. Growth of LAB on Cranberry A6
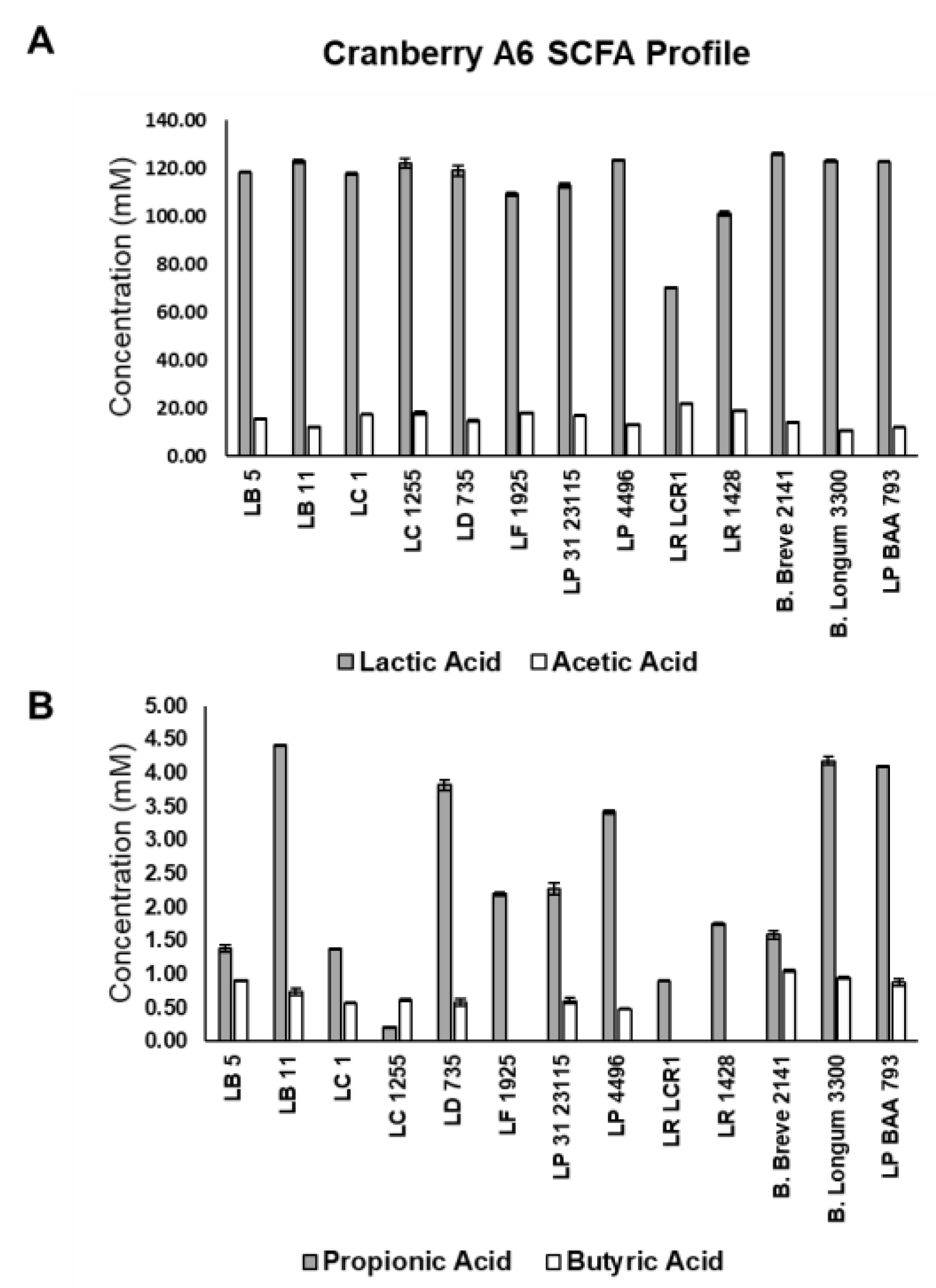
3.6. Short Chain Fatty Acid Production by LAB Grown on Cranberry A6
3.7. Effect of Cranberry A6 on Fecal Microbiota Growth and SCFA Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Statement
References
- Slavin, J. Fibers and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, A.T., Jr.; Nuñez, A.; Strahan, G.D.; Chau, H.K.; White, A.K.; Marais, J.P.J.; Hom, K.; Vakkalanka, M.S.; Di, R.; Yam, K.L.; et al. Cranberry xyloglucan structure and inhibition of Escherichia coli adhesion to epithelial cells. J. Agric. Food Chem. 2015, 63, 5622–5633. [Google Scholar] [CrossRef]
- Coleman, C.M.; Ferreira, D. Oligosaccharides and Complex Carbohydrates: A New Paradigm for Cranberry Bioactivity. Molecules 2020, 25, 881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jepson, R.G.; Williams, G.; Craig, J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev. 2012, 10, CD001321. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Liska, D.; Talan, D.; Chung, M. Cranberry reduces the risk of urinary tract infection recurrence in otherwise healthy women: A systematic review and meta-analysis. J. Nutr. 2017, 147, 2282–2288. [Google Scholar] [CrossRef] [Green Version]
- Luis, A.; Domingues, F.; Pereira, L. Can cranberries contribute to reduce the incidence of urinary tract infections? A systematic review with meta-analysis and trial sequential analysis of clinical trials. J. Urol. 2017, 198, 614–621. [Google Scholar] [CrossRef]
- Gardner, E. The health properties of cranberry juice. Nutr. Bull. 2014, 39, 223–230. [Google Scholar] [CrossRef]
- Hotchkiss, A.T.; Nunez, A.; Khoo, C.; Strahan, G.D. Cranberry Xyloglucan Oligosaccharide Composition. U.S. Patent 9,314,494, 19 April 2016. [Google Scholar]
- Sun, J.; Marais, J.P.J.; Khoo, C.; LaPlante, K.; Vejborg, R.M.; Givskov, M.; Tolker-Nielsen, T.; Seeram, N.P.; Rowley, D.C. Cranberry (Vaccinium macrocarpon) oligosaccharides decrease biofilm formation by uropathogenic Escherichia coli. J. Funct. Foods 2015, 17, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Neto, C.C.; Penndorf, K.A.; Feldman, M.; Meron-Sudaim, S.; Zakay-Rones, Z.; Steinberg, D.; Fridman, M.; Kashman, Y.; Ginsburg, I.; Ofek, I.; et al. Characterization of non-dialyzable constituents from cranberry juice that inhibit adhesion, co-aggregation and biofilm formation by oral bacteria. Food Funct. 2017, 8, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Deering, R.W.; Peng, Z.; Najia, L.; Khoo, C.; Cohen, P.S.; Seeram, N.P.; Rowley, D.C. Pectic oligosaccharides from cranberry prevent quiescence and persistence in the uropathogenic Escherichia coli CFT073. Sci. Rep. 2019, 9, 19590. [Google Scholar] [CrossRef] [PubMed]
- Guggenbichler, J.P.; Bettignies-Dutz, A.D.; Meissner, P.; Schellmoser, S.; Jurenitsch, J. Acidic oligosaccharides from natural sources block adherence of Escherichia coli on uroepithelial cells. Pharmaceut. Pharmacol. Lett. 1997, 7, 35–38. [Google Scholar]
- Al-Tamimi, M.A.H.M.; Palframan, R.J.; Cooper, J.M.; Gibson, G.R.; Rastall, R.A. In vitro fermentation of sugar beet arabinan and arabino-oligosaccharides by the human gut microflora. J. Appl. Microbiol. 2006, 100, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Chaluvadi, S.; Hotchkiss, A.T.; Call, J.E.; Luchansky, J.B.; Phillips, J.G.; Liu, L.S.; Yam, K.L. Protection of probiotic bacteria in synbiotic matrix following aerobic storage at 4°. Benefic. Microbes 2012, 3, 175–187. [Google Scholar] [CrossRef]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.K.; Rastall, R.A.; Yam, K.L.; Hotchkiss, A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef]
- Holck, J.; Lorentzen, A.; Vigsnæs, L.K.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Feruloylated and nonferuloylated arabino-oligosaccharides from sugar beet pectin selectively stimulate the growth of Bifidobacterium spp. in human fecal in vitro fermentations. J. Agric. Food Chem. 2011, 59, 6511–6519. [Google Scholar] [CrossRef]
- Hotchkiss, A.T., Jr.; Nunez, A.; Gibson, G.R.; Rastall, R.A. Methods of promoting the growth of beneficial bacteria in the gut. U.S. Patent 8,313,789, 20 November 2012. [Google Scholar]
- Mandalari, G.; Palop, C.N.; Tuohy, K.; Gibson, G.R.; Bennett, R.N.; Waldron, K.W.; Bisignano, G.; Narbad, A.; Faulds, C.B. In vitro evaluation of the prebiotic activity of a pectic oligosaccharide-rich extract enzymatically derived from bergamot peel. Appl. Microbiol. Biotechnol. 2007, 73, 1173–1179. [Google Scholar] [CrossRef]
- Manderson, K.; Pinart, M.; Tuohy, K.M.; Grace, W.E.; Hotchkiss, A.T.; Widmer, W.; Yadhav, M.P.; Gibson, G.R.; Rastall, R.A. In vitro determination of prebiotic properties of oligosaccharides derived from an orange juice manufacturing by-product stream. Appl. Environ. Microbiol. 2005, 71, 8383–8389. [Google Scholar] [CrossRef] [Green Version]
- Olano-Martin, E.; Gibson, G.R.; Rastall, R.A. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Olano-Martin, E.; Williams, M.R.; Gibson, G.R.; Rastall, R.A. Pectins and pectic-oligosaccharides inhibit Escherichia coli O157: H7 Shiga toxin as directed towards the human colonic cell line HT29. FEMS Microbiol. Lett. 2003, 218, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Onumpai, C.; Kolida, S.; Bonnin, E.; Rastall, R.A. Microbial utilization and selectivity of pectin fractions with various structures. Appl. Environ. Microbiol. 2011, 77, 5747–5754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigsnæs, L.K.; Holck, J.; Meyer, A.S.; Licht, T.R. In vitro fermentation of sugar beet arabino-oligosaccharides by fecal microbiota obtained from patients with ulcerative colitis to selectively stimulate the growth of Bifidobacterium spp. and Lactobacillus spp. Appl. Environ. Microbiol. 2011, 77, 8336–8344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinaki, E.; Kandylis, P.; Dimitrellou, D.; Zakynthinos, G.; Varzakas, T. Probiotic yogurt production with Lactobacillus casei and prebiotics. Curr. Res. Nutr. Food Sci. 2016, 4, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Langa, S.; Van Den Bulck, E.; Peirotén, A.; Gaya, P.; Schols, H.A.; Arqués, J.L. Application of lactobacilli and prebiotic oligosaccharides for the development of a synbiotic semi-hard cheese. LWT-Food Sci. Technol. 2019, 114, 108361. [Google Scholar] [CrossRef]
- Thongaram, T.; Hoeflinger, J.L.; Chow, J.; Miller, M.J. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J. Dairy Sci. 2017, 100, 7825–7833. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, O.; Muthaiyan, A.; Moreno, F.J.; Montilla, A.; Sanz, M.L.; Ricke, S.C. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol. 2012, 30, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, E.; Sun, J.; Rowley, D.C.; Sela, D.A. A human gut commensal ferments cranberry carbohydrates to produce formate. Appl. Environ. Microbiol. 2017, 83, e01097-17. [Google Scholar] [CrossRef] [Green Version]
- Zúñiga, M.; Yebra, M.J.; Monedero, V. Complex oligosaccharide utilization pathways in Lactobacillus. Curr. Issues Mol. Biol. 2021, 40, 49–80. [Google Scholar] [CrossRef] [Green Version]
- Iliev, I.; Vasileva, T.; Bivolarski, V.; Momchilova, A.; Ivanova, I. Metabolic profiling of xylooligosaccharides by Lactobacilli. Polymers 2020, 12, 2387. [Google Scholar] [CrossRef]
- Markowiak-Kopec, P.; Slizewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD. EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Cantu-Jungles, T.M.; do Nascimento, G.E.; Zhang, X.; Iacomini, M.; Cordeiro, L.M.C.; Hamaker, B.R. Soluble xyloglucan generates bigger bacterial community shifts than pectic polymers during in vitro fecal fermentation. Carbohydr. Polym. 2019, 206, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Liang, L.; Fan, X.; Yu, Z.; Hotchkiss, A.T.; Wilk, B.J.; Eliaz, I. The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Alternat. Therap. Health Med. 2008, 14, 34–38. [Google Scholar]
- Willis, L.M.; Stupak, J.; Richards, M.R.; Lowary, T.L.; Li, J.; Whitfield, C. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, 7868–7873. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; McLandsborough, L.A.; Weiss, J.; Peleg, M. Concentration and application order effects of sodium benzoate and eugenol mixtures on the growth inhibition of Saccharomyces cervisiae and Zygosaccharomyces bailii. J. Food Sci. 2010, 75, M482–M488. [Google Scholar] [CrossRef]
- Poveda, C.; Pereira, D.I.A.; Lewis, M.; Walton, G.E. The impact of low-level iron supplements on the faecal microbiota of irritable bowel syndrome and healthy donors using in vitro batch cultures. Nutrients 2020, 12, 3819. [Google Scholar] [CrossRef]
- Ozcan, E.; Rozycki, M.R.; Sela, D.A. Cranberry proanthocyanidins and dietary oligosaccharides synergistically modulate Lactobacillus plantarum physiology. Microorganisms 2021, 9, 656. [Google Scholar] [CrossRef]
- Renye Jr, J.A.; White, A.K.; Hotchkiss, A.T. Identification of Lactobacillus strains capable of fermenting fructo-oligosaccharides and inulin. Microorganisms 2021, 9, 2020. [Google Scholar] [CrossRef]
- Cselovszky, J.; Wolf, G.; Hammes, W.P. Production of formate, acetate, and succinate by anaerobic fermentation of Lactobacillus pentosus in the presence of citrate. Appl. Microbiol. Biotechnol. 1992, 37, 94–97. [Google Scholar] [CrossRef]
- Degnan, B.A.; Macfarlane, G.T. Effect of dilution rate and carbon availability on Bifidobacterium breve fermentation. Appl. Microbiol Biotechnol. 1994, 40, 800–805. [Google Scholar] [CrossRef]
- Usta-Gorgu, B.; Yilmaz-Ersan, L. Short-chain fatty acids production by Bifidobacterium species in the presence of salep. Elec. J. Biotechnol. 2020, 47, 29–35. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutrition Rev. 2021, 69, 245–258. [Google Scholar] [CrossRef]
- Nishina, P.M.; Freeland, R.A. Effects of propionate on lipid biosynthesis in isolated rat hepatocytes. J. Nutr. 1990, 120, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Jan, G.; Belzacq, A.S.; Haouzi, D.; Rouault, A.; Metivier, D.; Kroemer, G.; Brenner, C. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002, 9, 179–188. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Tian, Y.; Huang, C.; Li, D.; Zhong, Q.; Ma, X. Interaction between microbes and host intestinal health: Modulation by dietary nutrients and gut-brain-endocrine-immune axis. Curr. Protein Pept. Sci. 2015, 16, 592–603. [Google Scholar] [CrossRef]
- Canani, R.B.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanely, D.; Luong, S.; Maruya, M.; McKenzie, C.I.; Hijikata, A.; Wong, C. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fiber-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elamin, E.E.; Masclee, A.A.; Dekker, J.; Pieters, H.-J.; Jonkers, D.M. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J. Nutr. 2013, 143, 1872–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A double-edged sword for health? Adv. Nutr. 2021, 9, 21–29. [Google Scholar] [CrossRef] [Green Version]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Cai, D.; Zhao, R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016, 7, 56071–56082. [Google Scholar] [CrossRef] [Green Version]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [Green Version]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Michalek, S.M.; Hirasawa, M.; Kiyono, H.; Ochiai, K.; McGhee, J.R. Oral ecology and virulence of Lactobacillus casei and Streptococcus mutans in Gnotobiotic rats. Infect. Immun. 1981, 33, 690–696. [Google Scholar] [CrossRef] [Green Version]
- Slawik, S.; Staufenbiel, I.; Schilke, R.; Nicksch, S.; Weinspach, K.; Stiesch, M.; Eberhard, J. Probiotics affect the clinical inflammatory parameters of experimental gingivitis in humans. Eur. J. Clin. Nutr. 2011, 65, 857–863. [Google Scholar] [CrossRef]
- Chugh, P.; Dutt, R.; Sharma, A.; Bhagat, N.; Dhar, M. A critical appraisal of the effects of probiotics on oral health. J. Funct. Foods 2020, 70, 103985. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei group: History and health related applications. Front. Microbiol. 2018, 9, 2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naghmouchi, K.; Belguesmia, Y.; Bendali, F.; Spano, G.; Seal, B.S.; Drider, D. Lactobacillus fermentum: A bacterial species with potential for food preservation and biomedical applications. Critical Rev. Food Sci. Nutr. 2020, 60, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. Biomed. Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [Green Version]
- Moro-García, M.A.; Alonso-Arias, R.; Baltadjieva, M.; Fernández Benítez, C.; Fernández Barrial, M.A.; Díaz Ruisánchez, E.; Alonso Santos, R.; Alvarez Sánchez, M.; Saavedra Miján, J.; López-Larrea, C. Oral supplementation with Lactobacillus delbreuckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age 2013, 35, 1311–1326. [Google Scholar] [CrossRef]
- Evivie, S.E.; Abdelazez, A.; Li, B.; Lu, S.; Liu, F.; Huo, G. Lactobacillus delbreuckii subsp. bulgaricus KLDS 1.0207 exerts antimicrobial and cytotoxic effects in vitro and improves blood biochemical parameters in vivo against notable foodborne pathogens. Front. Microbiol. 2020, 11, 583070. [Google Scholar] [CrossRef]
- Cheon, M.-J.; Lim, S.-M.; Lee, N.K.; Paik, H.-D. Probiotic properties and neuroprotective effects of Lactobacillus buchneri KU200793 isolated from Korean fermented foods. Int. J. Mol. Sci. 2020, 21, 1227. [Google Scholar] [CrossRef] [Green Version]
- Sung, V.; D’Amico, F.; Cabana, M.D.; Chau, K.; Koren, G.; Savino, F.; Szajewska, H.; Deshpande, G.; Dupont, C.; Indrio, F.; et al. Lactobacillus reuteri to treat infant colic: A meta-analysis. Pediatrics 2018, 141, e20171811. [Google Scholar] [CrossRef] [Green Version]
- Cionci, N.B.; Baffoni, L.; Gaggia, F.; Di Gioia, D. Therapeutic microbiology: The role of Bifidobacterium breve as food supplement for the prevention/treatment of paediatric diseases. Nutr. 2018, 10, 1723. [Google Scholar] [CrossRef]
- O’Connor, K.; Morrissette, M.; Strandwitz, P.; Ghiglieri, M.; Caboni, M.; Liu, H.; Khoo, C.; D’Onofrio, A.; Lewis, K. Cranberry extracts promote growth of Bacteriodaceae and decrease abundance of Enterobacteriaceae in a human gut simulator model. PLoS ONE 2019, 14, e0224836. [Google Scholar] [CrossRef]
- Sasaki, D.; Sasaki, K.; Ikuta, N.; Yasuda, T.; Fukuda, I.; Kondo, A.; Osawa, R. Low amounts of dietary fibre increase in in vitro production of short-chain fatty acids without changing human colonic microbiota structure. Sci. Rep. 2018, 8, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| %Mass Fraction a | Mw/Mn | Mz/Mn | Mw (kDa) | ηw (dL/g) | Rhzv (nm) | M-H | |
|---|---|---|---|---|---|---|---|
| A6 | 97.8 (0.2) | 1.03 (0.03) | 1.07 (0.1) | 1.48 (0.3) | 0.025 (0.001) | 1.0 (0.1) | 0.260 (0.1) |
| A6 b | 103.0 (1.0) | 1.17 (0.04) | 1.64 (0.2) | 2.75 (0.2) | 0.044 (0.002) | 1.4 (0.01) | 0.701 (0.02) |
| Sample | Glc | Ara | Gal | Xyl | Rha | Fuc | GalA | GlcA |
|---|---|---|---|---|---|---|---|---|
| A6 A | 40.9 | 16.1 | 3.7 | 30.4 | 0.5 | 0.1 | 7.9 | 0.5 |
| A6 B | 38. 8 | 17.7 | 0.9 | 6.8 | 31.9 | 0.4 | 1.0 | 2.5 |
| Prep HPLC fraction 1 | 18.7 | 31.0 | 8.9 | 13.7 | 6.8 | 1.5 | 17.6 | 2.0 |
| Prep HPLC fraction 2 | 37.1 | 26.4 | 4.0 | 30.9 | ND | 0.2 | 1.3 | 0.0 |
| Prep HPLC fraction 3 | 40.1 | 14.5 | 7.6 | 35.3 | ND | 0.6 | 1.6 | 0.3 |
| Linkage | A6 A | A6 B | A6 C |
|---|---|---|---|
| t-Araf | 5.7 | 5.5 | 7.7 |
| t-Fucp | 0.1 | 0.3 | 0.2 |
| t-Arap | 0.1 | 0.3 | 0.1 |
| t-Xylp | 3.3 | 2.1 | 3.8 |
| 2-Rhap | 0.1 | 0.4 | 0.7 |
| 3-Rhap | - | 0.2 | 0.2 |
| t-Manp | 0.1 | - | - |
| t-Glcp | 15.5 | 26.9 | 8.1 |
| 3-Araf | 0.7 | 1.7 | 2.5 |
| t-GalfA | - | - | 0.6 |
| t-Galp | 2.5 | 1.6 | 2.8 |
| t-GalpA | 0.2 | 0.2 | 0.7 |
| 4-Arap or 5-Araf | 4.0 | 8.2 | 12.0 |
| 3’-Apiose | - | - | 0.1 |
| 2-Xylp | 9.9 | 4.5 | 7.0 |
| 4-Xylp | 1.2 | 0.9 | 1.0 |
| 3,4-Fucp | 0.1 | 0.1 | 0.2 |
| 3-Glcp | 0.3 | 1.3 | - |
| 2,4-Rhap | - | - | 0.5 |
| 2-Glcp | 0.2 | 0.4 | 0.1 |
| 2-GlcpA | - | - | 0.1 |
| 3-Galp | 0.1 | - | 0.2 |
| 4-Manp | 0.4 | 0.2 | 0.5 |
| 3,4-Arap or 3,5-Araf | 0.3 | 1.3 | 2.5 |
| 6-Glcp | 5.9 | 11.7 | 5.8 |
| 4-Galp | 0.1 | 0.1 | 0.3 |
| 4-GalpA | 0.4 | 0.2 | 1.7 |
| 4-Glcp | 20.0 | 17.7 | 17.4 |
| 2,4-Xylp | 0.1 | 0.1 | 0.1 |
| 6-Galp | 0.1 | 0.1 | 0.3 |
| 2,3,4-Arap or 2,3,5-Araf | 0.1 | 0.2 | 0.9 |
| 3,4-Glcp | 0.2 | 0.2 | 0.3 |
| 2,4-Glcp | 0.5 | 0.3 | 0.4 |
| 2,4-GlcpA | - | - | 0.1 |
| 3,6-Glcp | - | - | 0.1 |
| 4,6-Glcp | 27.7 | 13.2 | 20.6 |
| 3,6-Galp | 0.1 | - | 0.1 |
| Probe | Time (h) | Negative Control | Inulin | Cranberry Extract 2 |
|---|---|---|---|---|
| EUB | 0 | 7.25 (0.50) a | 7.25 (0.47) a | 7.25 (0.40) a |
| 24 | 7.33 (0.61) a | 7.73 (0.22) a* | 7.55 (0.41) a | |
| 48 | 7.06 (0.42) a | 7.69 (0.18) ab | 7.35 (0.20) ab | |
| BIF | 0 | 5.91 (0.85) a | 5.88 (0.85) a | 5.87 (0.67) a |
| 24 | 6.01 (1.01) a | 7.03 (0.59) a | 6.28 (0.47) a | |
| 48 | 5.86 (0.76) a | 7.32 (0.26) c* | 5.93 (0.22) ab | |
| LAB | 0 | 4.99 (0.51) a | 4.95 (0.34) a | 4.76 (0.73) a |
| 24 | 5.30 (0.72) a | 5.94 (0.21) a* | 5.57 (0.61) a | |
| 48 | 5.06 (0.85) a | 5.90 (0.38) a* | 5.65 (0.28) a | |
| BAC | 0 | 5.85 (0.16) a | 5.87 (0.20) a | 5.81 (0.34) a |
| 24 | 6.35 (0.43) a | 6.40 (0.39) a | 6.28 (0.55) a | |
| 48 | 5.92 (0.46) a | 6.21 (0.26) a | 5.98 (0.41) a | |
| EREC | 0 | 6.84 (0.45) a | 6.82 (0.47) a | 6.85 (0.33) a |
| 24 | 6.69 (0.47) a | 7.00 (0.22) a | 6.98 (0.64) a | |
| 48 | 6.02 (0.57) a | 6.56 (0.20) ab | 6.81 (0.22) ab | |
| RREC | 0 | 6.07 (0.47) a | 6.17 (0.50) a | 5.91 (0.50) a |
| 24 | 5.86 (0.63) a | 6.59 (0.25) a | 6.11 (0.81) a | |
| 48 | 5.10 (0.94) a | 5.95 (0.41) a | 5.96 (0.43) a | |
| ATO | 0 | 5.37 (0.33) a | 5.42 (0.35) a | 5.38 (0.54) a |
| 24 | 5.91 (0.64) a | 6.21 (0.46) a | 5.48 (0.81) a | |
| 48 | 5.64 (0.23) a | 6.02 (0.54) a | 5.56 (0.44) a | |
| PRO | 0 | 5.56 (0.55) a | 5.67 (0.41) a | 5.62 (0.59) a |
| 24 | 6.04 (0.89) a | 6.18 (0.38) a | 5.91 (0.68) a | |
| 48 | 5.56 (0.45) a | 5.97 (0.47) a | 5.78 (0.47) a | |
| FPRAU | 0 | 6.54 (0.60) a | 6.57 (0.49) a | 6.61 (0.43) a |
| 24 | 6.38 (0.86) a | 6.90 (0.40) a | 7.00 (0.14) a | |
| 48 | 5.85 (0.99) a | 6.82 (0.39) a | 6.70 (0.54) a | |
| DSV | 0 | 6.27 (0.54) a | 6.30 (0.37) a | 6.37 (0.41) a |
| 24 | 5.87 (0.84) a | 6.04 (0.37) a | 5.65 (1.01) a | |
| 48 | 5.22 (0.65) a | 6.11 (0.41) ab | 5.49 (0.37) ab | |
| CHIS | 0 | 4.86 (0.72) a | 4.61 (0.71) a | 4.41 (0.93) a |
| 24 | 5.02 (1.00) a | 5.40 (0.45) a | 5.91 (0.31) a± | |
| 48 | 4.87 (0.94) a | 5.73 (0.57) a | 5.74 (0.45) a± |
| Negative Control | Inulin | Cranberry Pomace | Cranberry Extract 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SCFA | Time | ||||||||
| Acetate | t0 | 10.55 | (3.29) | 12.62 | (3.15) | 7.67 | (1.57) | 4.49 | (0.82) |
| t24 | 8.98 | (1.38) | 21.93 | (2.12) a | 19.57 | (2.64) ± | 18.15 | (1.78) ± | |
| t48 | 14.23 | (0.87) | 20.11 | (2.14) a | 20.73 | (2.58) ± | 17.12 | (1.27) ± | |
| Propionate | t0 | 3.37 | (3.29) | 2.92 | (3.15) | 1.62 | (1.57) | 0.76 | (0.82) |
| t24 | 2.31 | (1.38) | 5.76 | (2.12) | 7.53 | (2.64) b | 7.73 | (1.78) *a | |
| t48 | 4.27 | (0.87) | 6.43 | (2.14) | 8.41 | (2.58) ± | 9.40 | (1.27) *a | |
| Butyrate | t0 | 3.90 | (2.54) | 3.39 | (2.17) | 2.34 | (1.02) | 0.36 | (0.62) |
| t24 | 4.54 | (3.96) | 26.23 | (15.58) ± | 18.39 | (3.55) *a | 17.01 | (6.51) *a | |
| t48 | 6.52 | (4.69) | 25.97 | (12.47) ± | 22.57 | (3.02) ** | 15.31 | (9.29) ± | |
| Valerate | t0 | 0.00 | (0.00) | 0.00 | (0.00) | 0.00 | (0.00) | 0.00 | (0.00) |
| t24 | 2.62 | (3.43) | 0.00 | (0.00) | 2.70 | (2.53) | 0.00 | (0.00) | |
| t48 | 2.88 | (2.96) | 0.47 | (0.81) | 4.52 | (0.91) * | 0.00 | (0.00) | |
| Isobutyrate | t0 | 0.00 | (0.00) | 0.00 | (0.00) | 0.00 | (0.00) | 0.00 | (0.00) |
| t24 | 2.26 | (1.32) ± | 0.00 | (0.00) a | 1.26 | (2.18) | 1.21 | (2.10) | |
| t48 | 2.12 | (1.68) | 0.95 | (1.64) | 1.72 | (2.98) | 1.79 | (2.46) | |
| Isovalerate | t0 | 0.00 | (0.00) | 0.00 | (0.00) | 0.00 | (0.00) | 0.00 | (0.00) |
| t24 | 2.74 | (0.85) * | 0.00 | (0.00) a | 1.09 | (1.89) | 1.17 | (1.30) | |
| t48 | 2.25 | (1.04) | 0.00 | (0.00) | 2.47 | (1.58) * | 0.85 | (1.48) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotchkiss, A.T., Jr.; Renye, J.A., Jr.; White, A.K.; Nunez, A.; Guron, G.K.P.; Chau, H.; Simon, S.; Poveda, C.; Walton, G.; Rastall, R.; et al. Cranberry Arabino-Xyloglucan and Pectic Oligosaccharides Induce Lactobacillus Growth and Short-Chain Fatty Acid Production. Microorganisms 2022, 10, 1346. https://doi.org/10.3390/microorganisms10071346
Hotchkiss AT Jr., Renye JA Jr., White AK, Nunez A, Guron GKP, Chau H, Simon S, Poveda C, Walton G, Rastall R, et al. Cranberry Arabino-Xyloglucan and Pectic Oligosaccharides Induce Lactobacillus Growth and Short-Chain Fatty Acid Production. Microorganisms. 2022; 10(7):1346. https://doi.org/10.3390/microorganisms10071346
Chicago/Turabian StyleHotchkiss, Arland T., Jr., John A. Renye, Jr., Andre K. White, Alberto Nunez, Giselle K. P. Guron, Hoa Chau, Stefanie Simon, Carlos Poveda, Gemma Walton, Robert Rastall, and et al. 2022. "Cranberry Arabino-Xyloglucan and Pectic Oligosaccharides Induce Lactobacillus Growth and Short-Chain Fatty Acid Production" Microorganisms 10, no. 7: 1346. https://doi.org/10.3390/microorganisms10071346






