Exploiting the Native Microorganisms from Different Food Matrices to Formulate Starter Cultures for Sourdough Bread Production
Abstract
:1. Introduction
2. Bread
2.1. Types of Bread
2.1.1. Commercial Bread
2.1.2. Sourdough Bread
3. Wheat Flour
4. Sourdough
4.1. Sourdough Fermentation
4.1.1. Lactic Acid Bacteria (LAB)
4.1.2. Yeast
4.1.3. Biochemical Transformations during Sourdough Fermentation
4.1.4. Fermentation Parameters
- Wheat Flour Type
- Dough Yield
- Temperature
- Fermentation Time
- Backslopping
- Environment
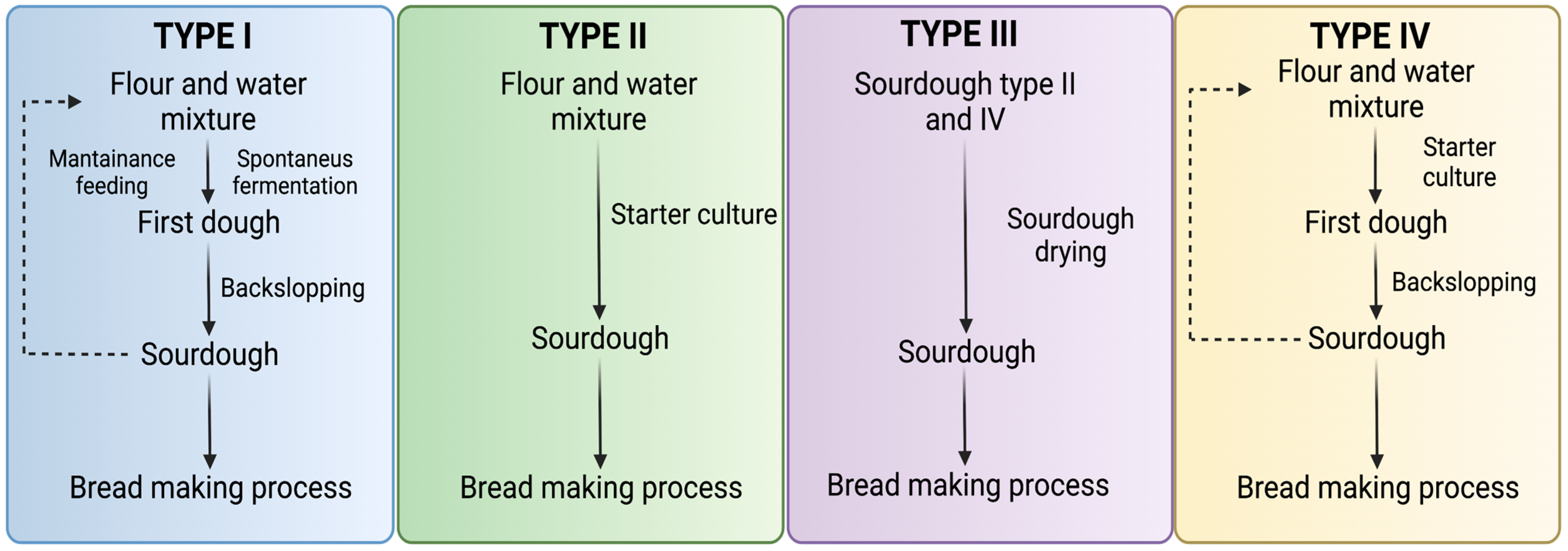
4.2. Classification of Sourdough
Sourdough Types
- Type I
- Type II
- Type III
- Type IV
5. Starter Cultures Used for Sourdough
5.1. Traditional Starter Cultures
5.2. Starter Cultures Formulated with Other Food Matrices Microorganisms
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landis, E.A.; Oliverio, A.M.; McKenney, E.A.; Nichols, L.M.; Kfoury, N.; Biango-Daniels, M.; Shell, L.K.; Madden, A.A.; Shapiro, L.; Sakunala, S.; et al. The diversity and function of sourdough starter microbiomes. eLife 2021, 10, e61644. [Google Scholar] [CrossRef] [PubMed]
- Montanari, C.; Bargossi, E.; Lanciotti, R.; Chinnici, F.; Gardini, F.; Tabanelli, G. Effects of two different sourdoughs on the characteristics of Pandoro, a typical Italian sweet leavened baked good. LWT 2014, 59, 289–299. [Google Scholar] [CrossRef]
- Calvert, M.D.; Madden, A.A.; Nichols, L.M.; Haddad, N.M.; Lahne, J.; Dunn, R.R.; McKenney, E.A. A review of sourdough starters: Ecology, practices, and sensory quality with applications for baking and recommendations for future research. PeerJ 2021, 9, e11389. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Sadiq, F.A.; Yang, H.; Gu, J.; Yuan, L.; Lee, Y.K.; He, G. Predominant yeasts in Chinese traditional sourdough and their influence on aroma formation in Chinese steamed bread. Food Chem. 2018, 242, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Reese, A.T.; Madden, A.A.; Joossens, M.; Lacaze, G.; Dunn, R.R. Influences of ingredients and bakers on the bacteria and fungi in sourdough starters and bread. mSphere 2020, 5, e00950-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nionelli, L.; Rizzello, C.G. Sourdough-based biotechnologies for the production of gluten-free foods. Foods 2016, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Münch, P.; Schieberle, P. Quantitative studies on the formation of key odorants in thermally treated yeast extracts using stable isotope dilution assays. J. Agric. Food Chem. 1998, 46, 4695–4701. [Google Scholar] [CrossRef]
- Raimondi, S.; Amaretti, A.; Rossi, M.; Fall, P.A.; Tabanelli, G.; Gardini, F.; Montanari, C. Evolution of microbial community and chemical properties of a sourdough during the production of Colomba, an Italian sweet leavened baked product. LWT 2017, 86, 31–39. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, H.; Xi, J.; Jin, Y.; Chen, Y.; Guo, L.; Jin, Z.; Xu, X. Improving bread aroma using low-temperature sourdough fermentation. Food Biosci. 2020, 37, 100704. [Google Scholar] [CrossRef]
- Montemurro, M.; Coda, R.; Rizzello, C.G. Recent advances in the use of sourdough biotechnology in pasta making. Foods 2019, 8, 129. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K.; Adesulu-Dahunsi, A.T. Sensory and antioxidant properties and in-vitro digestibility of gluten-free sourdough made with selected starter cultures. LWT 2020, 129, 109576. [Google Scholar] [CrossRef]
- Catzeddu, P. Sourdough breads. In Flour and Breads and Their Fortification in Health and Disease Prevention, 1st ed.; Preedy, V., Watson, R., Patel, V., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 37–46. [Google Scholar]
- Cappelle, S.; Guylaine, L.; Gänzle, M.; Gobbetti, M. History and Social Aspects of Sourdough. In Handbook on Sourdough Biotechnology, 1st ed.; Gobbetti, M., Gänzle, M., Eds.; Springer: NewYork, NY, USA, 2013; pp. 1–10. [Google Scholar]
- Comasio, A.; van Kerrebroeck, S.; Harth, H.; Verté, F.; de Vuyst, L. Potential of bacteria from alternative fermented foods as starter cultures for the production of wheat sourdoughs. Microorganisms 2020, 8, 1534. [Google Scholar] [CrossRef]
- Plessas, S.; Alexopoulos, A.; Mantzourani, I.; Koutinas, A.; Voidarou, C.; Stavropoulou, E.; Bezirtzoglou, E. Application of novel starter cultures for sourdough bread production. Anaerobe 2011, 17, 486–489. [Google Scholar] [CrossRef]
- Graça, C.; Edelmann, M.; Raymundo, A.; Sousa, I.; Coda, R.; Sontag-Strohm, T.; Huang, X. Yoghurt as a starter in sourdough fermentation to improve the technological and functional properties of sourdough-wheat bread. J. Funct. Foods 2022, 88, 104877. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Jagelaviciute, J.; Stankevicius, M.; Maruska, A. Thermophilic lactic acid bacteria affect the characteristics of sourdough and whole-grain wheat bread. Food Biosci. 2020, 38, 100791. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Ceoromila (Cantaragiu), A.-M.; Vasile, M.A.; Bahrim, G.-E. Novel insights into different kefir grains usefulness as valuable multiple starter cultures to achieve bioactive gluten-free sourdoughs. LWT 2022, 165, 113670. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ciudad-Mulero, M.; Fernández-Ruiz, V.; Ferreira, E.; Heleno, S.; Rodrigues, P.; Barros, L.; Ferreira, I.C.F.R. Comparison of different bread types: Chemical and physical parameters. Food Chem. 2020, 310, 125954. [Google Scholar] [CrossRef]
- Cauvain, S.P. Bread—The Product. In Technology of Breadmaking, 3rd ed.; Cauvain, S.P., Young, L.S., Eds.; Springer: Boston, MA, USA, 2007; pp. 1–17. [Google Scholar]
- Hermans, W.; Mutlu, S.; Michalski, A.; Langenaeken, N.A.; Courtin, C.M. The Contribution of sub-aleurone cells to wheat endosperm protein content and gradient is dependent on cultivar and n-fertilization level. J. Agric. Food Chem. 2021, 69, 6444–6454. [Google Scholar] [CrossRef]
- Gartaula, G.; Dhital, S.; Netzel, G.; Flanagan, B.M.; Yakubov, G.E.; Beahan, C.T.; Collins, H.M.; Burton, R.A.; Bacic, A.; Gidley, M.J. Quantitative structural organisation model for wheat endosperm cell walls: Cellulose as an important constituent. Carbohydr. Polym. 2018, 196, 199–208. [Google Scholar] [CrossRef]
- Finnie, S.; Atwell, W.A. Wheat Flour, 2nd ed.; Atwell, W., Finnie, S., Eds.; Woodhead Publishing: Sawston, UK; AACC International Press: St. Paul, MN, USA, 2016. [Google Scholar]
- Surget, A.; Barron, C. Histologie du grain de blé. Ind. Céréales 2005, 145, 3–7. [Google Scholar]
- De Vuyst, L.; van Kerrebroeck, S.; Leroy, F. Microbial ecology and process technology of sourdough fermentation. Adv. Appl. Microbiol. 2017, 100, 49–160. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications—A review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Effect of small granules in potato starch and wheat starch on quality changes of direct heated surimi gels after freezing. Food Hydrocoll. 2020, 104, 105732. [Google Scholar] [CrossRef]
- Su, C.; Saleh, A.S.M.; Zhang, B.; Zhao, K.; Ge, X.; Zhang, Q.; Li, W. Changes in structural, physicochemical, and digestive properties of normal and waxy wheat starch during repeated and continuous annealing. Carbohydr. Polym. 2020, 247, 116675. [Google Scholar] [CrossRef]
- Hong, J.; An, D.; Li, L.; Liu, C.; Li, M.; Buckow, R.; Zheng, X.; Bian, K. Structural, rheological and gelatinization properties of wheat starch granules separated from different noodle-making process. J. Cereal Sci. 2020, 91, 102897. [Google Scholar] [CrossRef]
- Rocha, J.M.; Kalo, P.J.; Malcata, F.X. Composition of neutral lipid classes and content of fatty acids throughout sourdough breadmaking. Eur. J. Lipid Sci. Technol. 2012, 114, 294–305. [Google Scholar] [CrossRef]
- Janssen, F.; Wouters, A.G.B.; Pareyt, B.; Gerits, L.R.; Delcour, J.A.; Waelkens, E.; Derua, R. Wheat (Triticum aestivum L.) lipid species distribution in the different stages of straight dough bread making. Food Res. Int. 2018, 112, 299–311. [Google Scholar] [CrossRef]
- Kumar, A.; Nayak, R.; Purohit, S.R.; Rao, P.S. Impact of UV-C Irradiation on solubility of Osborne protein fractions in wheat flour. Food Hydrocoll. 2021, 110, 105845. [Google Scholar] [CrossRef]
- Roussel, P.; Onno, B.; Michel, E.; Sicard, D. La Panification au Levain Naturel, 1st ed.; Roussel, P., Onno, B., Michel, E., Sicard, D., Eds.; Éditions Quae: Versailles, France, 2020. [Google Scholar]
- Bonnot, T.; Bancel, E.; Alvarez, D.; Davanture, M.; Boudet, J.; Pailloux, M.; Zivy, M.; Ravel, C.; Martre, P. Grain subproteome responses to nitrogen and sulfur supply in diploid wheat Triticum monococcum ssp. monococcum. Plant J. 2017, 91, 894–910. [Google Scholar] [CrossRef] [Green Version]
- Shumoy, H.; van Bockstaele, F.; Devecioglu, D.; Raes, K. Effect of sourdough addition and storage time on in vitro starch digestibility and estimated glycemic index of tef bread. Food Chem. 2018, 264, 34–40. [Google Scholar] [CrossRef]
- Mondal, S.; Hays, D.B.; Alviola, N.J.; Mason, R.E.; Tilley, M.; Waniska, R.D.; Bean, S.R.; Glover, K.D. Functionality of gliadin proteins in wheat flour tortillas. J. Agric. Food Chem. 2009, 57, 1600–1605. [Google Scholar] [CrossRef]
- Ooms, N.; Delcour, J.A. How to impact gluten protein network formation during wheat flour dough making. Curr. Opin. Food Sci. 2019, 25, 88–97. [Google Scholar] [CrossRef]
- Urade, R.; Sato, N.; Sugiyama, M. Gliadins from wheat grain: An overview, from primary structure to nanostructures of aggregates. Biophys. Rev. 2018, 10, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Uthayakumaran, S.; Tömösközi, S.; Tatham, A.S.; Savage, A.W.J.; Gianibelli, M.C.; Stoddard, F.L.; Bekes, F. Effects of gliadin fractions on functional properties of wheat dough depending on molecular size and hydrophobicity. Cereal Chem. 2001, 78, 138–141. [Google Scholar] [CrossRef]
- Noma, S.; Hayakawa, K.; Abe, C.; Suzuki, S.; Kawaura, K. Contribution of α-gliadin alleles to the extensibility of flour dough in japanese wheat cultivars. J. Cereal Sci. 2019, 86, 15–21. [Google Scholar] [CrossRef]
- Dupont, F.M.; Altenbach, S.B. Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J. Cereal Sci. 2003, 38, 133–146. [Google Scholar] [CrossRef]
- Yoo, M.S.; Lee, Y.T. Pasting properties of crude ss-glucan from spent brewer’s yeast on wheat flour and starch. Food Sci. Biotechnol. 2007, 16, 485–488. [Google Scholar]
- Ma, S.; Wang, Z.; Guo, X.; Wang, F.; Huang, J.; Sun, B.; Wang, X. Sourdough improves the quality of whole-wheat flour products: Mechanisms and challenges—A review. Food Chem. 2021, 360, 130038. [Google Scholar] [CrossRef]
- Lau, S.W.; Chong, A.Q.; Chin, N.L.; Talib, R.A.; Basha, R.K. Sourdough microbiome comparison and benefits. Microorganisms 2021, 9, 1355. [Google Scholar] [CrossRef]
- Sevgili, A.; Erkmen, O.; Koçaslan, S. Identification of lactic acid bacteria and yeasts from traditional sourdoughs and sourdough production by enrichment. Czech J. Food Sci. 2021, 39, 312–318. [Google Scholar] [CrossRef]
- Syrokou, M.K.; Stasinopoulou, P.; Paramithiotis, S.; Bosnea, L.; Mataragas, M.; Papadopoulos, G.K.; Skandamis, P.N.; Drosinos, E.H. The effect of incubation temperature, substrate and initial pH value on plantaricin activity and the relative transcription of PLN genes of six sourdough derived Lactiplantibacillus plantarum strains. Fermentation 2021, 7, 320. [Google Scholar] [CrossRef]
- Dos Santos, J.G.; de Ávila, P.M.; Schimitberger, R.; da Cunha, L.R.; Gomes, R.A.B.; Vieira, M.C.; de Souza Monteiro, R.; Vieira, S.M.; Pereira, P.A.P. Evaluation of the effect of substrates and types of wheat flour on microbiological characteristics, pH values, levels of total phenolic compounds, antioxidant capacity and fermentative capacity of sourdough. Res. Soc. Dev. 2022, 11, e13211932401. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Hajinia, F.; Sadeghi, A.; Sadeghi Mahoonak, A. The use of antifungal oat-sourdough lactic acid bacteria to improve safety and technological functionalities of the supplemented wheat bread. J. Food Saf. 2021, 41, e12873. [Google Scholar] [CrossRef]
- Oshiro, M.; Zendo, T.; Nakayama, J. Diversity and dynamics of sourdough lactic acid bacteriota created by a slow food fermentation system. J. Biosci. Bioeng. 2021, 131, 333–340. [Google Scholar] [CrossRef]
- Rocken, W.; Voysey, P.A. Sourdough fermentation in bread making. J. Appl. Bacteriol. Suppl. 1995, 79, 38S–48S. [Google Scholar]
- Jekle, M.; Houben, A.; Mitzscherling, M.; Becker, T. Effects of selected lactic acid bacteria on the characteristics of amaranth sourdough. J. Sci. Food Agric. 2010, 90, 2326–2332. [Google Scholar] [CrossRef]
- Corsetti, A. Technology of Sourdough Fermentation and Sourdough Applications. In Handbook on Sourdough Biotechnology, 1st ed.; Gobbetti, M., Gänzle, M., Eds.; Springer: New York, NY, USA, 2013; pp. 85–103. ISBN 9781461454250. [Google Scholar]
- Suo, B.; Chen, X.; Wang, Y. Recent research advances of lactic acid bacteria in sourdough: Origin, diversity, and function. Curr. Opin. Food Sci. 2021, 37, 66–75. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [Green Version]
- Oshiro, M.; Tanaka, M.; Momoda, R.; Zendo, T.; Nakayama, J. Mechanistic insight into yeast bloom in a lactic acid bacteria relaying-community in the start of sourdough microbiota evolution. Microbiol. Spectr. 2021, 9, e00662-21. [Google Scholar] [CrossRef]
- Demirgul, F.; Simsek, O.; Sagdic, O. Amino acid, mineral, vitamin B contents and bioactivities of extracts of yeasts isolated from sourdough. Food Biosci. 2022, 50, 102040. [Google Scholar] [CrossRef]
- Aydın, F.; Özer, G.; Alkan, M.; Çakır, İ. Start codon targeted (SCoT) markers for the assessment of genetic diversity in yeast isolated from Turkish sourdough. Food Microbiol. 2022, 107, 104081. [Google Scholar] [CrossRef]
- Yang, Q.; Rutherfurd-Markwick, K.; Mutukumira, A.N. Identification of dominant lactic acid bacteria and yeast in rice sourdough produced in New Zealand. Curr. Res. Food Sci. 2021, 4, 729–736. [Google Scholar] [CrossRef]
- Jin, J.; Nguyen, T.T.H.; Humayun, S.; Park, S.H.; Oh, H.; Lim, S.; Mok, I.K.; Li, Y.; Pal, K.; Kim, D. Characteristics of sourdough bread fermented with Pediococcus pentosaceus and Saccharomyces cerevisiae and its bio-preservative effect against Aspergillus flavus. Food Chem. 2021, 345, 128787. [Google Scholar] [CrossRef]
- Pino, A.; Russo, N.; Solieri, L.; Sola, L.; Caggia, C.; Randazzo, C.L. Microbial consortia involved in traditional Sicilian sourdough: Characterization of lactic acid bacteria and yeast populations. Microorganisms 2022, 10, 283. [Google Scholar] [CrossRef]
- Oshiro, M.; Momoda, R.; Tanaka, M.; Zendo, T.; Nakayama, J. Dense tracking of the dynamics of the microbial community and chemicals constituents in spontaneous wheat sourdough during two months of backslopping. J. Biosci. Bioeng. 2019, 128, 170–176. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, L.; Qian, H.; Zhang, H.; Qi, X. Contribution of spontaneously-fermented sourdoughs with pear and navel orange for the bread making. LWT 2018, 89, 336–343. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Bi, Y.; Zhao, R.; Nie, Y.; Yuan, W. Dynamics of microbial community and changes of metabolites during production of type I sourdough steamed bread made by retarded sponge-dough method. Food Chem. 2020, 330, 127316. [Google Scholar] [CrossRef]
- Fujimoto, A.; Ito, K.; Narushima, N.; Miyamoto, T. Identification of lactic acid bacteria and yeasts, and characterization of food components of sourdoughs used in Japanese bakeries. J. Biosci. Bioeng. 2019, 127, 575–581. [Google Scholar] [CrossRef]
- Lhomme, E.; Urien, C.; Legrand, J.; Dousset, X.; Onno, B.; Sicard, D. Sourdough microbial community dynamics: An analysis during French organic bread making processes. Food Microbiol. 2016, 53, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Yaǧmur, G.; Tanguler, H.; Leventdurur, S.; Elmaci, S.B.; Turhan, E.Ü.; Francesca, N.; Settanni, L.; Moschetti, G.; Erten, H. Identification of predominant lactic acid bacteria and yeasts of Turkish sourdoughs and selection of starter cultures for liquid sourdough production using different flours and dough yields. Pol. J. Food Nutr. Sci. 2016, 66, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Yan, B.; Sadiq, F.A.; Cai, Y.; Fan, D.; Chen, W.; Zhang, H.; Zhao, J. Microbial diversity in traditional type I sourdough and Jiaozi and its influence on volatiles in Chinese steamed bread. LWT 2019, 101, 764–773. [Google Scholar] [CrossRef]
- Karaman, K.; Sagdic, O.; Durak, M.Z. Use of phytase active yeasts and lactic acid bacteria isolated from sourdough in the production of whole wheat bread. LWT 2018, 91, 557–567. [Google Scholar] [CrossRef]
- Korcari, D.; Secchiero, R.; Laureati, M.; Marti, A.; Cardone, G.; Rabitti, N.S.; Ricci, G.; Fortina, M.G. Technological properties, shelf life and consumer preference of spelt-based sourdough bread using novel, selected starter cultures. LWT 2021, 151, 112097. [Google Scholar] [CrossRef]
- Von Gastrow, L.; Amelot, R.; Segond, D.; Guézennec, S.; Valence, F.; Sicard, D. Microbial community dispersal in sourdough. bioRxiv 2021. [Google Scholar] [CrossRef]
- Palla, M.; Cristani, C.; Giovannetti, M.; Agnolucci, M. Identification and characterization of lactic acid bacteria and yeasts of PDO Tuscan bread sourdough by culture dependent and independent methods. Int. J. Food Microbiol. 2017, 250, 19–26. [Google Scholar] [CrossRef]
- Harth, H.; van Kerrebroeck, S.; de Vuyst, L. Community dynamics and metabolite target analysis of spontaneous, backslopped barley sourdough fermentations under laboratory and bakery conditions. Int. J. Food Microbiol. 2016, 228, 22–32. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Maes, D.; de Vuyst, L. Sourdoughs as a function of their species diversity and process conditions, a meta-analysis. Trends Food Sci. Technol. 2017, 68, 152–159. [Google Scholar] [CrossRef]
- Reale, A.; di Stasio, L.; di Renzo, T.; de Caro, S.; Ferranti, P.; Picariello, G.; Addeo, F.; Mamone, G. Bacteria do it better! Proteomics suggests the molecular basis for improved digestibility of sourdough products. Food Chem. 2021, 359, 129955. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Gioulatos, S.; Tsakalidou, E.; Kalantzopoulos, G. Interactions between Saccharomyces cerevisiae and lactic acid bacteria in sourdough. Process Biochem. 2006, 41, 2429–2433. [Google Scholar] [CrossRef]
- Rogalski, E.; Ehrmann, M.A.; Vogel, R.F. Role of Kazachstania humilis and Saccharomyces cerevisiae in the strain-specific assertiveness of Fructilactobacillus sanfranciscensis strains in rye sourdough. Eur. Food Res. Technol. 2020, 246, 1817–1827. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Vermeulen, N.; Vogel, R.F. Carbohydrate, peptide and lipid metabolism of lactic acid bacteria in sourdough. Food Microbiol. 2007, 24, 128–138. [Google Scholar] [CrossRef]
- Petkova, M.; Stefanova, P.; Gotcheva, V.; Angelov, A. Isolation and characterization of lactic acid bacteria and yeasts from typical Bulgarian sourdoughs. Microorganisms 2021, 9, 1346. [Google Scholar] [CrossRef]
- Amapu, T.; Ameh, J.; Ado, S.; Abdullahi, I.; Dapiya, H. Amylolytic potential of lactic acid bacteria isolated from wet milled cereals, cassava flour and fruits. Br. Microbiol. Res. J. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Reddy, G.; Altaf, M.; Naveena, B.J.; Venkateshwar, M.; Kumar, E.V. Amylolytic bacterial lactic acid fermentation—A review. Biotechnol. Adv. 2008, 26, 22–34. [Google Scholar] [CrossRef]
- Kanpiengjai, A.; Lumyong, S.; Nguyen, T.H.; Haltrich, D.; Khanongnuch, C. Characterization of a maltose-forming α-amylase from an amylolytic lactic acid bacterium Lactobacillus plantarum S21. J. Mol. Catal. B Enzym. 2015, 120, 1–8. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K.; Stoyancheva, G. Starch-modifying enzymes of lactic acid bacteria—Structures, properties, and applications. Starch 2013, 65, 34–47. [Google Scholar] [CrossRef]
- Rodríguez-Sanoja, R.; Ruiz, B.; Guyot, J.P.; Sanchez, S. Starch-binding domain affects catalysis in two Lactobacillus α-amylases. Appl. Environ. Microbiol. 2005, 71, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez Sanoja, R.; Morlon-Guyot, J.; Jore, J.; Pintado, J.; Juge, N.; Guyot, J.P. Comparative characterization of complete and truncated forms of Lactobacillus amylovorus α-amylase and role of the C-terminal direct repeats in raw-starch binding. Appl. Environ. Microbiol. 2000, 66, 3350–3356. [Google Scholar] [CrossRef] [Green Version]
- Giraud, E.; Gosselin, L.; Marin, B.; Parada, J.L.; Raimbault, M. Purification and characterization of an extracellular amylase from Lactobacillus plantarum strain A6. J. Appl. Bacteriol. 1993, 75, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, G.; Morlon-Guyot, J.; Trejo-Aguilar, B.; Guyot, J.P. Purification and characterization of an extracellular α-amylase produced by Lactobacillus manihotivorans LMG 18010(T), an amylolytic lactic acid bacterium. Enzyme Microb. Technol. 2000, 27, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. From microbial ecology to innovative applications in food quality improvements: The case of sourdough as a model matrix. J—Multidiscip. Sci. J. 2020, 3, 9–19. [Google Scholar] [CrossRef]
- Zotta, T.; Piraino, P.; Ricciardi, A.; McSweeney, P.L.H.; Parente, E. Proteolysis in model sourdough fermentations. J. Agric. Food Chem. 2006, 54, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; de Angelis, M.; di Cagno, R.; Rizzello, C.G. Sourdough/Lactic Acid Bacteria. In Gluten-Free Cereal Products and Beverages, 1st ed.; Arendt, E.K., Dal Bello, F., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 267–288. ISBN 9780123737397. [Google Scholar]
- Di Cagno, R.; de Angelis, M.; Lavermicocca, P.; de Vincenzi, M.; Giovannini, C.; Faccia, M.; Gobbetti, M. Proteolysis by sourdough lactic acid bacteria: Effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl. Environ. Microbiol. 2002, 68, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Zoumpopoulou, G.; Georgalaki, M.; Alexandraki, V.; Kazou, M.; Anastasiou, R.; Tsakalidou, E. Sourdough Bread. In Innovations in Traditional Foods, 1st ed.; Galanakis, C.M., Ed.; Woodhead Publishing: Duxford, UK, 2019; pp. 127–158. ISBN 9780128148884. [Google Scholar]
- Boudaoud, S.; Aouf, C.; Devillers, H.; Sicard, D.; Segond, D. Sourdough yeast-bacteria interactions can change ferulic acid metabolism during fermentation. Food Microbiol. 2021, 98, 103790. [Google Scholar] [CrossRef]
- Xu, D.; Yin, Y.; Ali, B.; Zhang, Y.; Guo, L.; Xu, X. Isolation of yeast strains from Chinese liquor Daqu and its use in the wheat sourdough bread making. Food Biosci. 2019, 31, 100443. [Google Scholar] [CrossRef]
- Pétel, C.; Onno, B.; Prost, C. Sourdough volatile compounds and their contribution to bread: A review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Plessas, S. Innovations in sourdough bread making. Fermentation 2021, 7, 29. [Google Scholar] [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty years of knowledge on sourdough fermentation: A systematic review. Trends Food Sci. Technol. 2021, 108, 71–83. [Google Scholar] [CrossRef]
- Zahra, A.; Farooq, U.; Saeed, M.T.; Quddoos, M.Y.; Hameed, A.; Iftikhar, M.; Noreen, A.; Mahvish, S.; Bukhari, S.R.; Naqvi, S.N.; et al. Enhancement of sensory attributes and mineral content of sourdough bread by means of microbial culture and yeast (Saccharomyces cerevisiae). Food Chem. Adv. 2022, 1, 100094. [Google Scholar] [CrossRef]
- Minervini, F.; Celano, G.; Lattanzi, A.; de Angelis, M.; Gobbetti, M. Added ingredients affect the microbiota and biochemical characteristics of durum wheat type-I sourdough. Food Microbiol. 2016, 60, 112–123. [Google Scholar] [CrossRef]
- Gobbetti, M.; de Angelis, M.; di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Martín-Garcia, A.; Riu-Aumatell, M.; López-Tamames, E. Influence of process parameters on sourdough microbiota, physical properties and sensory profile. Food Rev. Int. 2021, 37, 1–15. [Google Scholar] [CrossRef]
- Siepmann, F.B.; Ripari, V.; Waszczynskyj, N.; Spier, M.R. Overview of sourdough technology: From production to marketing. Food Bioprocess Technol. 2018, 11, 242–270. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Cini, E. A Systematic review of gluten-free dough and bread: Dough rheology, bread characteristics, and improvement strategies. Appl. Sci. 2020, 10, 6559. [Google Scholar] [CrossRef]
- Comasio, A.; Verce, M.; van Kerrebroeck, S.; de Vuyst, L. Diverse microbial composition of sourdoughs from different origins. Front. Microbiol. 2020, 11, 1212. [Google Scholar] [CrossRef]
- Palla, M.; Agnolucci, M.; Calzone, A.; Giovannetti, M.; di Cagno, R.; Gobbetti, M.; Rizzello, C.G.; Pontonio, E. Exploitation of autochthonous Tuscan sourdough yeasts as potential starters. Int. J. Food Microbiol. 2019, 302, 59–68. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; de Marco, I.; Neves Oliveira dos Santos, N.; Costa Nunes, C.; Leite Cartabiano, C.E.; Molognoni, L.; de Melo Pereira, G.V.; Daguer, H.; de Dea Lindner, J. Reducing FODMAPs and improving bread quality using type II sourdough with selected starter cultures. Int. J. Food Sci. Nutr. 2021, 72, 912–922. [Google Scholar] [CrossRef]
- Voinea, A.; Codină, G.G. Effect of Dry Sourdough addition in wheat flour on dynamic rheological properties and bread quality. J. Agroaliment. Process. Technol. 2021, 27, 9–14. [Google Scholar]
- Albagli, G.; do Monte Schwartz, I.; Amaral, P.F.F.; Ferreira, T.F.; Finotelli, P.V. How dried sourdough starter can enable and spread the use of sourdough bread. LWT 2021, 149, 111888. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Hussain, R.; Kubow, S.; Sadiq, F.A.; Huang, W.; Imran, M. Sourdough bread: A contemporary cereal fermented product. J. Food Process. Preserv. 2019, 43, e13883. [Google Scholar] [CrossRef]
- Ventimiglia, G.; Alfonzo, A.; Galluzzo, P.; Corona, O.; Francesca, N.; Caracappa, S.; Moschetti, G.; Settanni, L. Codominance of Lactobacillus plantarum and obligate heterofermentative lactic acid bacteria during sourdough fermentation. Food Microbiol. 2015, 51, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, V.; Osimani, A.; Garofalo, C.; Belleggia, L.; Maoloni, A.; Cardinali, F.; Mozzon, M.; Foligni, R.; Aquilanti, L.; Clementi, F. Selection of cereal-sourced lactic acid bacteria as candidate starters for the baking industry. PLoS ONE 2020, 15, e0236190. [Google Scholar] [CrossRef]
- Denkova, R.; Ilieva, S.; Denkova, Z.; Georgieva, L.; Krastanov, A. Examination of the technological properties of newly isolated strains of the genus Lactobacillus and possibilities for their application in the composition of starters. Biotechnol. Biotechnol. Equip. 2014, 28, 487–494. [Google Scholar] [CrossRef]
- Falasconi, I.; Fontana, A.; Patrone, V.; Rebecchi, A.; Garrido, G.D.; Principato, L.; Callegari, M.L.; Spigno, G.; Morelli, L. Genome-Assisted characterization of Lactobacillus fermentum, Weissella cibaria, and Weissella confusa strains isolated from sorghum as starters for sourdough fermentation. Microorganisms 2020, 8, 1388. [Google Scholar] [CrossRef]
- Medina-Pradas, E.; Pérez-Díaz, I.M.; Garrido-Fernández, A.; Arroyo-López, F.N. Review of vegetable fermentations with particular emphasis on processing modifications, microbial ecology, and spoilage. In The Microbiological Quality of Food: Foodborne Spoilers; Woodhead Publishing: Sawston, UK, 2017; pp. 211–236. ISBN 9780081005033. [Google Scholar]
- Moroni, A.V.; Iametti, S.; Bonomi, F.; Arendt, E.K.; Dal Bello, F. Solubility of proteins from non-gluten cereals: A comparative study on combinations of solubilising agents. Food Chem. 2010, 121, 1225–1230. [Google Scholar] [CrossRef]
- Coda, R.; di Cagno, R.; Edema, M.O.; Nionelli, L.; Gobbetti, M. Exploitation of Acha (Digitaria exiliis) and Iburu (Digitaria Iburua) flours: Chemical characterization and their use for sourdough fermentation. Food Microbiol. 2010, 27, 1043–1050. [Google Scholar] [CrossRef]
- Carbó, R.; Gordún, E.; Fernández, A.; Ginovart, M. Elaboration of a spontaneous gluten-free sourdough with a mixture of amaranth, buckwheat, and quinoa flours analyzing microbial load, acidity, and pH. Food Sci. Technol. Int. 2020, 26, 344–352. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Arendt, E.; Gänzle, M. Exopolysaccharide-forming Weissella strains as starter cultures for sorghum and wheat sourdoughs. J. Agric. Food Chem. 2010, 58, 5834–5841. [Google Scholar] [CrossRef]
- Katina, K.; Maina, N.H.; Juvonen, R.; Flander, L.; Johansson, L.; Virkki, L.; Tenkanen, M.; Laitila, A. In Situ production and analysis of Weissella confusa dextran in wheat sourdough. Food Microbiol. 2009, 26, 734–743. [Google Scholar] [CrossRef]
- Di Cagno, R.; Rizzello, C.G.; de Angelis, M.; Cassone, A.; Giuliani, G.; Benedusi, A.; Limitone, A.; Surico, R.F.; Gobbetti, M. Use of selected sourdough strains of Lactobacillus for removing gluten and enhancing the nutritional properties of gluten-free bread. J. Food Prot. 2008, 71, 1491–1495. [Google Scholar] [CrossRef]
- De Vuyst, L.; van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vancanneyt, M. Biodiversity and identification of sourdough lactic acid bacteria. Food Microbiol. 2007, 24, 120–127. [Google Scholar] [CrossRef]
- Siepmann, F.B.; Sousa de Almeida, B.; Waszczynskyj, N.; Spier, M.R. Influence of temperature and of starter culture on biochemical characteristics and the aromatic compounds evolution on Type II sourdough and wheat bread. LWT 2019, 108, 199–206. [Google Scholar] [CrossRef]
- Ehrmann, M.A.; Brandt, M.; Stolz, P.; Vogel, R.F.; Korakli, M. Lactobacillus secaliphilus sp. Nov., isolated from Type II sourdough fermentation. Int. J. Syst. Evol. Microbiol. 2007, 57, 745–750. [Google Scholar] [CrossRef]
- Gaggiano, M.; di Cagno, R.; de Angelis, M.; Arnault, P.; Tossut, P.; Fox, P.F.; Gobbetti, M. Defined multi-species semi-liquid ready-to-use sourdough starter. Food Microbiol. 2007, 24, 15–24. [Google Scholar] [CrossRef]
- Burns, P.; Borgo, M.F.; Binetti, A.; Puntillo, M.; Bergamini, C.; Páez, R.; Mazzoni, R.; Reinheimer, J.; Vinderola, G. Isolation, characterization and performance of autochthonous spray dried lactic acid bacteria in maize micro and bucket-silos. Front. Microbiol. 2018, 9, 2861. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Chouliaras, Y.; Tsakalidou, E.; Kalantzopoulos, G. Application of selected starter cultures for the production of wheat sourdough bread using a traditional three-stage procedure. Process Biochem. 2005, 40, 2813–2819. [Google Scholar] [CrossRef]
- Sidari, R.; Martorana, A.; Zappia, C.; Mincione, A.; Giuffrè, A.M. Persistence and effect of a multistrain starter culture on antioxidant and rheological properties of novel wheat sourdoughs and bread. Foods 2020, 9, 1258. [Google Scholar] [CrossRef] [PubMed]
- Fekri, A.; Torbati, M.; Yari Khosrowshahi, A.; Bagherpour Shamloo, H.; Azadmard-Damirchi, S. Functional effects of phytate-degrading, probiotic lactic acid bacteria and yeast strains isolated from Iranian traditional sourdough on the technological and nutritional properties of whole wheat bread. Food Chem. 2020, 306, 125620. [Google Scholar] [CrossRef] [PubMed]
- Boyaci Gunduz, C.P.; Agirman, B.; Gaglio, R.; Franciosi, E.; Francesca, N.; Settanni, L.; Erten, H. Evaluation of the variations in chemical and microbiological properties of the sourdoughs produced with selected lactic acid bacteria strains during fermentation. Food Chem. X 2022, 14, 100357. [Google Scholar] [CrossRef] [PubMed]
- Limbad, M.; Maddox, N.G.; Hamid, N.; Kantono, K. Sensory and physicochemical characterization of sourdough bread prepared with a coconut water kefir starter. Foods 2020, 9, 1165. [Google Scholar] [CrossRef]
- Choi, H.; Kim, Y.W.; Hwang, I.; Kim, J.; Yoon, S. Evaluation of Leuconostoc citreum HO12 and Weissella koreensis HO20 isolated from kimchi as a starter culture for whole wheat sourdough. Food Chem. 2012, 134, 2208–2216. [Google Scholar] [CrossRef]
- Decimo, M.; Quattrini, M.; Ricci, G.; Fortina, M.G.; Brasca, M.; Silvetti, T.; Manini, F.; Erba, D.; Criscuoli, F.; Casiraghi, M.C. Evaluation of microbial consortia and chemical changes in spontaneous maize bran fermentation. AMB Express 2017, 7, 205. [Google Scholar] [CrossRef]



| Protein | Percentage (% w/w) |
|---|---|
| Albumins | 5–15 |
| Globulins | 5–10 |
| Prolamins (gliadins) | 40–50 |
| Glutelins (glutenins) | 30–40 |
| Country/ Region | Sourdough Type * | Lactic Acid Bacteria (LAB) | Yeast | References |
|---|---|---|---|---|
| Japan /Asia | Type II | Lc. citreum, L. lactis, W. confusa, W. cibaria, Lpb. plantarum, Lpb. paraplantarum, Lvb. brevis | W. anomalus, Ks. unispora, S. cerevisiae | [63] |
| China /Asia | Type II | Lat. curvatus, Ped. pentosaceus, Lvb. brevis, Lpb. plantarum, Lc. mesenteroides, Flb. rossiae | S. cerevisiae | [64] |
| China /Asia | Type I | F. sanfranciscensis, Lim. pontis | S. cerevisiae, C. humilis | [65] |
| Italy /Europe | Type I | Lvb. brevis, Lpb. plantarum, Lcb. rhamnosus | W. anomalus, S. cerevisiae, T. delbruekii, P. kluyveri, C. boidinii, C. diddensiae | [62] |
| Japan /Asia | Type I | Lvb. brevis, Co. alimentarius, Lpb. pentosus | S. cerevisiae, C. humilis | [66] |
| France /Europe | Type I | F. sanfranciscensis, Lpb. plantarum, Co. kimchi, Lat. sakei, Lev. hamesii, Lpb. pentosus | K. bulderi, C. humilis, K. unispora, T. delbruekii, R. mucilaginosa, C. carpophila, S. cerevisiae, H. pseudoburtonii | [67] |
| Turkey /Asia | Type I | Lpb. plantarum, F. sanfranciscensis, Lev. spicheri, Flb. rossiae, Lev. namurensis, Lev. zymae, Lcb. casei, Co. mindensis, Lb. acetotolerans, Co. farciminis, Co. paralimentarius, Ped. pentosaceus, E. durans, E. faecium, Lc. mesenteroides, W. confusa | S. cerevisiae, P. guillermondii, T. delbrueckii, C. parapsilosis, C. pararugosa | [68] |
| China /Asia | Type I | F. sanfranciscensis, W. cibaria, Lim. fermentum, Lpb. plantarum, Lim. pontis, Co. paralimentarius | S. cerevisiae, C. humilis, W. anomalus | [69] |
| Turkey /Asia | Type I | W. viridescens, Ped. pentosaceus, Ped. acidilactici, Lvb. brevis, Len. parabuchneri | S. cerevisiae, P. membranifaciens | [70] |
| Italy /Europe | Type I | Ped. pentosaceus, Lat. curvatus, Lvb. brevis, Lim. fermentum, Lpb. plantarum, Ped. acidilactici | W. anomalus, P. fermentans, C. lusitaniae, S. cerevisiae | [71] |
| France /Europe | Type I | F. sanfranciscensis, Co. paralimentarius, Lvb. brevis | S. cerevisiae, K. humilis, K. bulderi | [72] |
| Italy/Europe | Type I | Lat. curvatus, F. sanfranciscensis, Lc. citreum, Lc. mesenteroides, Lc. pseudomesenteroides, Ped. pentosaceus, Lev. acidifarinae | C. humilis, T. delbrueckii, S. cerevisiae, K. marxianus | [8] |
| Italy/Europe | Type I | F. sanfranciscensis | C. milleri, S. cerevisiae | [73] |
| Belgium/Europe | Type II | Lim. fermentum, Lpb. plantarum, Lvb. brevis, W. confusa, Ped. pentosaceus | S. cerevisiae | [74] |
| Belgium/Europe | Type I | F. fructivorans, Lpb. plantarum, Lim. reuteri, Lb. delbrueckii, Lc. spp., Weisella | C. humilis, S. cerevisiae, K. sp, P. kudriavzevii | [75] |
| Type II | Lpb. plantarum, Lc. spp., Lim. reuteri, Lb. delbrueckii | S. cerevisiae, W. anomalus, S. bayanus, T. delbrueckii | ||
| Italy/Europe | Type I | F. sanfranciscensis, W. cibaria, Lpb. plantarum, Lim. reuteri, Lim. pontis | S. cerevisiae, K. exigua | [76] |
| Food Matrix Used | Microorganisms | Inoculum Size | Main Results | References |
|---|---|---|---|---|
| Yogurt | S. thermophilus and Lb. delbrueckii subsp. bulgaricus | 40% (w/w) | Enhanced bread has bread crumb softness, retarded staling, and increased antioxidant activity compared with yeast-sourdough | [16] |
| Corn bran | Two consortia: (1) K. unispora + W. cibaria+ Ped. pentosaceus and (2) S. cerevisiae (commercial) + W. cibaria + Ped. pentosaceus | 6 log UFC/mL for all microorganisms except for W. cibaria | Spelt-sourdough bread obtained with the consortium (2) had a superior crumb texture that was maintained during five days of storage and has better consumer acceptation. Both consortia improved shelf life by preventing the growth of common cereal-contaminating fungi | [71] |
| Coconut water kefir | Lim. fermentum with and without yeast Lpb. plantarum with and without yeast | 4.90 and 8.30 log UFC/mL 5.00 and 9.69 log UFC/mL | Sourdough bread inoculated with Lpb. plantarum at 9.60 log CFU/mL without yeast and fermented during 24 h showed a higher concentration of organic acids and amino acid, and better quality in terms of taste, shelf life, and texture | [133] |
| Cocoa bean fermentation, fermented sausage and water kefir | Lim. fermentum IMDO 222 (cocoa bean fermentation) Lat. sakei CTC 494 (fermented sausage) Acetobacter pasteurianus IMDO 386B and Gluconobacter oxydans IMDO A845 | 6–7 log UFC/mL of wheat flour-water mixture | Lim. fermentum IMDO 222 from cocoa bean fermentation and Lat. sakei CTC 494 from fermented sausage were potential starters for sourdough, as well AAB strains (A. pasteurianus IMDO 386B and Gluconobacter oxydans IMDO A845), both strains from cocoa bean fermentation), due to their competitiveness in the dough. | [14] |
| Water (WKG1, WKG2) and milk (MKG) kefir grains | Len. kefiri and P. acidilactici strains isolated from MKG | 0.20% (w/w) | Using WKG2 as starter for sourdough in liquid and solid fermentation was exhibited a higher concentration of organic acids, flavonoids, and polyphenolic compounds with antioxidant and antifungal properties. | [18] |
| Pear and orange | Lvb. brevis, Lpb. plantarum, Flb. rossiae, and S. cerevisiae | 200 g of fruit | The use of pear and orange as sourdough starters significantly decreased bread’s pH, acidity, and gas production, and increased free amino acids (FAA) content and gas holding capacity. Moreover, compared to the use of orange as starter, pear can achieve acidic conditions that are more suitable for the good performance of LAB and yeast during fermentation, resulting in a bread with a higher specific volume and a softer crumb. | [64] |
| Kimchi | Lc. citreum and W. koreensis | 6 log CFU/g dough | The bread prepared with sourdough inoculated with kimchi LAB strains had significant effect on texture and could lead to an extended shelf life, by delaying bread staling and microbial spoilage. | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Parada, N.; González-Ríos, O.; Suárez-Quiroz, M.L.; Hernández-Estrada, Z.J.; Figueroa-Hernández, C.Y.; Figueroa-Cárdenas, J.d.D.; Rayas-Duarte, P.; Figueroa-Espinoza, M.C. Exploiting the Native Microorganisms from Different Food Matrices to Formulate Starter Cultures for Sourdough Bread Production. Microorganisms 2023, 11, 109. https://doi.org/10.3390/microorganisms11010109
Hernández-Parada N, González-Ríos O, Suárez-Quiroz ML, Hernández-Estrada ZJ, Figueroa-Hernández CY, Figueroa-Cárdenas JdD, Rayas-Duarte P, Figueroa-Espinoza MC. Exploiting the Native Microorganisms from Different Food Matrices to Formulate Starter Cultures for Sourdough Bread Production. Microorganisms. 2023; 11(1):109. https://doi.org/10.3390/microorganisms11010109
Chicago/Turabian StyleHernández-Parada, Natali, Oscar González-Ríos, Mirna Leonor Suárez-Quiroz, Zorba Josué Hernández-Estrada, Claudia Yuritzi Figueroa-Hernández, Juan de Dios Figueroa-Cárdenas, Patricia Rayas-Duarte, and María Cruz Figueroa-Espinoza. 2023. "Exploiting the Native Microorganisms from Different Food Matrices to Formulate Starter Cultures for Sourdough Bread Production" Microorganisms 11, no. 1: 109. https://doi.org/10.3390/microorganisms11010109







