LncRNA 8244-ssc-miR-320-CCR7 Regulates IFN-β during SVA Infecting PK-15 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses and Clinical Samples of Piglets
2.2. Plasmids and Antibodies
2.3. Quantitative Real-Time PCR (qPCR)
2.4. Cell Infection
2.5. Transduction and Western Blotting
2.6. Dual Luciferase Activity Detection
2.7. Statistical Analysis
3. Results
3.1. SVA Infection Inhibits the Expression of lncRNA 8244
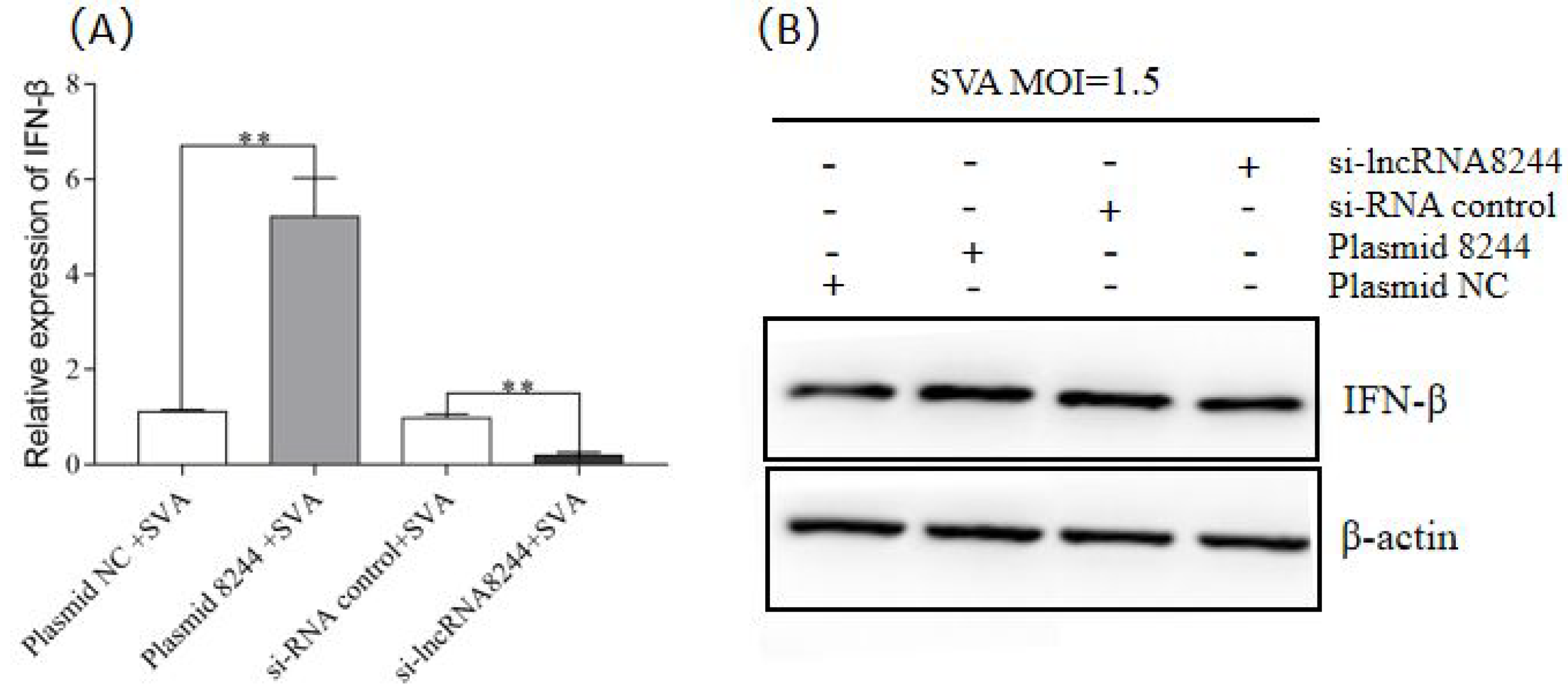
3.2. LncRNA 8244 Promotes the Production of IFN-β after SVA Infection in PK-15 Cells
3.3. LncRNA8244 Promotes the Expression of Key Signal Molecules in the TLR Signaling Pathway
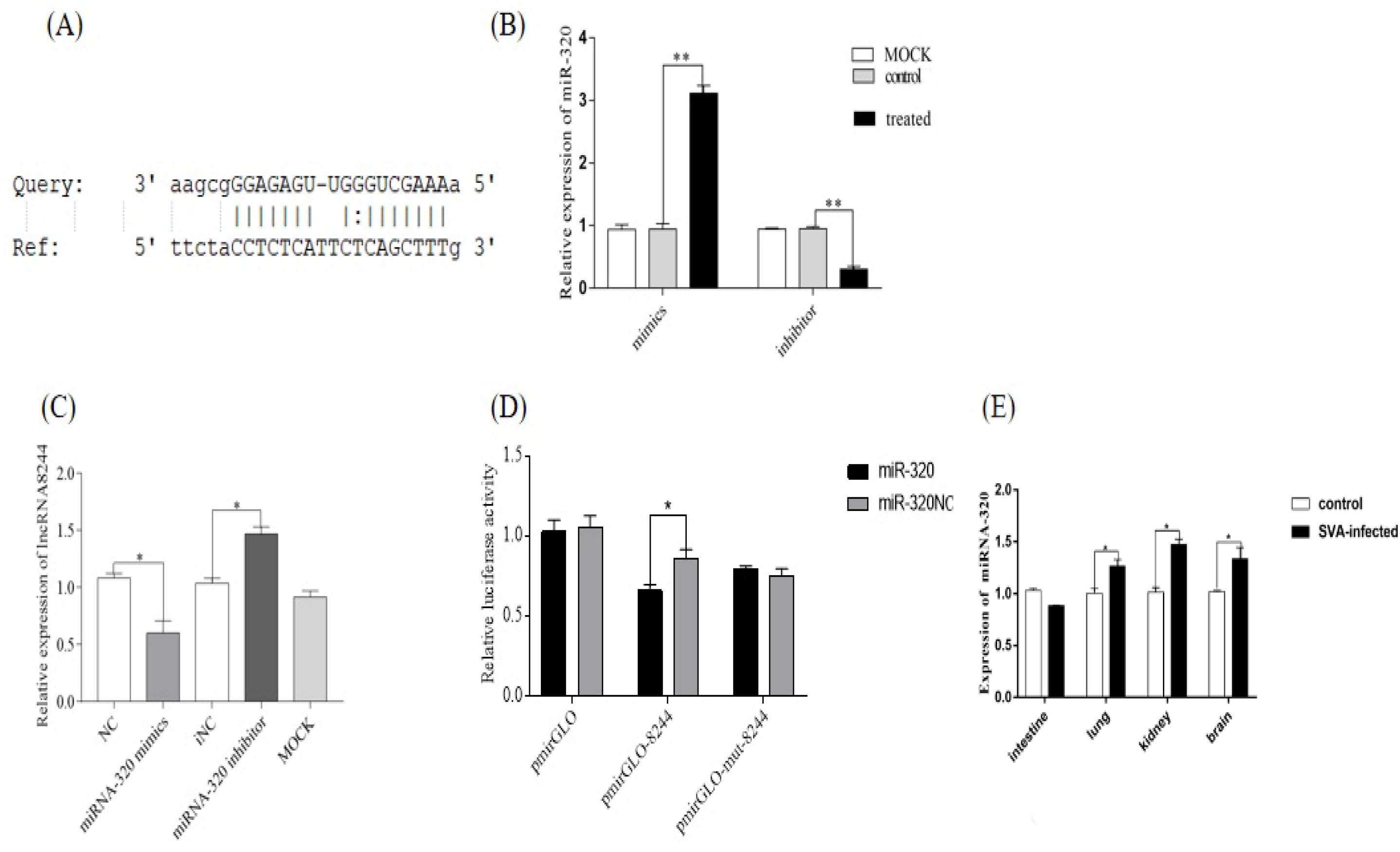
3.4. LncRNA 8244 Targets miR-320
3.5. LncRNA 8244 Affects IFN-β Secretion by Regulating the Expression of ssc-miR-320 in PK-15 Cells Infected by SVA
3.6. LncRNA 8244 Affects the Expression of Key Signal Molecules in TLR3-Related Signaling Pathways by Regulating the Expression of ssc-miR-320
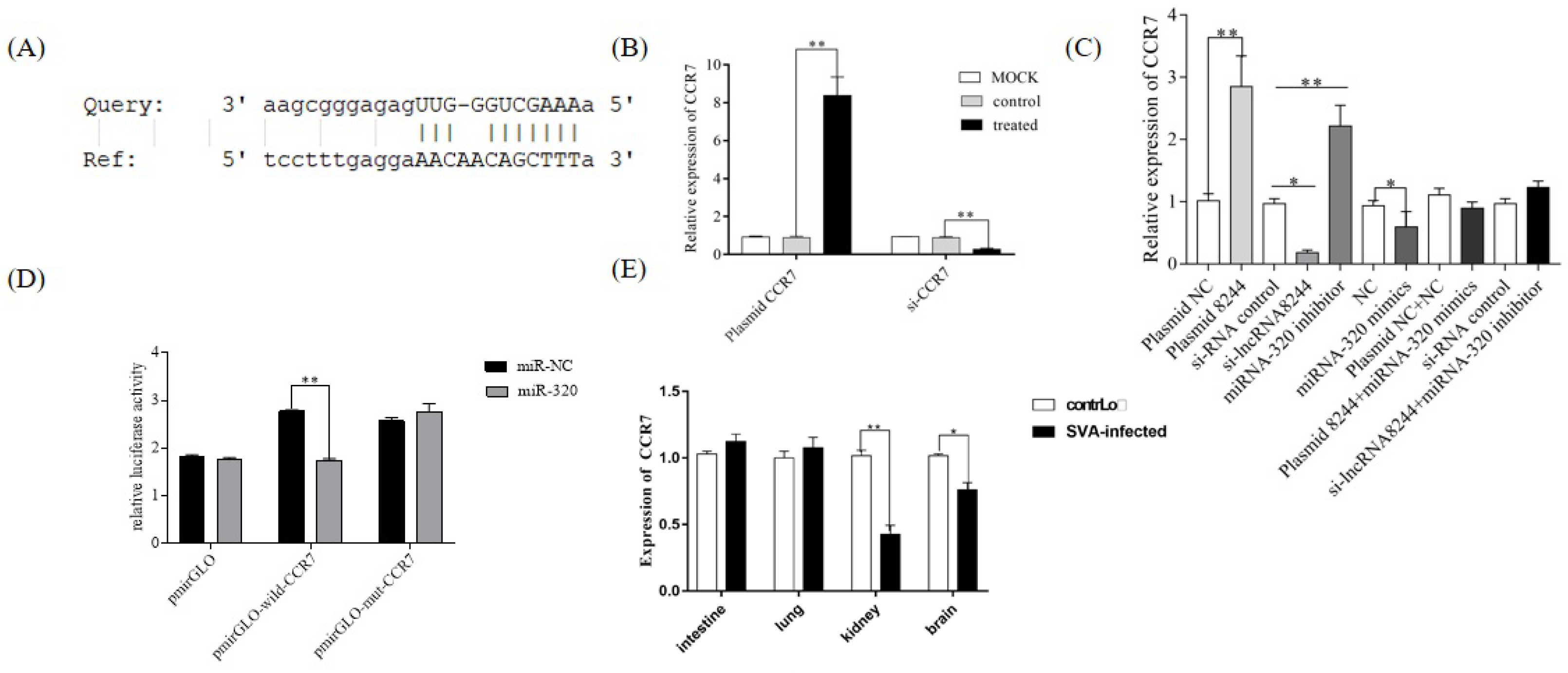
3.7. CCR7 Was the Target Gene of ssc-miR-320
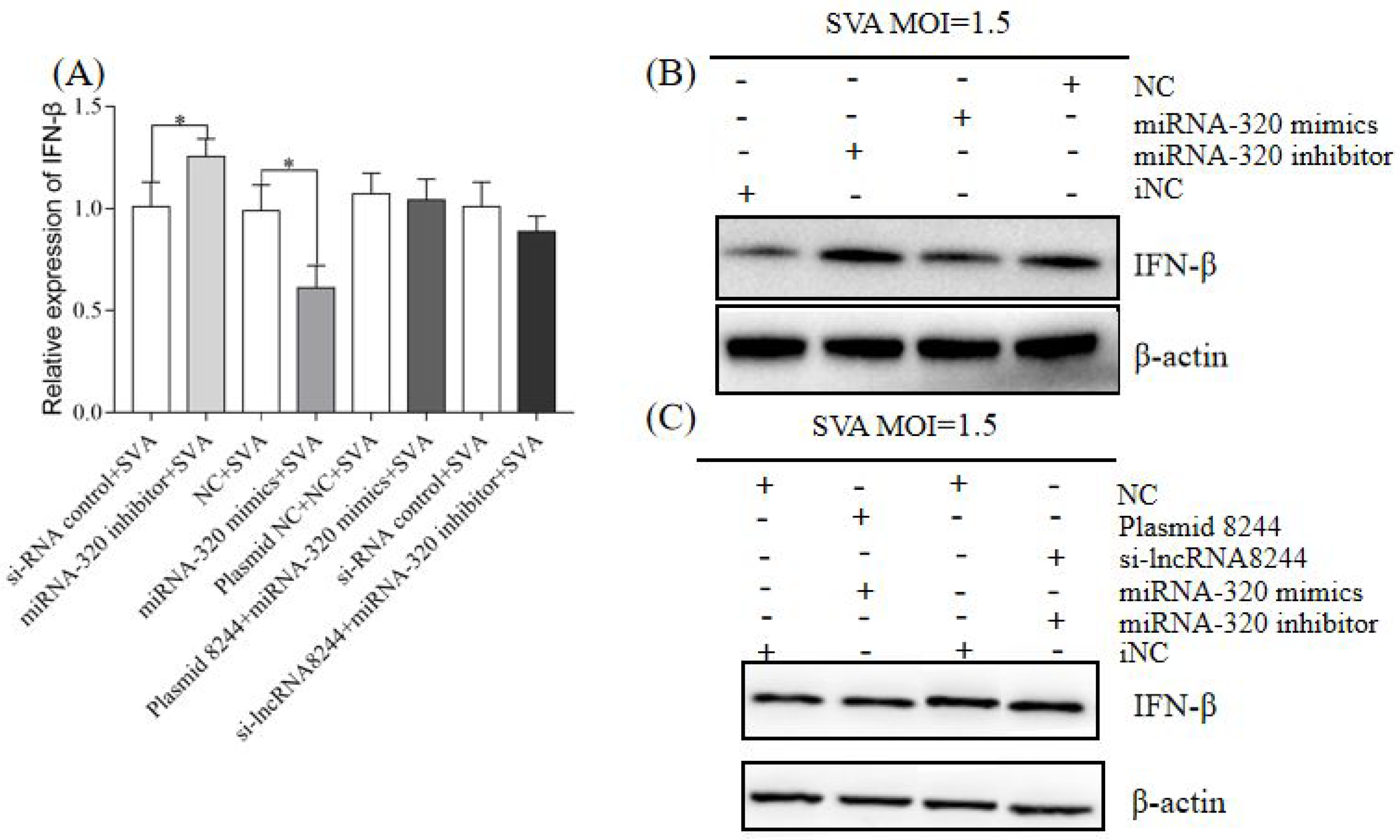
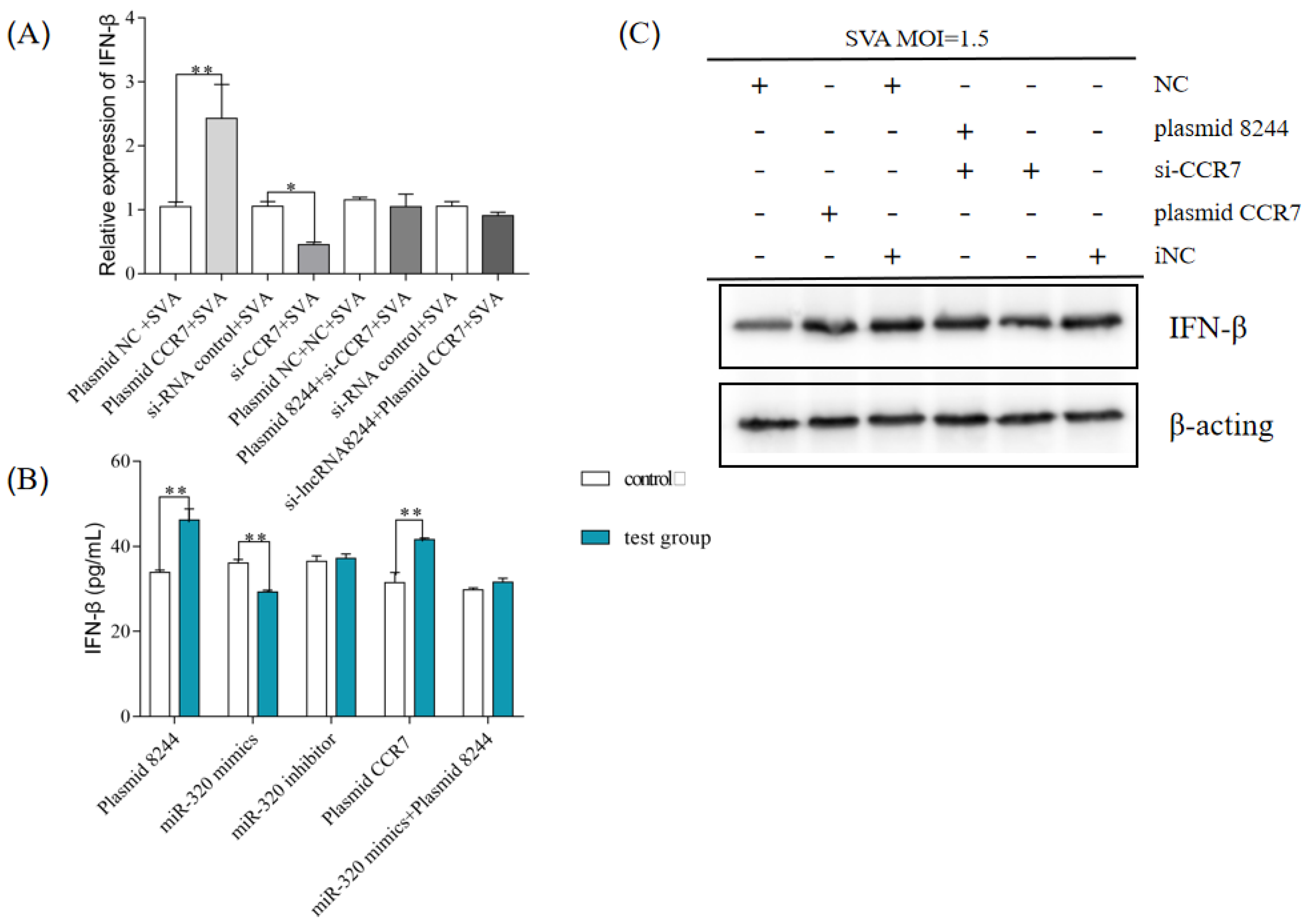
3.8. After SVA Infection, the lncRNA 8244-ssc-miR-320-CCR7 Axis Can Regulate the Production of IFN-β by PK-15 Cells
3.9. The LncRNA 8244-ssc-miR-320-CCR7 Axis Regulates the Expression of Key Signal Molecules in TLR3 Involved the Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hales, L.; Knowles, N.; Reddy, S.; Xu, L.; Hallenbeck, P. Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. J. Gen. Virol. 2008, 89 Pt 5, 1265. [Google Scholar] [CrossRef] [PubMed]
- Canning, P.; Canon, A.; Bates, J.; Gerardy, K.; Linhares, D.; Piñeyro, P.; Schwartz, K.; Yoon, K.; Rademacher, C.; Holtkamp, D. Neonatal Mortality, Vesicular Lesions and Lameness Associated with Senecavirus A in a U.S. Sow Farm. Transbound. Emerg. Dis. 2016, 63, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Leme, R.A.; Zotti, E.; Alcantara, B.K.; Oliveira, M.V.; Freitas, L.A.; Alfieri, A.F.; Alfieri, A.A. Clinical Manifestations of Senecavirus A Infection in Neonatal Pigs, Brazil. Emerg. Infect. Dis. J. 2015, 22, 1238–1241. [Google Scholar] [CrossRef]
- Leme, R.; Oliveira, T.; Alfieri, A.; Headley, S.; Alfieri, A. Pathological, Immunohistochemical and Molecular Findings Associated with Senecavirus A-Induced Lesions in Neonatal Piglets. J. Comp. Pathol. 2016, 155, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Leme, R.; Zotti, E.; Alcantara, B.; Oliveira, M.; Freitas, L.; Alfieri, A.; Alfieri, A. Senecavirus A: An Emerging Vesicular Infection in Brazilian Pig Herds. Transbound. Emerg. Dis. 2015, 62, 603–611. [Google Scholar] [CrossRef]
- Yang, M.; Van Bruggen, R.; Xu, W. Generation and diagnostic application of monoclonal antibodies against Seneca Valley virus. J. Vet. Diagn. Investig. 2012, 24, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Zhu, Z.; Cao, W.; Liu, H.; Zhang, K.; Tian, H.; Liu, X.; Zheng, H. Immunogenicity and protective efficacy of an inactivated cell culture-derived Seneca Valley virus vaccine in pigs. Vaccine 2018, 36, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, F.; Linhares, D.; Barcellos, D.; Lam, H.; Collins, J.; Marthaler, D. Identification and Complete Genome of Seneca Valley Virus in Vesicular Fluid and Sera of Pigs Affected with Idiopathic Vesicular Disease, Brazil. Transbound. Emerg. Dis. 2015, 62, 589–593. [Google Scholar] [CrossRef]
- Segales, J.; Barcellos, D.; Alfieri, A.; Burrough, E.; Marthaler, D. Senecavirus A: An Emerging Pathogen Causing Vesicular Disease and Mortality in Pigs? Vet. Pathol. 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Rapicavoli, N.; Kun, Q.; Zhang, J.; Megan, M.; Remi-Martin, L.; Chang, H. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. eLife 2013, 2, e00762. [Google Scholar] [CrossRef]
- Xiong, Y.; Yuan, J.; Zhang, C.; Zhu, Y.; Kuang, X. The STAT3-regulated long non-coding RNA Lethe promote the HCV replication. Biomed. Pharmacother. 2015, 72, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, J.; Xiao, J.; Yang, L.; Wang, X. Lnc-mg is a long non-coding RNA that promotes myogenesis. Nat. Commun. 2017, 8, 14718. [Google Scholar] [CrossRef] [Green Version]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, J.; Wang, Y.; Cao, X. An interferon-independent lncRNA promotes viral replication by modulating cellular metabolism. Science 2017, 358, 1051–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.; Hu, J.; Chen, J.L. lncRNAs regulate the innate immune response to viral infection. Wiley Interdisciplinary Reviews. Comput. Mol. Sci. 2017, 7, 129–143. [Google Scholar]
- Jiang, M.; Zhang, S.; Yang, Z.; Lin, H.; Zhu, J.; Liu, L.; Wang, W.; Liu, S.; Liu, W.; Ma, Y.; et al. Self-Recognition of an Inducible Host lncRNA by RIG-I Feedback Restricts Innate Immune Response. Cell 2018, 173, 906–919.e13. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Zhao, Y.; Yin, Z.; Wang, D.W.; Chen, C. The role of miR-320 in glucose and lipid metabolism disorder-associated diseases. Int. J. Biol. Sci. 2021, 17, 402–416. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Li, J.; Geng, H.; Zhou, B.; Zhang, B.; Chen, H. miR320/ELF3 axis inhibits the progression of breast cancer via the PI3K/AKT pathway. Oncol. Lett. 2019, 19, 3239–3248. [Google Scholar]
- Chang, T.H.; Huang, H.-Y.; Hsu, J.B.-K.; Weng, S.-L.; Horng, J.-T. An enhanced computational platform for investigating the roles of regulatory RNA and for identifying functional RNA motifs. BMC Bioinform. 2013, 14 (Suppl. 2), S4. [Google Scholar] [CrossRef] [Green Version]
- Pasma, T.; Davidson, S.; Shaw, S.L. Idiopathic vesicular disease in swine in Manitoba. Can. Vet. J.-Rev. Vet. Can. 2008, 49, 84–85. [Google Scholar]
- Meng, X.; Luo, Y.; Anwar, M.; Yao, G.; Qiu, H. Long Non-Coding RNAs: Emerging and Versatile Regulators in Host–Virus Interactions. Front. Immunol. 2017, 8, 1663. [Google Scholar] [CrossRef]
- Gomez, J.A.; Wapinski, O.; Yang, Y.; Bureau, J.F.; Gopinath, S.; Monack, D.; Chang, H.; Brahic, M.; Kirkegaard, K. The NeST Long ncRNA Controls Microbial Susceptibility and Epigenetic Activation of the Interferon-γ Locus. Cell 2013, 152, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Zhu, X.; Chen, Y.; Wei, H.; Chen, Q.; Chi, X.; Qi, B.; Zhang, L.; Zhao, Y.; Gao, G.F.; et al. NRAV, a Long Noncoding RNA, Modulates Antiviral Responses through Suppression of Interferon-Stimulated Gene Transcription. Cell Host Microbe 2014, 16, 616–626. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Sun, L.; Hua, Y.; Yang, C.; Teng, Y. Overexpression of long non-coding rna h19 protects lung fibroblasts from lps-induced injury by targeting mir-181a and runx2 via activation of notch and jnk pathways. J. Cell. Biochem. 2018, 120, 12075. [Google Scholar]
- Zhang, H.; Lu, W. LncRNA SNHG12 regulates gastric cancer progression by acting as a molecular sponge of miR-320. Mol. Med. Rep. 2018, 2, 2743–2749. [Google Scholar] [CrossRef] [Green Version]
- Takekoshi, T.; Fang, L.; Paragh, G.; Hwang, S.T. CCR7-expressing B16 melanoma cells downregulate interferon-γ-mediated inflammation and increase lymphangiogenesis in the tumor microenvironment. Oncogenesis 2012, 1, e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlos, T.S.; Young, D.; Stertz, S.; Kochs, G.; Randall, R.E. Interferon-induced inhibition of parainfluenza virus type 5; the roles of MxA, PKR and oligo A synthetase/RNase L. Virology 2007, 363, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. Innate immune recognition of viral infection. Uirusu 2006, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hiroyuki Oshiumi, M.O.K.F. The TLR3/TICAM-1 Pathway Is Mandatoryfor Innate Immune Responses to Poliovirus Infection. J. Immunol. 2011, 10, 5320–5327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikkert, M. Innate Immune Evasion by Human Respiratory RNA Viruses. J. Innate Immun. 2020, 12, 4–20. [Google Scholar] [CrossRef]
- Xue, Q.; Liu, H.; Zhu, Z.; Yang, F.; Xue, Q.; Cai, X.; Liu, X.; Zheng, H. Seneca Valley Virus 3C protease negatively regulates the type I interferon pathway by acting as a viral deubiquitinase. Antivir. Res. 2018, 160, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Liu, H.; Zhu, Z.; Yang, F.; Ma, L.; Cai, X.; Xue, Q.; Zheng, H. Seneca Valley Virus 3Cpro abrogates the IRF3- and IRF7-mediated innate immune response by degrading IRF3 and IRF7. Virology 2018, 518, 1–7. [Google Scholar] [CrossRef] [PubMed]




| Primers | Sequence (5′-3′) | Amplification Length |
|---|---|---|
| IFN-β-F | GCTAACAAGTGCATCCTCCAAA | 77 bp |
| IFN-β-R | AGCACATCATAGCTCATGGAAAGA | |
| IRF3-F | CCAGTGGTGCCTACACTCCT | 191 bp |
| IRF3-R | AGAGGTGTCTGGCTCAGGAA | |
| TLR3-F | CGCCTCCTGGAAAACCAA | 76 bp |
| TLR3-R | CCCTGAGTTGTCCTGCAACA | |
| TBK1-F | GCCTTTCTCGGGGTCTTCAA | 74 bp |
| TBK1-R | ACACTTTTCCTGATCCGCCT | |
| TLR7-F | CGGTGTTTGTGATGACAGAC | 134 bp |
| TLR7-R | AACTCCCACAGAGCCTCTTC | |
| TLR8-F | CACATTTGCCCGGTATCAAG | 145 bp |
| TLR8-R | TGTGTCACTCCTGCTATTCG | |
| TLR9-F | GGCCTTCAGCTTCACCTTGG | 151 bp |
| TLR9-R | GGTCAGCGGCACAAACTGAG | |
| MyD88-F | TGATGAACCGCAGGAT | 438 bp |
| MyD88-R | ACTGTGCTACGGGCTGGATT | |
| TRIF-F | TGGGACATCCTTAGGGACATG | 158 bp |
| TRIF-R | CCAGTGGACCTCAGGGAATG | |
| TRAF3-F | GTGTCAAGAAGGCATCG | 164 bp |
| TRAF3-R | CCTCAAACTGGCAATCA | |
| CCR7-F | TGCTGGTGGTGGCTCTCCTTG | 87 bp |
| CCR7-R | CCGTGGTGTTGTCGCCGATG | |
| LncRNA 8244-F | CAGAGGCAGGAACTGTGATGGC | 145 bp |
| LncRNA 8244-R | GTGGTAGGTGAATCTGCGGAAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Zhang, R.; Gao, L.; Lv, X.; Sun, Y.; Ma, J. LncRNA 8244-ssc-miR-320-CCR7 Regulates IFN-β during SVA Infecting PK-15 Cells. Microorganisms 2023, 11, 688. https://doi.org/10.3390/microorganisms11030688
Tang X, Zhang R, Gao L, Lv X, Sun Y, Ma J. LncRNA 8244-ssc-miR-320-CCR7 Regulates IFN-β during SVA Infecting PK-15 Cells. Microorganisms. 2023; 11(3):688. https://doi.org/10.3390/microorganisms11030688
Chicago/Turabian StyleTang, Xiaoyu, Ruiyu Zhang, Long Gao, Xiaocheng Lv, Yuan Sun, and Jingyun Ma. 2023. "LncRNA 8244-ssc-miR-320-CCR7 Regulates IFN-β during SVA Infecting PK-15 Cells" Microorganisms 11, no. 3: 688. https://doi.org/10.3390/microorganisms11030688





