Effects of Two Feeding Patterns on Growth Performance, Rumen Fermentation Parameters, and Bacterial Community Composition in Yak Calves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Growth Performance and Survival Rate
2.3. Milk Replacer Nutrient Content
2.4. Ruminal Chyme Collection
2.5. Ruminal Analysis of Fermentation Parameters
2.6. DNA Extraction, PCR Amplification, and 16S rRNA Sequencing
2.7. Sequencing Data Processing
2.8. Statistical Analyses
3. Results
3.1. Growth Performance and Survival of Yak Calves under Two Feeding Modes
3.2. Rumen Fermentation Parameters for Yak Calves under Two Feeding Modes
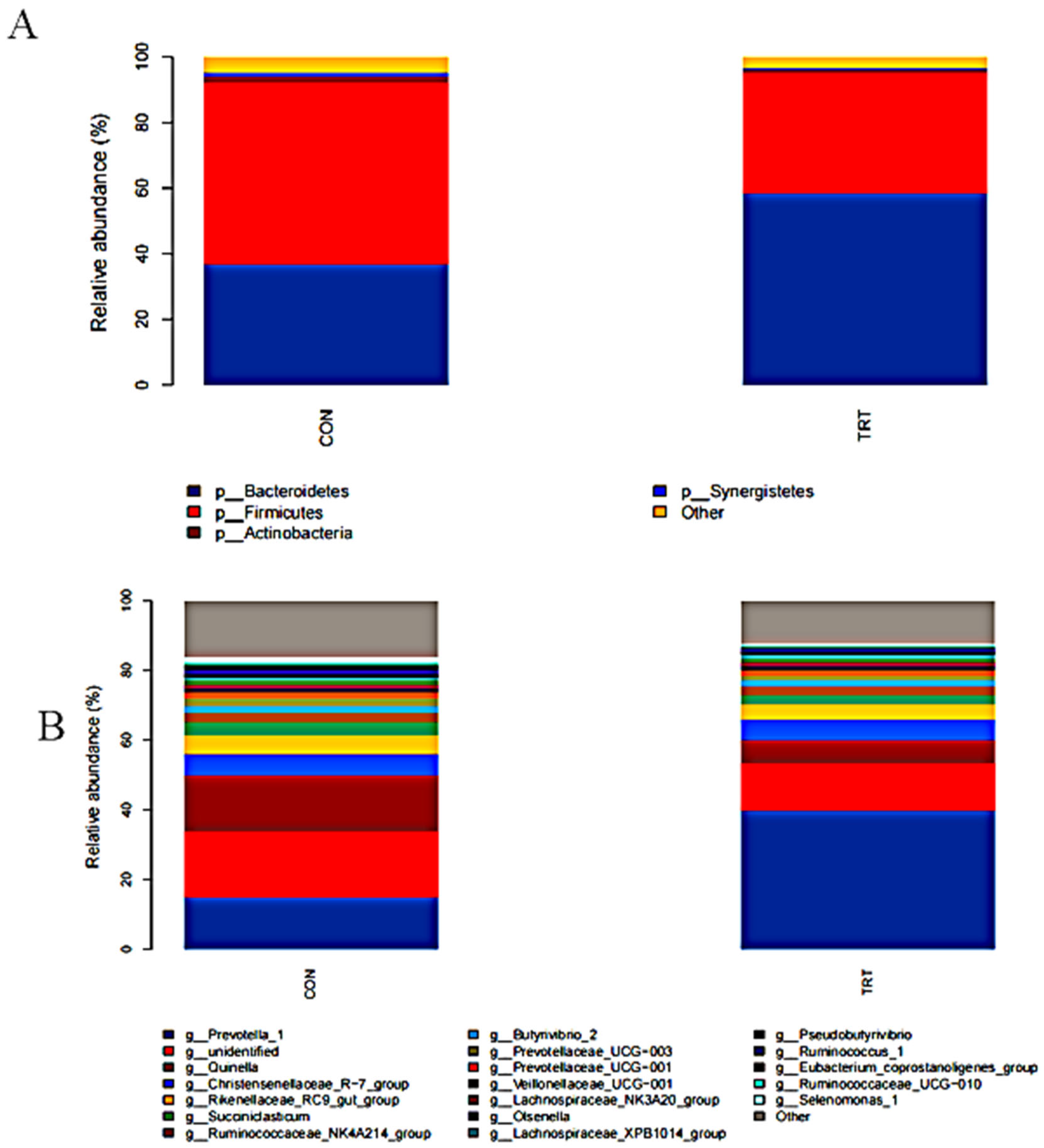
3.3. Composition of Rumen Bacteria
3.3.1. Overview of Sequencing Data for Rumen Microorganisms
3.3.2. Relative Abundance of Bacterial Populations
3.3.3. LEfSe Analysis
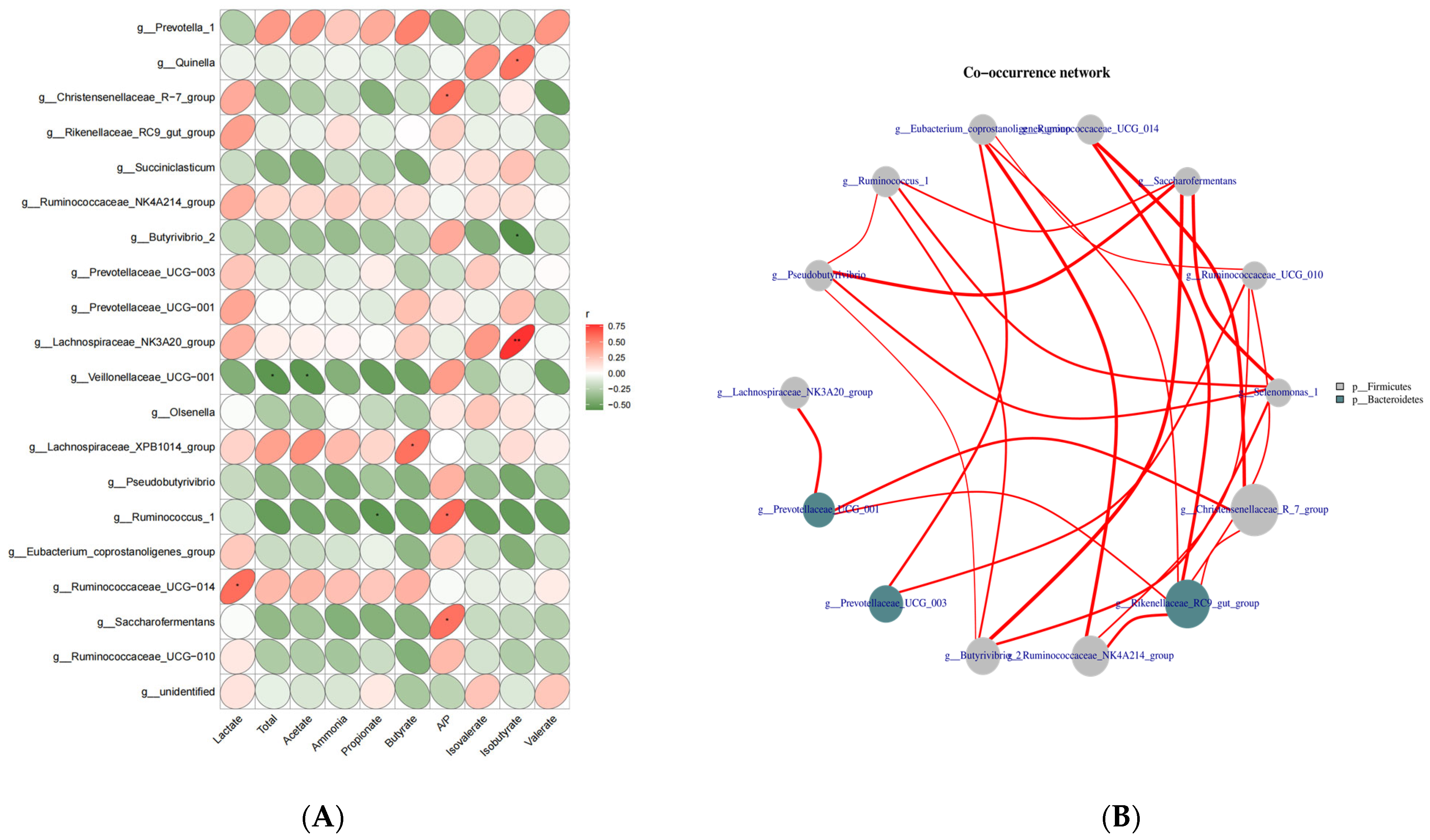
3.4. The Correlation of Ruminal Fermentation Parameters with Bacterial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, M.U.; Zhang, H.; Huang, S.; Iqbal, M.K.; Mehmood, K.; Luo, H.; Li, J. Characteristics of Integrons and Associated Gene Cassettes in Antibiotic-Resistant Escherichia coli Isolated from Free-Ranging Food Animals in China. J. Food Sci. 2017, 82, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Long, R.J.; Dong, S.K.; Hu, Z.Z.; Shi, J.J.; Dong, Q.M.; Han, X.T. Digestibility, nutrient balance and urinary purine derivative excretion in dry yak cows fed oat hay at dif-ferent levels of intake. Livest. Prod. Sci. 2004, 88, 27–32. [Google Scholar] [CrossRef]
- Long, R.J.; Ding, L.M.; Shang, Z.H.; Guo, X. The yak grazing system on the Qinghai-Tibetan plateau and its status. Rangel. J. 2008, 30, 241–246. [Google Scholar] [CrossRef]
- Lan, D.; Xiong, X.; Huang, C.; Mipam, T.D.; Li, J. Toward Understanding the Genetic Basis of Yak Ovary Reproduction: A Characterization and Comparative Analyses of Estrus Ovary Transcriptiome in Yak and Cattle. PLoS ONE 2016, 11, e0152675. [Google Scholar] [CrossRef]
- Long, R.; Dong, S.; Wei, X.; Pu, X. The effect of supplementary feeds on the bodyweight of yaks in cold season. Livest. Prod. Sci. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- Long, R.J.; Dong, S.K.; Hu, Z.Z.; Shi, J.J.; Dong, Q.M.; Han, X.T. Effects of different breeding patterns on growth performance, rumen fermentation parameters and serum bio-chemical indices of yak calves. J. Anim. Nutr. 2021, 33, 2055–2062. [Google Scholar]
- Zhou, B.C. Status Analysis on Genetic Resources of Domestic Animal in Qinghai Province. Acta Ecol. Anim. Domastici 2010. [Google Scholar]
- Chapman, C.; Erickson, P.; Quigley, J.; Hill, T.; Bateman, H.; Suarez-Mena, F.; Schlotterbeck, R. Effect of milk replacer program on calf performance and digestion of nutrients with age of the dairy calf. J. Dairy Sci. 2016, 99, 2740–2747. [Google Scholar] [CrossRef] [Green Version]
- Rosenberger, K.; Costa, J.H.C.; Neave, H.W.; Weary, D.M.; von Keyserlingk, M.A.G. Corrigendum to “The effect of milk allowance on behavior and weight gains in dairy calves” (J. Dairy Sci. 100:504–512). J. Dairy Sci. 2017, 100, 3327. [Google Scholar] [CrossRef]
- Bhatt, R.S.; Tripathi, M.K.; Verma, D.L.; Karim, S.A. Effect of different feeding regimes on pre-weaning growth rumen fermentation and its influence on post-weaning performance of lambs. J. Anim. Physiol. Anim. Nutr. 2009, 93, 568–576. [Google Scholar] [CrossRef]
- Whalin, L.; Neave, H.W.; Johnsen, J.F.; Mejdell, C.M.; Ellingsen-Dalskau, K. The influence of personality and weaning method on early feeding behavior and growth of Norwegian Red calves. J. Dairy Sci. 2022, 105, 1369–1386. [Google Scholar] [CrossRef] [PubMed]
- Abecia, L.; Ramos-Morales, E.; Martínez-Fernandez, G.; Arco, A.; Martín-García, A.I.; Newbold, C.J.; Yáñez-Ruiz, D.R. Feeding management in early life influences microbial colonisation and fermentation in the rumen of newborn goat kids. Anim. Prod. Sci. 2014; in press. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Global Rumen Census Collaborators; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, C.; Bell, R.; Klag, K.A.; Lee, S.-H.; Soto, R.; Ghazaryan, A.; Buhrke, K.; Ekiz, H.A.; Ost, K.S.; Boudina, S.; et al. T cell–mediated regulation of the microbiota protects against obesity. Science 2019, 365, eaat9351. [Google Scholar] [CrossRef]
- Bickhart, D.; Weimer, P. Symposium review: Host–rumen microbe interactions may be leveraged to improve the productivity of dairy cows. J. Dairy Sci. 2018, 101, 7680–7689. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Asnicar, F.; Weingart, G.; Tickle, T.; Huttenhower, C.; Segata, N. Compact graphical representation of phylogenetic data and metadata with GraPhlAn. Peerj 2015, 3, e1029. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Struc-ture Microbial Communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Chen, X.; Li, J.; Yang, Y.; Cui, Z.; Yao, J. Effect of alfalfa hay and starter feed supplementation on caecal microbiota and fermentation, growth, and health of yak calves. Animal 2021, 15, 100019. [Google Scholar] [CrossRef]
- Weimer, P.; Stevenson, D.; Mertens, D. Shifts in bacterial community composition in the rumen of lactating dairy cows under milk fat-depressing conditions. J. Dairy Sci. 2010, 93, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Shabat, S.K.B.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Berg Miller, M.E.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016, 10, 2958–2972. [Google Scholar] [CrossRef] [Green Version]
- Shibata, M.; Terada, F. Factors affecting methane production and mitigation in ruminants. Anim. Sci. J. 2010, 81, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.P.; Zhou, H.Y. Effect of dry matter and water intake on some rumen parameters in yaks. Foreign Anim. Husb. Herbiv. 1992, 47. [Google Scholar] [CrossRef]
- Wen, Y.L.; Zhao, J.Q. Measurement of lactation and its milk characteristics in yaks. China Dairy Ind. 2019, 12–18. [Google Scholar]
- Moore, T.; Mao, I. Prediction of Total Intake of Dry Matter and Net Energy in a Lactation. J. Dairy Sci. 1990, 73, 1255–1262. [Google Scholar] [CrossRef]
- Górka, P.; Kowalski, Z.M.; Zabielski, R.; Guilloteau, P. Invited review: Use of butyrate to promote gastrointestinal tract development in calves. J. Dairy Sci. 2018, 101, 4785–4800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.; Bach, A.; Weary, D.M.; Von Keyserlingk, M.A. Invited review: Transitioning from milk to solid feed in dairy heifers. J. Dairy Sci. 2016, 99, 885–902. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.; Trabi, E.B.; Zhu, C.; Mao, S. Role of butyrate as part of milk replacer and starter diet on intestinal development in pre-weaned calves. A systematic review. Anim. Feed. Sci. Technol. 2022, 292, 115423. [Google Scholar] [CrossRef]
- Górka, P.; Kowalski, Z.; Pietrzak, P.; Kotunia, A.; Jagusiak, W.; Holst, J.; Guilloteau, P.; Zabielski, R. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J. Dairy Sci. 2011, 94, 5578–5588. [Google Scholar] [CrossRef] [Green Version]
- Saegusa, A.; Inouchi, K.; Ueno, M.; Inabu, Y.; Koike, S.; Sugino, T.; Oba, M. Effects of partial replacement of corn grain with lactose in calf starters on ruminal fermentation and growth performance. J. Dairy Sci. 2017, 100, 6177–6186. [Google Scholar] [CrossRef]
- Koike, S.; Ueno, M.; Miura, H.; Saegusa, A.; Inouchi, K.; Inabu, Y.; Sugino, T.; Guan, L.; Oba, M.; Kobayashi, Y. Rumen microbiota and its relation to fermentation in lactose-fed calves. J. Dairy Sci. 2021, 104, 10744–10752. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Zhu, M.-X.; Wang, C.-F. Progress of research on the effects of allotropic acid on rumen metabolism and produc-tion performance in ruminants. J. Anim. Nutr. 2022, 34, 1408–1415. [Google Scholar]
- Ren, Y.; Zhao, S. Progress of research on the effect of allotropic acid on rumen metabolism in ruminants. Feed. Res. 2008, 10–12. [Google Scholar]
- Broderick, G.A. In Vitro determination of rates of ruminal protein degradation. Can. J. Anim. Sci. 1984, 64, 31–32. [Google Scholar] [CrossRef]
- Van Es, A. Matching ruminant production systems with available resources in the tropics and sub-tropics. Livest. Prod. Sci. 1988, 19, 532–533. [Google Scholar] [CrossRef]
- Shen, F. Effects of different feeding methods on growth performance, rumen fermentation and flora structure of yak calves. J. Anim. Nutr. 2022, 34, 5931–5941. [Google Scholar]
- Davis, C.L.; Drackley, J.K. The Development, Nutrition, and Management of the Young Calf; Iowa State University Press: Ames, IA, USA, 1998. [Google Scholar]
- Guzman, C.E.; Bereza-Malcolm, L.T.; De Groef, B.; Franks, A.E. Uptake of milk with and without solid feed during the monogastric phase: Effect on fibrolytic and methanogenic microorganisms in the gastrointestinal tract of calves. Anim. Sci. J. 2016, 87, 378–388. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, X.; Yang, X.; Wang, J.; Li, T.; Hua, G.; Han, D.; Da, L.; Li, R.; Rong, W.; et al. Characteristics of Bacterial Microbiota in Different Intestinal Segments of Aohan Fine-Wool Sheep. Front. Microbiol. 2022, 13, 874536. [Google Scholar] [CrossRef]
- Lin, B.; Henderson, G.; Zou, C.; Cox, F.; Liang, X.; Janssen, P.H.; Attwood, G.T. Characterization of the rumen microbial community composition of buffalo breeds consuming diets typical of dairy production systems in Southern China. Anim. Feed. Sci. Technol. 2015, 207, 75–84. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Song, J.; Xin, J.; Zhang, S.; Lei, Y.; Yang, Y.; Xie, P.; Suo, H. Comparison of Gut Microbiota of Yaks From Different Geographical Regions. Front. Microbiol. 2021, 12, 666940. [Google Scholar] [CrossRef]
- Guo, N.; Wu, Q.; Shi, F.; Niu, J.; Zhang, T.; Degen, A.A.; Fang, Q.; Ding, L.; Shang, Z.; Zhang, Z.; et al. Seasonal dynamics of diet–gut microbiota interaction in adaptation of yaks to life at high altitude. Npj Biofilms Microbiomes 2021, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Dong, X.; Dong, Z. Prokaryote diversity in the rumen of yak (Bos grunniens) and Jinnan cattle (Bos taurus) esti-mated by 16S rDNA homology analyses. Anaerobe 2005, 11, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.-L.; Cai, Q.; Shen, Y.-Y.; San, A.; Ma, L.; Zhang, Y.; Yi, X.; Chen, Y.; Yang, L.; Huang, Y.; et al. Draft genome sequence of the Tibetan antelope. Nat. Commun. 2013, 4, 1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Liu, H.; Hu, L.; Zhao, N.; Xu, S.; Lin, Z.; Chen, Y. Bacterial Community Characteristics in the Gastrointestinal Tract of Yak (Bos grunniens) Fully Grazed on Pasture of the Qinghai-Tibetan Plateau of China. Animals 2021, 11, 2243. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent Evolution of Rumen Microbiomes in High-Altitude Mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, Z.; Pei, C.; Degen, A.; Hao, L.; Cao, X.; Liu, H.; Zhou, J.; Long, R. A comparison between yaks and Qaidam cattle in in vitro rumen fermentation, methane emission, and bacte-rial community composition with poor quality substrate. Anim. Feed. Sci. Technol. 2022, 291, 115395. [Google Scholar] [CrossRef]
- Peng, B.; Huang, S.; Liu, T.; Geng, A. Bacterial xylose isomerases from the mammal gut Bacteroidetes cluster function in Saccharomyces cerevisiae for effective xylose fermentation. Microb. Cell Fact. 2015, 14, 70. [Google Scholar] [CrossRef] [Green Version]
- Cummins, K.; Papas, A. Effect of Isocarbon-4 and Isocarbon-5 Volatile Fatty Acids on Microbial Protein Synthesis and Dry Matter Digestibility In Vitro. J. Dairy Sci. 1985, 68, 2588–2595. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Zhang, Y.L.; Pei, C.X.; Wang, Y.X.; Zhang, Z.W.; Yang, W.Z.; Wang, H.; Guo, G.; Huo, W.J. Effects of isovalerate supplementation on growth performance and ruminal fermentation in pre- and post-weaning dairy calves. J. Agric. Sci. 2016, 154, 1499–1508. [Google Scholar] [CrossRef]
- Xue, D.; Chen, H.; Luo, X.; Guan, J.; He, Y.; Zhao, X. Microbial diversity in the rumen, reticulum, omasum, and abomasum of yak on a rapid fattening regime in an agro-pastoral transition zone. J. Microbiol. 2018, 56, 734–743. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, M.; Wang, H.; Zhang, J.; Yu, L. The effect of acetic acid to propionic acid ratio on the volatile fatty acid fermentation pattern and the diversity of microbial populations in vitro rumen fluid. J. Anim. Nutr. 2011, 23, 2129–2135. [Google Scholar]
- Guo, Y.-X.; Yang, R.-C.; Duan, C.-H.; Wang, Y.; Hao, Q.-H.; Ji, S.-K.; Yan, H.; Zhang, Y.-J.; Liu, Y.-Q. Effect of Dioscorea Opposite Waste on Growth Performance, Blood Parameters, Rumen Fermentation and Rumen Bacterial Community in Weaned Lambs. J. Integr. Agric. 2022; in press. [Google Scholar] [CrossRef]
- Du, R. Probiotics on the gastrointestinal flora, metabolites and meat quality in Sunite sheep. Food Sci. 2020, 41, 14–21. [Google Scholar]


| Nutrient 1 | Milk Replacer 2 Composition (%) |
|---|---|
| DM | 94.0 |
| CP | 24.0 |
| EE | 16.0 |
| Ash | 10.0 |
| Ga | 2.0 |
| P | 0.9 |
| NaCl | 1.0 |
| Lysine | 2.2 |
| Vitamin E, IU | 70.0 |
| Items | Groups 1 | SEM | p-Value | |
|---|---|---|---|---|
| CON | TRT | |||
| n1 | 30 | 30 | ||
| n2 | 14 | 22 | ||
| Survival rate,% | 46.47 | 73.33 | ||
| IBM, kg | 22.71 | 22.34 | 0.89 | 0.819 |
| FBM, kg | 43.73 | 43.84 | 1.21 | 0.924 |
| ADG, g/d | 165.23 | 159.94 | 6.77 | 0.529 |
| Items | Groups 1 | SEM | p-Value | |
|---|---|---|---|---|
| CON | TRT | |||
| n | 7 | 7 | ||
| TVFA, mmol/L | 50.19 | 79.18 | 6.03 | 0.017 |
| Molar proportion | ||||
| Acetate, % | 65.87 | 62.96 | 1.15 | 0.252 |
| Propionate, % | 20.28 | 22.19 | 1.21 | 0.482 |
| Butyrate, % | 8.52 | 10.93 | 0.50 | 0.016 |
| Iso-butyrate, % | 1.96 | 1.29 | 0.19 | 0.095 |
| Valerate, % | 1.04 | 1.32 | 0.13 | 0.342 |
| Iso-valerate, % | 2.32 | 1.31 | 0.20 | 0.013 |
| A/P | 3.5 | 2.92 | 0.27 | 0.220 |
| NH3-N concentration, mg/dL | 10.10 | 18.10 | 2.61 | 0.082 |
| Lactate, mmol/dL | 3033.20 | 3944.48 | 182.80 | 0.021 |
| Items | Group 1 | SEM | p-Value | |
|---|---|---|---|---|
| CON | TRT | |||
| Chao1 | 2335.36 | 2059.59 | 272.70 | 0.055 |
| Goods_coverage,% | 0.98 | 0.98 | 0.00 | 0.847 |
| Observed_species | 1845.26 | 1534.2 | 275.24 | 0.029 |
| PD_whole_tree | 155.41 | 134.27 | 18.75 | 0.029 |
| Shannon | 7.96 | 7.49 | 1.18 | 0.48 |
| Simpson | 0.95 | 0.97 | 0.06 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Tu, Y.; Ma, T.; Cui, K.; Zhang, J.; Diao, Q.; Bi, Y. Effects of Two Feeding Patterns on Growth Performance, Rumen Fermentation Parameters, and Bacterial Community Composition in Yak Calves. Microorganisms 2023, 11, 576. https://doi.org/10.3390/microorganisms11030576
Li Q, Tu Y, Ma T, Cui K, Zhang J, Diao Q, Bi Y. Effects of Two Feeding Patterns on Growth Performance, Rumen Fermentation Parameters, and Bacterial Community Composition in Yak Calves. Microorganisms. 2023; 11(3):576. https://doi.org/10.3390/microorganisms11030576
Chicago/Turabian StyleLi, Qin, Yan Tu, Tao Ma, Kai Cui, Jianxin Zhang, Qiyu Diao, and Yanliang Bi. 2023. "Effects of Two Feeding Patterns on Growth Performance, Rumen Fermentation Parameters, and Bacterial Community Composition in Yak Calves" Microorganisms 11, no. 3: 576. https://doi.org/10.3390/microorganisms11030576






