Adaptation of Lacticaseibacillus rhamnosus CM MSU 529 to Aerobic Growth: A Proteomic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of L. rhamnosus CM MSU 529
2.2. Protein Extraction
2.3. Protein Digest
2.4. Liquid Chromatography-Mass Spectrometry
2.5. Data Acquisition
2.6. Peptide Identification, Quantification, and Statistical Analysis
2.7. Cell Dry Weight Determination
2.8. Protein–Protein Interaction Analysis
3. Results
3.1. Effect of Aeration on the Proteome of Strain CM MSU 529
3.2. Protein–Protein Interaction Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Husain, S. Clinical applications of probiotics. Int. J. Adv. Biol. Res. 2016, 6, 427–434. [Google Scholar]
- Forestier, C.; De Champs, C.; Vatoux, C.; Joly, B. Probiotic activities of Lactobacillus casei rhamnosus: In vitro adherence to intestinal cells and antimicrobial properties. Res. Microbiol. 2001, 152, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Du, M.; Yi, H.; Guo, C.; Tuo, Y.; Han, X.; Li, J.; Zhang, L.; Yang, L. Antimicrobial activity against Shigella sonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol. Res. 2011, 167, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P.; Mostert, M.; Leonessa, M.L.; Priolo, C.; Farina, D.; Monetti, C.; Latino, M.A.; Gomirato, G. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: A randomized study. Clin. Infect. Dis. 2006, 42, 1735–1742. [Google Scholar] [CrossRef]
- Allonsius, C.N.; van den Broek, M.F.L.; De Boeck, I.; Kiekens, S.; Oerlemans, E.F.M.; Kiekens, F.; Foubert, K.; Vandenheuvel, D.; Cos, P.; Delputte, P.; et al. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb. Biotechnol. 2017, 10, 1753–1763. [Google Scholar] [CrossRef]
- Boonma, P.; Spinler, J.K.; Venable, S.F.; Versalovic, J.; Tumwasorn, S. Lactobacillus rhamnosus L34 and Lactobacillus casei L39 suppress Clostridium difficile-induced IL-8 production by colonic epithelial cells. BMC Microbiol. 2014, 14, 177. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Shao, L.; Liu, R.; Msuthwana, P.; Hu, J.; Wang, C. Lactobacillus rhamnosus GG defense against Salmonella enterica serovar Typhimurium infection through modulation of M1 macrophage polarization. Microb. Pathog. 2021, 156, e104939. [Google Scholar] [CrossRef]
- Moslem, P.; Hossein, N.; Mahdi, R.; Seyed, N.H.; Seyed, A.S. Lactobacillus rhamnosus Gorbach-Goldin (GG): A top well-researched probiotic strain. J. Med. Microbiol. 2017, 5, 46–59. [Google Scholar]
- Zotta, T.; Guidone, A.; Ianniello, R.G.; Parente, E.; Ricciardi, A. Temperature and respiration affect the growth and stress resistance of Lactobacillus plantarum C17. J. Appl. Microbiol. 2013, 115, 848–858. [Google Scholar] [CrossRef]
- Ianniello, R.G.; Zotta, T.; Matera, A.; Genovese, F.; Parente, E.; Ricciardi, A. Investigation of factors affecting aerobic and respiratory growth in the oxygen-tolerant strain Lactobacillus casei N87. PLoS ONE 2016, 11, e0164065. [Google Scholar] [CrossRef] [Green Version]
- Johanson, A.; Goel, A.; Olsson, L.; Franzén, C.J. Respiratory physiology of Lactococcus lactis in chemostat cultures and its effect on cellular robustness in frozen and freeze-dried starter cultures. Appl. Environ. Microbiol. 2020, 86, e02785-19. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.B.; Gaudu, P.; Lechardeur, D.; Petit, M.A.; Gruss, A. Aerobic respiration metabolism in lactic acid bacteria and uses in biotechnology. Annu. Rev. Food Sci. Technol. 2012, 3, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Zotta, T.; Parente, E.; Ricciardi, A. Aerobic metabolism in the genus Lactobacillus: Impact on stress response and potential applications in the food industry. J. Appl. Microbiol. 2017, 122, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.B.; Garrigues, C.; Tuphile, K.; Brun, C.; Vido, K.; Bennedsen, M.; Møllgaard, H.; Gaudu, P.; Gruss, A. Impact of aeration and heme-activated respiration on Lactococcus lactis gene expression: Identification of a heme-responsive operon. J. Bacteriol. 2008, 190, 4903–4911. [Google Scholar] [CrossRef] [Green Version]
- Larsen, N.; Werner, B.B.; Jespersen, L. Transcriptional responses in Lactococcus lactis subsp. cremoris to the changes in oxygen and redox potential during milk acidification. Lett. Appl. Microbiol. 2016, 63, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, M.J.A.; Wiersma, A.; de Vos, W.M.; Kuipers, O.P.; Smid, E.J.; Molenaar, D.; Kleerebezem, M. Improvement of Lactobacillus plantarum aerobic growth as directed by comprehensive transcriptome analysis. Appl. Environ. Microbiol. 2008, 74, 4776–4778. [Google Scholar] [CrossRef] [Green Version]
- Eikmeyer, F.G.; Heinl, S.; Marx, H.; Puhler, A.; Grabherr, R.; Schluter, A. Identification of oxygen-responsive transcripts in the silage inoculant Lactobacillus buchneri CD034 by RNA sequencing. PLoS ONE 2015, 10, e0134149. [Google Scholar] [CrossRef] [PubMed]
- Vido, K.; Le Bars, D.; Mistou, M.Y.; Anglade, P.; Gruss, A.; Gaudu, P. Proteome analyses of heme-dependent respiration in Lactococcus lactis: Involvement of the proteolytic system. J. Bacteriol. 2004, 186, 1648–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzeo, M.F.; Cacace, G.; Peluso, A.; Zotta, T.; Muscariello, L.; Vastano, V.; Parente, E.; Siciliano, R.A. Effect of inactivation of ccpA and aerobic growth in Lactobacillus plantarum: A proteomic perspective. J. Proteom. 2012, 75, 4050–4061. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Pannella, G.; Lippolis, R.; Ricciardi, A.; Mazzeo, M.F.; Zotta, T. Impact of aerobic and respirative life-style on Lactobacillus casei N87 proteome. Int. J. Food Microbiol. 2019, 298, 51–62. [Google Scholar] [CrossRef]
- Kolbeck, S.; Ludwig, C.; Meng, C.; Hilgarth, M.; Vogel, R.F. Comparative proteomics of meat spoilage bacteria predicts drivers for their coexistence on modified atmosphere packaged meat. Front. Microbiol. 2020, 11, e209. [Google Scholar] [CrossRef] [PubMed]
- Dinarieva, T.Y.; Klimko, A.I.; Cherdyntseva, T.A.; Bryukhanov, A.L.; Netrusov, A.I. Vitamin K2 mediates electron transport from NADH dehydrogenase 2 to bd-type quinol oxidase in Lacticaseibacillus rhamnosus CM MSU 529. Mosc. Univ. Biol. Sci. Bull. 2022, 77, 172–177. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- di Salvo, M.L.; Safo, M.K.; Musayev, F.N.; Bossa, F.; Schirch, V. Structure and mechanism of Escherichia coli pyridoxine 5′-phosphate oxidase. Biochim. Biophis. Acta 2003, 1647, 76–82. [Google Scholar] [CrossRef]
- Lorquet, F.; Goffin, P.; Muscariello, L.; Baudry, J.B.; Ladero, V.; Sacco, M.; Kleerebezem, M.; Hols, P. Characterization and functional analysis of the poxB gene, which encodes pyruvate oxidase in Lactobacillus plantarum. J. Bacteriol. 2004, 186, 3749–3759. [Google Scholar] [CrossRef] [Green Version]
- Bron, P.A.; Wels, M.; Bongers, R.S.; van Bokhorst-van de Veen, H.; Wiersma, A.; Overmars, L.; Kleerebezem, M. Transcriptomes reveal genetic signatures underlying physiological variations imposed by different fermentation conditions in Lactobacillus plantarum. PLoS ONE 2012, 7, e38720. [Google Scholar] [CrossRef] [Green Version]
- Ianniello, R.G.; Zheng, J.; Zotta, T.; Ricciardi, A.; Gänzle, M.G. Biochemical analysis of respiratory metabolism in the heterofermentative Lactobacillus spicheri and Lactobacillus reuteri. J. Appl. Microbiol. 2015, 119, 763–775. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, L.; Xin, Y.; Xu, Z.; He, H.; Kong, J. Oxygen-inducible conversion of lactate to acetate in heterofermentative Lactobacillus brevis ATCC 367. Appl. Environ. Microbiol. 2017, 83, e01659-17. [Google Scholar] [CrossRef] [Green Version]
- Odamaki, T.; Xiao, J.Z.; Yonezawa, S.; Yaeshima, T.; Iwatsuki, K. Improved viability of bifidobacteria in fermented milk by cocultivation with Lactococcus lactis subspecies lactis. J. Dairy Sci. 2011, 94, 1112–1121. [Google Scholar] [CrossRef]
- Goto, S.; Kawamoto, J.; Sato, S.B.; Iki, T.; Watanabe, I.; Kudo, K.; Esaki, N.; Kurihara, T. Alkyl hydroperoxide reductase enhances the growth of Leuconostoc mesenteroides lactic acid bacteria at low temperatures. AMB Express 2015, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and utility of the antioxidant potential of probiotic lactobacilli and bifidobacteria of the human gut microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Lorca, G.L.; Font de Valdez, G.; Ljungh, A. Characterization of the protein-synthesis dependent adaptive acid tolerance response in Lactobacillus acidophilus. J. Mol. Microbiol. Biotechnol. 2002, 4, 525–532. [Google Scholar] [PubMed]
- Fernandez, A.; Ogawa, J.; Penaud, S.; Boudebbouze, S.; Ehrlich, D.; van de Guchte, M.; Maguin, E. Rerouting of pyruvate metabolism during acid adaptation in Lactobacillus bulgaricus. Proteomics 2008, 8, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Gury, J.; Seraut, H.; Tran, N.P.; Barthelmebs, L.; Weidmann, S.; Gervais, P.; Cavin, J.F. Inactivation of PadR, the repressor of the phenolic acid stress response, by molecular interaction with Usp1, a universal stress protein from Lactobacillus plantarum, in Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 5273–5283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankapalli, K.; Vishwanathan, V.; Susarla, G.; Sunayana, N.; Saladi, S.; Peethambaram, D.; D’Silva, P. Redox-dependent regulation of mitochondrial dynamics by DJ-1 paralogs in Saccharomyces cerevisiae. Redox Biol. 2020, 32, e101451. [Google Scholar] [CrossRef]
- Sastry, M.S.R.; Quigley, P.M.; Hol, W.G.J.; Baneyx, F. The linker-loop region of Escherichia coli chaperone Hsp31 functions as a gate that modulates high affinity substrate binding at elevated temperatures. Proc. Natl. Acad. Sci. USA 2004, 101, 8587–8592. [Google Scholar] [CrossRef] [Green Version]
- Zhan, D.; Bai, A.; Yu, L.; Han, W.; Feng, Y. Characterization of the PH1704 protease from Pyrococcus horikoshii OT3 and the critical functions of Tyr120. PLoS ONE 2014, 9, e103902. [Google Scholar] [CrossRef]
- Weber, A.; Kogl, S.A.; Jung, K. Time-dependent proteome alterations under osmotic stress during aerobic and anaerobic growth in Escherichia coli. J. Bacteriol. 2006, 188, 7165–7175. [Google Scholar] [CrossRef] [Green Version]
- Bankapalli, K.; Saladi, S.; Awadia, S.S.; Goswami, A.V.; Samaddar, M.; D’Silva, P. Robust glyoxalase activity of Hsp31, a ThiJ/DJ-1/PfpI family member protein, is critical for oxidative stress resistance in Saccharomyces cerevisiae. J. Biol. Chem. 2015, 290, 26491–26507. [Google Scholar] [CrossRef] [Green Version]
- Scholtissek, A.; Tischler, D.; Westphal, A.H.; van Berkel, W.J.H.; Paul, C.E. Old yellow enzyme-catalysed asymmetric hydrogenation: Linking family roots with improved catalysis. Catalysts 2017, 7, 130. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, E.S.; Ohlsen, K. The old yellow enzyme OfrA fosters Staphylococcus aureus survival via affecting thiol-dependent redox homeostasis. Front. Microbiol. 2022, 13, e888140. [Google Scholar] [CrossRef]
- Allan, W.L.; Breitkreuz, K.E.; Waller, J.C.; Simpson, J.P.; Hoover, G.J.; Rochon, A.; Wolyn, D.J.; Rentsch, D.; Snedden, W.A.; Shelp, B.J. Detoxification of succinate semialdehyde in Arabidopsis glyoxylate reductase and NAD kinase mutants subjected to submergence stress. Botany 2011, 90, 51–61. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Zheng, Y.; Garavito, R.M. Identification of succinic semialdehyde reductases from Geobacter: Expression, purification, crystallization, preliminary functional, and crystallographic analysis. Acta Biochim. Biophys. Sin. 2011, 43, 996–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, M.; Schweiger, P.; Deppenmeier, U. Succinic semialdehyde reductase Gox1801 from Gluconobacter oxydans in comparison to other succinic semialdehyde-reducing enzymes. Appl. Microbiol. Biotechnol. 2015, 99, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Kumsab, J.; Tobe, R.; Kurihara, T.; Hirose, Y.; Omori, T.; Mihara, H. Characterization of a novel class of glyoxylate reductase belonging to the β-hydroxyacid dehydrogenase family in Acetobacter aceti. Biosci. Biotechnol. Biochem. 2020, 84, 2303–2310. [Google Scholar] [CrossRef]
- Naraki, S.; Igimi, S.; Sasaki, Y. NADH peroxidase plays a crucial role in consuming H2O2 in Lactobacillus casei IGM394. Biosci. Microbiota Food Health 2020, 39, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Zuljan, F.A.; Repizo, G.D.; Alarcón, S.H.; Magni, C. α-Acetolactate synthase of Lactococcus lactis contributes to pH homeostasis in acid stress conditions. Int. J. Food Microbiol. 2014, 188, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bringel, F.; Hubert, J.C. Extent of genetic lesions of the arginine and pyrimidine biosynthetic pathways in Lactobacillus plantarum, L. paraplantarum, L. pentosus, and L. casei: Prevalence of CO(2)-dependent auxotrophs and characterization of deficient arg genes in L. plantarum. Appl. Environ. Microbiol. 2003, 69, 2674–2683. [Google Scholar] [CrossRef] [Green Version]
- Munch-Petersen, A. Deoxyribonucleoside catabolism and thymine incorporation in mutants of Escherichia coli lacking deoxyriboaldolase. Eur. J. Biochem. 1970, 15, 191–202. [Google Scholar] [CrossRef]
- Pricer, W.E.; Horecker, B.L. Deoxyribose aldolase from Lactobacillus plantarum. J. Biol. Chem. 1960, 235, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.-S.; Groth, D.P. Polycarboxylic acid activation of rat liver deoxyribose phosphate aldolase. J. Biol. Chem. 1962, 237, 3339–3341. [Google Scholar] [CrossRef] [PubMed]
- Kilstrup, M.; Hammer, K.; Jensen, P.R.; Martinussen, J. Nucleotide metabolism and its control in lactic acid bacteria. FEMS Microbiol. Rev. 2005, 29, 555–590. [Google Scholar] [CrossRef] [PubMed]
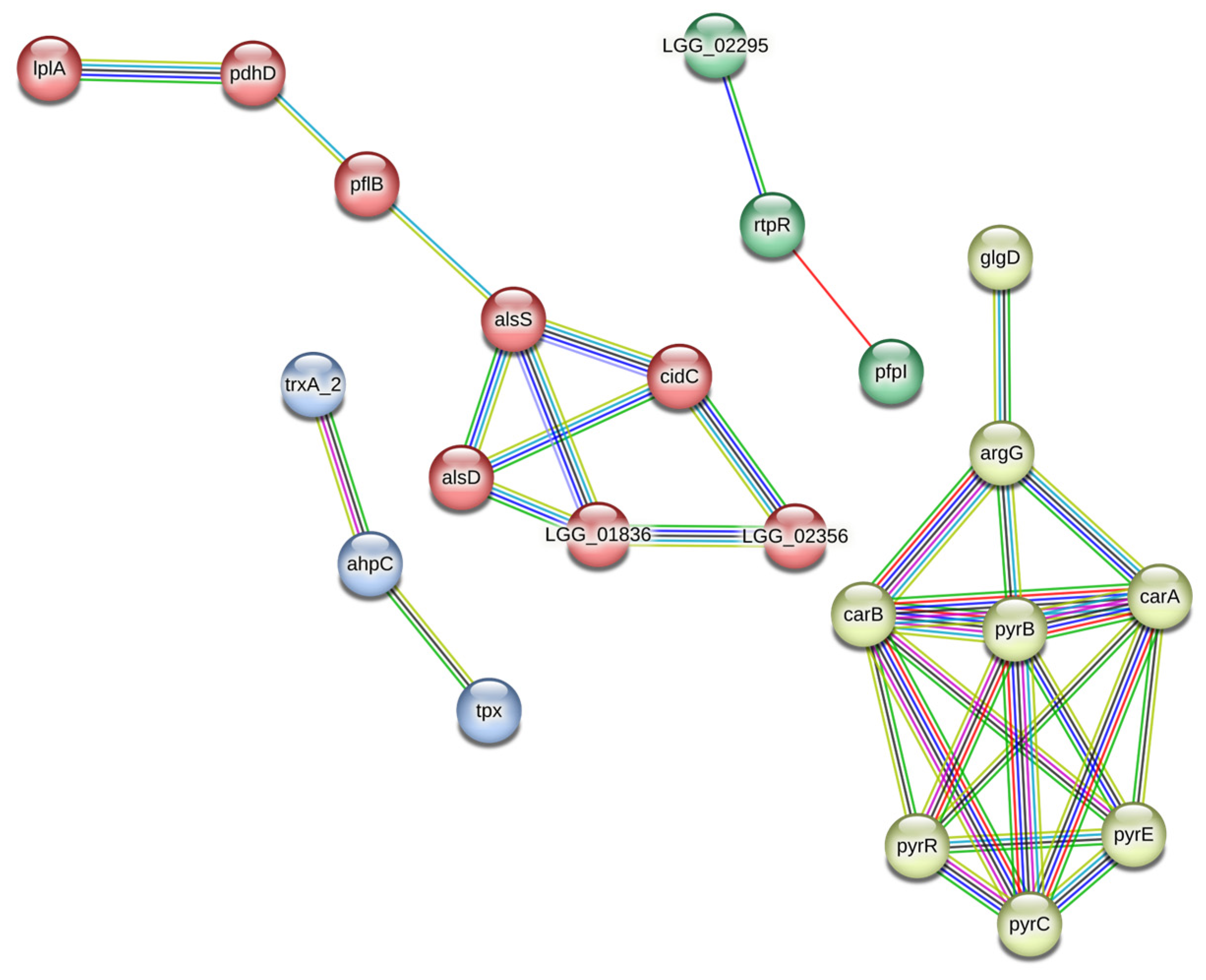
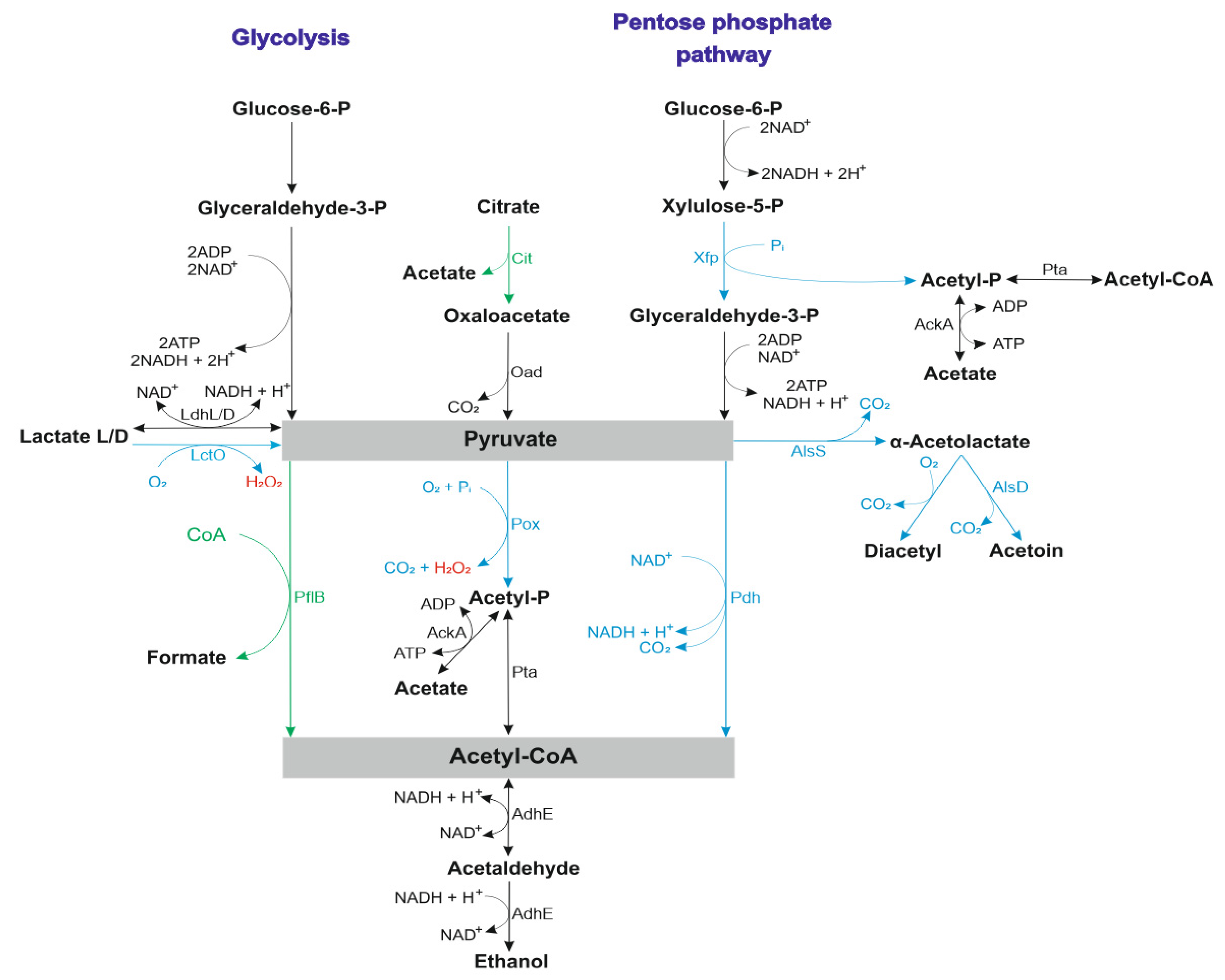
| Identified Proteins | Protein AC | Gene | MW, kDa | log2 Fold Change A/S 1 |
|---|---|---|---|---|
| CELLULAR PROCESSES AND SIGNALING | ||||
| [D] Cell cycle control, cell division | ||||
| Cell division protein SepF | K8QLA2 | sepF | 16.8 | 0.6 |
| [O] Post-translational modification, protein turnover, and chaperones | ||||
| Thioredoxin | K8Q3Z2 | trxA, trxA_2 | 11.5 | 1.2 |
| Thiol peroxidase Tpx-type | K8QQU7 | tpx | 18.5 | 1.6 |
| 10 kDa chaperonin | K8QSC4 | groES, groS | 10.0 | 0.9 |
| [T] Signal transduction mechanisms | ||||
| Universal stress protein | K8QLD2 | LRHMDP2_1718 | 18.2 | 0.7 |
| [V] Defense mechanisms | ||||
| ThiJ/PfpI family protein | K8QE31 | yfkM, pfpI | 18.4 | 0.7 |
| Alkyl hydroperoxide reductase C | K8QI55 | ahpC | 20.5 | −0.9 |
| INFORMATION STORAGE AND PROCESSING | ||||
| [J] Translation, ribosomal structure and biogenesis | ||||
| Ribosome-binding factor A | K8QHI9 | rbfA | 16.7 | 0.7 |
| Probable tRNA sulfurtransferase | K8QLD7 | thiI | 45.2 | 0.6 |
| Lactamase_B domain-containing protein | K8QEJ1 | vicX | 46.3 | 0.9 |
| [K] Transcription | ||||
| Organic hydroperoxide resistance transcriptional regulator | K8QM79 | ohrR | 16.1 | 0.8 |
| Transcriptional regulator, MarR family | K8QLH4 | LRHMDP2_405 | 17.4 | −0.6 |
| METABOLISM | ||||
| [C] Energy production and conversion | ||||
| NADH dehydrogenase | K8Q7J1 | LRHMDP2_2182 | 42.7 | 1.0 |
| Dihydrolipoyl dehydrogenase (E3) | K8QFD2 | pdhD, lpdA | 49.1 | 1.2 |
| NADH:flavin oxidoreductase Old Yellow Enzyme family | K8Q900 | namA, yqiG | 41.8 | 1.3 |
| Putative NAD+(FAD)-dependent dehydrogenase | K8QIY4 | nox_2 | 49.1 | 1.7 |
| L-lactate oxidase | K8Q6V5 | lctO | 39.3 | 1.2 |
| NADH peroxidase Npx | K8QQ66 | npx, npr | 49.3 | 2.5 |
| Pyruvate oxidase [C/H/R] | K8QGX3 | ydaP, cidC | 62.7 | 2.2 |
| Alcohol dehydrogenase | K8QNN2 | xylB_1, xylB_3 | 39.8 | −1.6 |
| Formate acetyltransferase | K8QD01 | pflB | 85.2 | −1.4 |
| [E] Amino acid transport and metabolism | ||||
| Carbamoyl-phosphate synthase small chain [E/F] | K8Q6G7 | carA | 39.4 | 0.9 |
| Carbamoyl-phosphate synthase large chain [E/F] | K8QCB0 | carB | 116.2 | 1.5 |
| Acetolactate synthase catabolic [E/H] | K8QB15 | alsS | 60.5 | 2.9 |
| Oligopeptide ABC transporter periplasmic oligopeptide-binding protein OppA | K8QLE7 | oppA, oppA_2 | 60.4 | 1.0 |
| Neutral endopeptidase | K8Q9I4 | pepO | 71.7 | 0.6 |
| Glycine cleavage system H protein | K8QLC1 | gcvH | 10.8 | 0.6 |
| Cysteine synthase | K8QQD2 | cysK | 32.6 | 0.7 |
| Transcriptional regulator, GntR family domain/Aspartate aminotransferase [E/K] | K8Q1F8 | avtA | 44.2 | −0.9 |
| Argininosuccinate synthase | K8QAT7 | argG | 44.7 | −0.7 |
| Cell division transporter ATP-binding protein FtsE | K8QCV1 | glnQ | 27.3 | −0.7 |
| [F] Nucleotide transport and metabolism | ||||
| Orotate phosphoribosyltransferase | K8Q3P7 | pyrE | 22.4 | 2.3 |
| Dihydroorotase | K8Q3Q2 | pyrC | 45.1 | 1.8 |
| Aspartate carbamoyltransferase | K8QFX4 | pyrB | 35.1 | 1.4 |
| Bifunctional protein PyrR | K8QCB4 | pyrR | 19.2 | 1.6 |
| Deoxyribose-phosphate aldolase | K8QGF6 | deoC | 23.0 | 1.1 |
| Adenosylcobalamin-dependent ribonucleoside-triphosphate reductase | K8Q4T0 | rtpR, nrdJ | 82.5 | −2.0 |
| Deoxyadenosine kinase/Deoxyguanosine kinase | K8QBP7 | LRHMDP2_1731 | 27.2 | −1.1 |
| [G] Carbohydrate transport and metabolism | ||||
| Probable phosphoketolase | K8Q7D7 | xfp, xpkA | 89.8 | 1.2 |
| Glycogen biosynthesis protein GlgD, glucose-1-phosphate adenylyltransferase family | K8QJ10 | glgD | 43.1 | 0.9 |
| Pyruvate oxidase [G/H/R] | K8Q595 | spxB, pox | 64.0 | 1.0 |
| ABC transporter substrate-binding protein | K8QJU7 | LRHMDP2_357 | 34.0 | −1.1 |
| Citrate lyase beta chain | K8Q549 | citE | 31.4 | −2.9 |
| [H] Coenzyme transport and metabolism | ||||
| Lipoate-protein ligase | K8Q5G3 | lplJ, lplA | 38.4 | 0.9 |
| [I] Lipid transport and metabolism | ||||
| 3-hydroxyisobutyrate dehydrogenase related beta-hydroxyacid dehydrogenase | K8QD39 | LRHMDP2_2289 | 30.1 | 1.3 |
| [P] Inorganic ion transport and metabolism | ||||
| Phosphate-binding protein | K8QH56 | pstS | 32.0 | −0.8 |
| [Q] Secondary metabolites biosynthesis, transport, and catabolism | ||||
| Oxidoreductase of aldo/keto reductase family, subgroup 1 | K8Q5E9 | dkgA, yqhE | 31.6 | 0.7 |
| Alpha-acetolactate decarboxylase | K8QD20 | budA, alsD | 25.7 | 1.2 |
| POORLY CHARACTERIZED | ||||
| [R] General function prediction only | ||||
| Pyridoxine 5’-phosphate oxidase V related favin-nucleotide-binding protein | K8Q7A0 | LRHMDP2_2560 | 14.7 | 2.9 |
| [S] Function unknown | ||||
| UPF0297 protein | K8QCI9 | alaRS | 10.1 | 1.1 |
| UPF0291 protein | K8QHM8 | ynzC | 9.7 | 0.7 |
| DUF124 domain-containing protein | K8QS65 | yfhL | 25.9 | 0.9 |
| Multi-ubiq domain-containing protein | K8Q6T6 | LRHMDP2_1960 | 20.1 | 1.7 |
| YfIT domain-containing protein | K8QDE0 | LRHMDP2_784 | 14.3 | 1.2 |
| Fe-S_biosyn domain-containing protein | K8Q441 | ykuJ | 10.5 | 0.9 |
| Putative pheromone lipoprotein (FMN-binding domain protein) | K8Q706 | cad | 32.6 | −0.9 |
| Additional lipoprotein component of putative cobalamin ECF transporter | K8Q211 | LRHMDP2_2713 | 14.3 | −1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinarieva, T.Y.; Klimko, A.I.; Kahnt, J.; Cherdyntseva, T.A.; Netrusov, A.I. Adaptation of Lacticaseibacillus rhamnosus CM MSU 529 to Aerobic Growth: A Proteomic Approach. Microorganisms 2023, 11, 313. https://doi.org/10.3390/microorganisms11020313
Dinarieva TY, Klimko AI, Kahnt J, Cherdyntseva TA, Netrusov AI. Adaptation of Lacticaseibacillus rhamnosus CM MSU 529 to Aerobic Growth: A Proteomic Approach. Microorganisms. 2023; 11(2):313. https://doi.org/10.3390/microorganisms11020313
Chicago/Turabian StyleDinarieva, Tatiana Yu., Alena I. Klimko, Jörg Kahnt, Tatiana A. Cherdyntseva, and Alexander I. Netrusov. 2023. "Adaptation of Lacticaseibacillus rhamnosus CM MSU 529 to Aerobic Growth: A Proteomic Approach" Microorganisms 11, no. 2: 313. https://doi.org/10.3390/microorganisms11020313





