Extraction Bottleneck in the Diagnosis of SARS-CoV-2: Evaluation of an Alternative Protocol Derived from Veterinary Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Total RNA Extraction
2.3. RT-qPCR
2.4. Data Analysis
3. Results
3.1. Clinical Performance
3.2. Cycle Threshold Analysis
3.3. Limit of Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Liu, C.; Martínez-Acuña, N.; Arellanos-Soto, D.; Galan-Huerta, K.; Lozano-Sepulveda, S.; Martínez-Guzmán, M.d.C.; Rivas-Estilla, A.M. SARS-CoV-2 in Mexico: Beyond Detection Methods, Scope and Limitations. Diagnostics 2021, 11, 124. [Google Scholar] [CrossRef]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, N.W.; Brooks, J.T.; Sobel, J. Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic. Emerg. Infect. Dis. 2020, 26, E1–E6. [Google Scholar] [CrossRef]
- García-Basteiro, A.L.; Chaccour, C.; Guinovart, C.; Llupià, A.; Brew, J.; Trilla, A.; Plasencia, A. Monitoring the COVID-19 Epidemic in the Context of Widespread Local Transmission. Lancet Respir. Med. 2020, 8, 440–442. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843. [Google Scholar] [CrossRef] [Green Version]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 Novel Coronavirus (2019-NCoV) by Real-Time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [Green Version]
- Rangaiah, A.; Shankar, S.M.; Basawarajappa, S.G.; Shah, P.A.; Chandrashekar, A.; Munegowda, A.; Padukone, S. Detection of SARS-CoV-2 in Clinical Samples: Target-Specific Analysis of Qualitative Reverse Transcription–Polymerase Chain Reaction(RT-PCR) Diagnostic Kits. IJID Reg. 2021, 1, 163–169. [Google Scholar] [CrossRef]
- Petrillo, S.; Carrà, G.; Bottino, P.; Zanotto, E.; De Santis, M.C.; Margaria, J.P.; Giorgio, A.; Mandili, G.; Martini, M.; Cavallo, R.; et al. A Novel Multiplex QRT-PCR Assay to Detect SARS-CoV-2 Infection: High Sensitivity and Increased Testing Capacity. Microorganisms 2020, 8, 1064. [Google Scholar] [CrossRef]
- Martín, G.; Rojo-Alba, S.; Castelló-Abietar, C.; Abreu-Salinas, F.; Costales, I.; Boga, J.A.; Melón, S.; Álvarez-Argüelles, M.E. Comparison of In-House SARS-CoV-2 Genome Extraction Procedures. A Need for COVID-19 Pandemic. J. Virol. Methods 2022, 300, 114415. [Google Scholar] [CrossRef]
- Wozniak, A.; Cerda, A.; Ibarra-Henríquez, C.; Sebastian, V.; Armijo, G.; Lamig, L.; Miranda, C.; Lagos, M.; Solari, S.; Guzmán, A.M.; et al. A Simple RnA Preparation Method for SARS-CoV-2 Detection by Rt-QpcR. Sci. Rep. 2020, 10, 16608. [Google Scholar] [CrossRef]
- Lim, H.-J.; Jung, H.-S.; Park, M.-Y.; Baek, Y.-H.; Kannappan, B.; Park, J.-Y.; Yang, J.-H.; Seol, J.-H.; Lee, M.-W.; Jung, S.-K.; et al. Evaluation of Three Automated Extraction Systems for the Detection of SARS-CoV-2 from Clinical Respiratory Specimens. Life 2022, 12, 68. [Google Scholar] [CrossRef]
- Perez, V.P.; Pessoa, W.F.B.; Galvão, B.H.A.; Sousa, E.S.S.; Dejani, N.N.; Campana, E.H.; Cavalcanti, M.G.d.S.; Cantarelli, V.V. Evaluation of Alternative RNA Extraction Methods for Detection of SARS-CoV-2 in Nasopharyngeal Samples Using the Recommended CDC Primer-Probe Set. J. Clin. Virol. Plus 2021, 1, 100032. [Google Scholar] [CrossRef]
- Ambrosi, C.; Prezioso, C.; Checconi, P.; Scribano, D.; Sarshar, M.; Capannari, M.; Tomino, C.; Fini, M.; Garaci, E.; Palamara, A.T.; et al. SARS-CoV-2: Comparative Analysis of Different RNA Extraction Methods. J. Virol. Methods 2021, 287, 114008. [Google Scholar] [CrossRef]
- Haile, S.; Nikiforuk, A.M.; Pandoh, P.K.; Twa, D.D.W.; Smailus, D.E.; Nguyen, J.; Pleasance, S.; Wong, A.; Zhao, Y.; Eisler, D.; et al. Optimization of Magnetic Bead-Based Nucleic Acid Extraction for SARS-CoV-2 Testing Using Readily Available Reagents. J. Virol. Methods 2022, 299, 114339. [Google Scholar] [CrossRef]
- Technical Guidance Publications. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications (accessed on 2 May 2021).
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; DaSilva, J.; Muecksch, F.; Gaebler, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 384, 2212–2218. [Google Scholar] [CrossRef]
- Suh, I.B.; Lim, J.; Kim, H.S.; Rhim, G.; Kim, H.; Kim, H.; Lee, S.M.; Park, H.S.; Song, H.J.; Hong, M.K.; et al. Development and Evaluation of AccuPower COVID-19 Multiplex Real-Time RT-PCR Kit and AccuPower SARS-CoV-2 Multiplex Real-Time RT-PCR Kit for SARS-CoV-2 Detection in Sputum, NPS/OPS, Saliva and Pooled Samples. PLoS ONE 2022, 17, e0263341. [Google Scholar] [CrossRef]
- Al-Saud, H.; Al-Romaih, K.; Bakheet, R.; Mahmoud, L.; Al-Harbi, N.; Alshareef, I.; Judia, S.B.; Aharbi, L.; Alzayed, A.; Jabaan, A.; et al. Automated SARS-COV-2 RNA Extraction from Patient Nasopharyngeal Samples Using a Modified DNA Extraction Kit for High Throughput Testing. Ann. Saudi Med. 2020, 40, 373–381. [Google Scholar] [CrossRef]
- Lázaro-Perona, F.; Rodriguez-Antolín, C.; Alguacil-Guillén, M.; Gutiérrez-Arroyo, A.; Mingorance, J.; García-Rodriguez, J. Evaluation of Two Automated Low-Cost RNA Extraction Protocols for SARS-CoV-2 Detection. PLoS ONE 2021, 16, e0246302. [Google Scholar] [CrossRef]
- Villanueva-Cañas, J.L.; Gonzalez-Roca, E.; Unanue, A.G.; Titos, E.; Yoldi, M.J.M.; Gómez, A.V.; Butillé, J.A.P. ROBOCOV: An Affordable Open-Source Robotic Platform for SARS-CoV-2 Testing by RT-QPCR. bioRxiv 2020. [Google Scholar] [CrossRef]


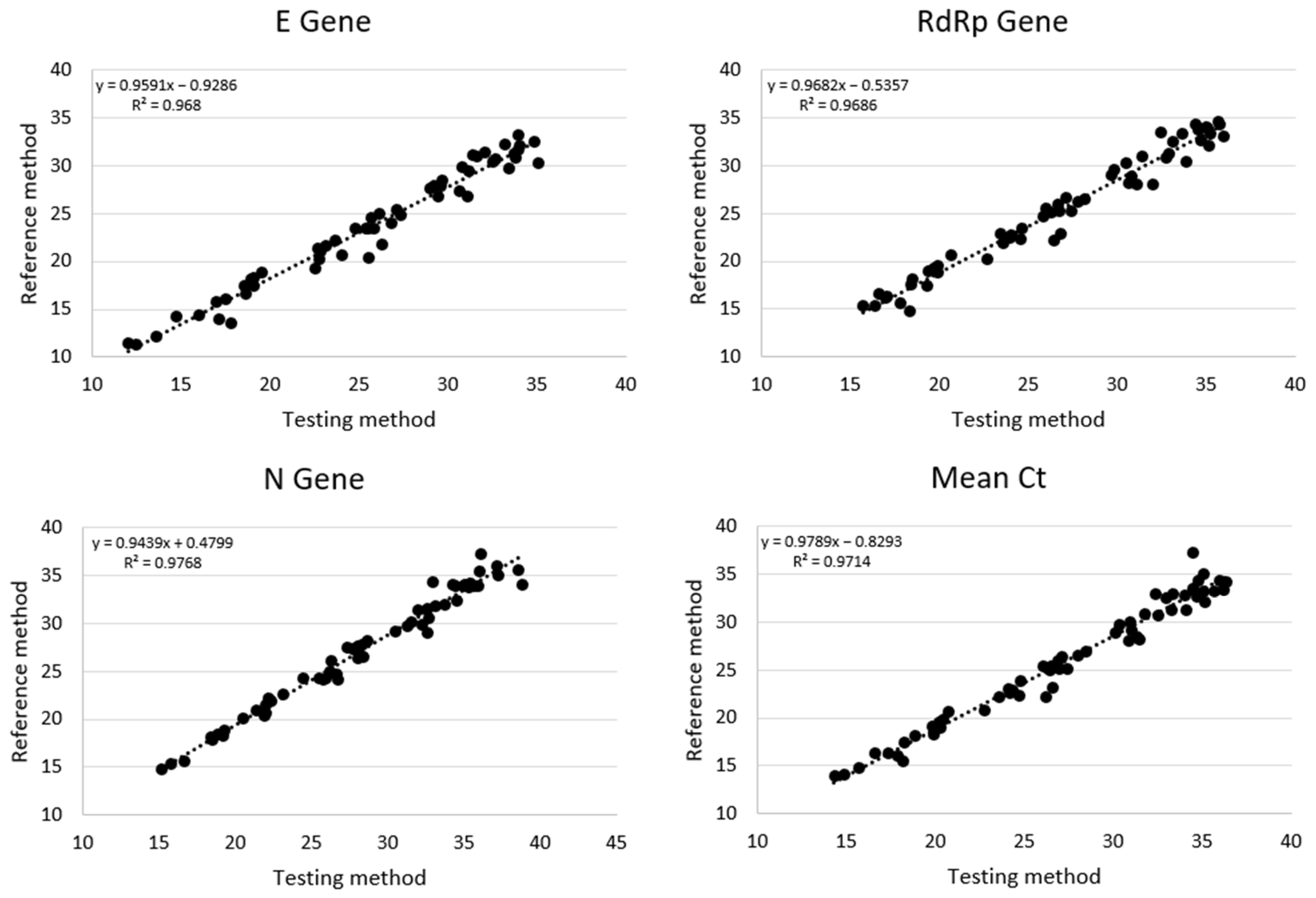
| Testing Method | Reference Method | |
|---|---|---|
| Positive samples | 59 | 60 |
| 3/3 genes | 56 | 57 |
| 2/3 genes | 2 | 2 |
| 1/3 genes | 1 | 1 |
| Negative samples | 13 (+1 *) | 13 |
| Total | 73 | 73 |
| Overall Sensitivity | 98.33% | |
| Overall Specificity | 100% | |
| Cohen’s kappa coefficient | 0.955 (excellent) | |
| Concentration (In TCID50/mL) | Reference Method | Testing Method | ||||
|---|---|---|---|---|---|---|
| Rep 1 | Rep 2 | Rep 3 | Rep 1 | Rep 2 | Rep 3 | |
| 1000 | + | + | + | + | + | + |
| 100 | + | + | + | + | + | + |
| 10 | + | + | + | + | + | + |
| 1 | + | + | + | + | + * | + |
| 0.1 | + | + | + | + | + | + |
| 0.01 | + | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bottino, P.; Zanotto, E.; Sidoti, F.; Pastrone, L.; Piva, R.; Mereu, E.; Costa, C.; Cavallo, R. Extraction Bottleneck in the Diagnosis of SARS-CoV-2: Evaluation of an Alternative Protocol Derived from Veterinary Use. Microorganisms 2023, 11, 535. https://doi.org/10.3390/microorganisms11020535
Bottino P, Zanotto E, Sidoti F, Pastrone L, Piva R, Mereu E, Costa C, Cavallo R. Extraction Bottleneck in the Diagnosis of SARS-CoV-2: Evaluation of an Alternative Protocol Derived from Veterinary Use. Microorganisms. 2023; 11(2):535. https://doi.org/10.3390/microorganisms11020535
Chicago/Turabian StyleBottino, Paolo, Elisa Zanotto, Francesca Sidoti, Lisa Pastrone, Roberto Piva, Elisabetta Mereu, Cristina Costa, and Rossana Cavallo. 2023. "Extraction Bottleneck in the Diagnosis of SARS-CoV-2: Evaluation of an Alternative Protocol Derived from Veterinary Use" Microorganisms 11, no. 2: 535. https://doi.org/10.3390/microorganisms11020535






