IL-27 Signaling Promotes Th1 Response by Downregulating IL-10 Production in DCs during Chlamydial Respiratory Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Bacterial Strains, Chlamydial Infection and Administration of rIL-27
2.3. Lung, Spleen, and Lymph Nodes (LNs) Single-Cell Preparation
2.4. Antibodies and Flow Cytometry
2.5. Generation and Stimulation of Bone-Marrow-Derived DCs (BMDCs)
2.6. Splenic DCs Isolation and Adoptive Transfer
2.7. Co-Culture of Splenic DCs and CD4+ T Cells
2.8. Pulmonary Chlamydial Loads
2.9. Histology Analysis and Semi-Quantitative Pathological Scoring
2.10. ELISA
2.11. RNA Extraction and Quantitative Real-Time PCR (qPCR)
2.12. Statistical Analysis
3. Results
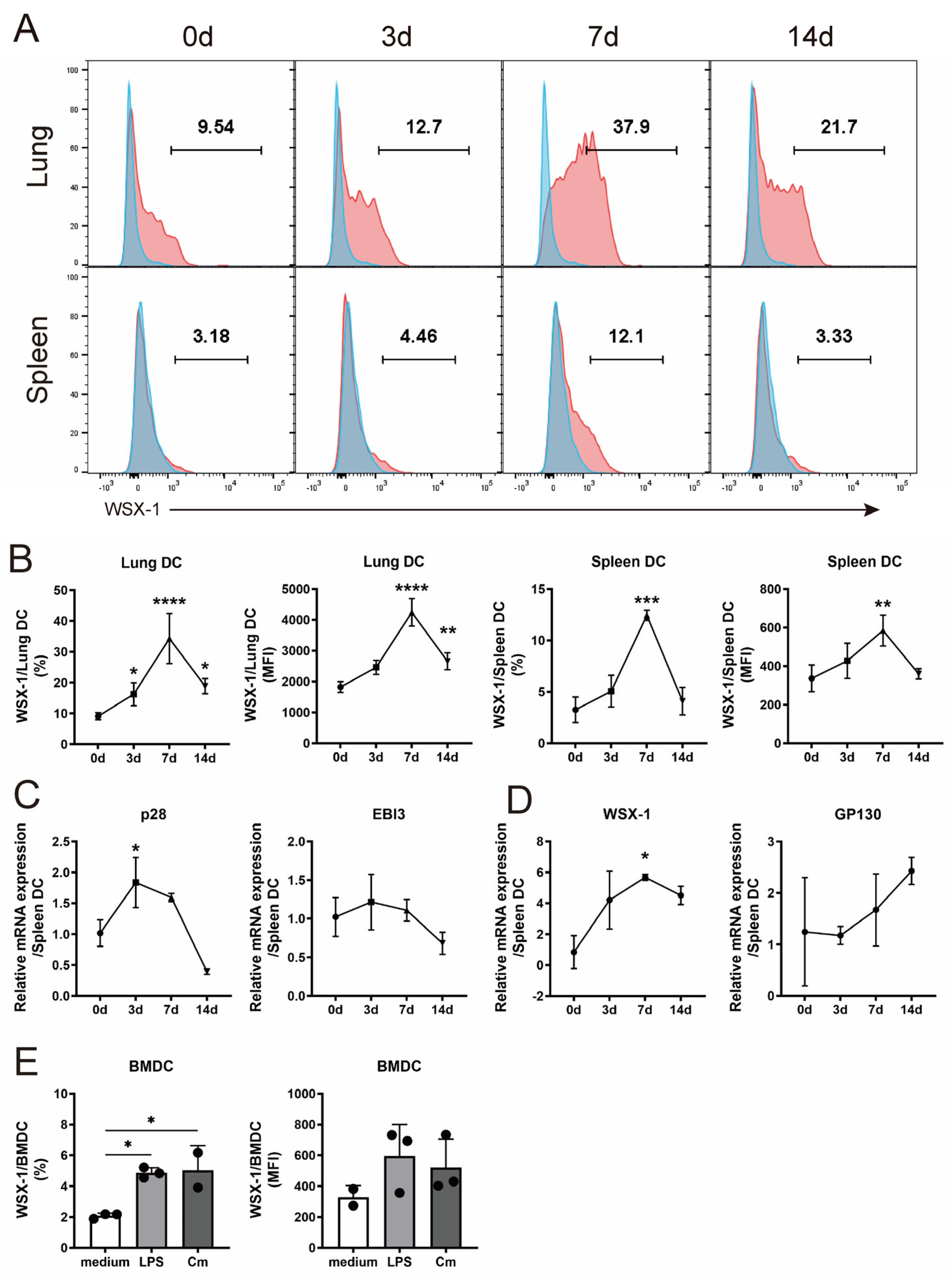
3.1. C. muridarum Respiratory Infection Induces IL-27/IL-27R Expression of DC
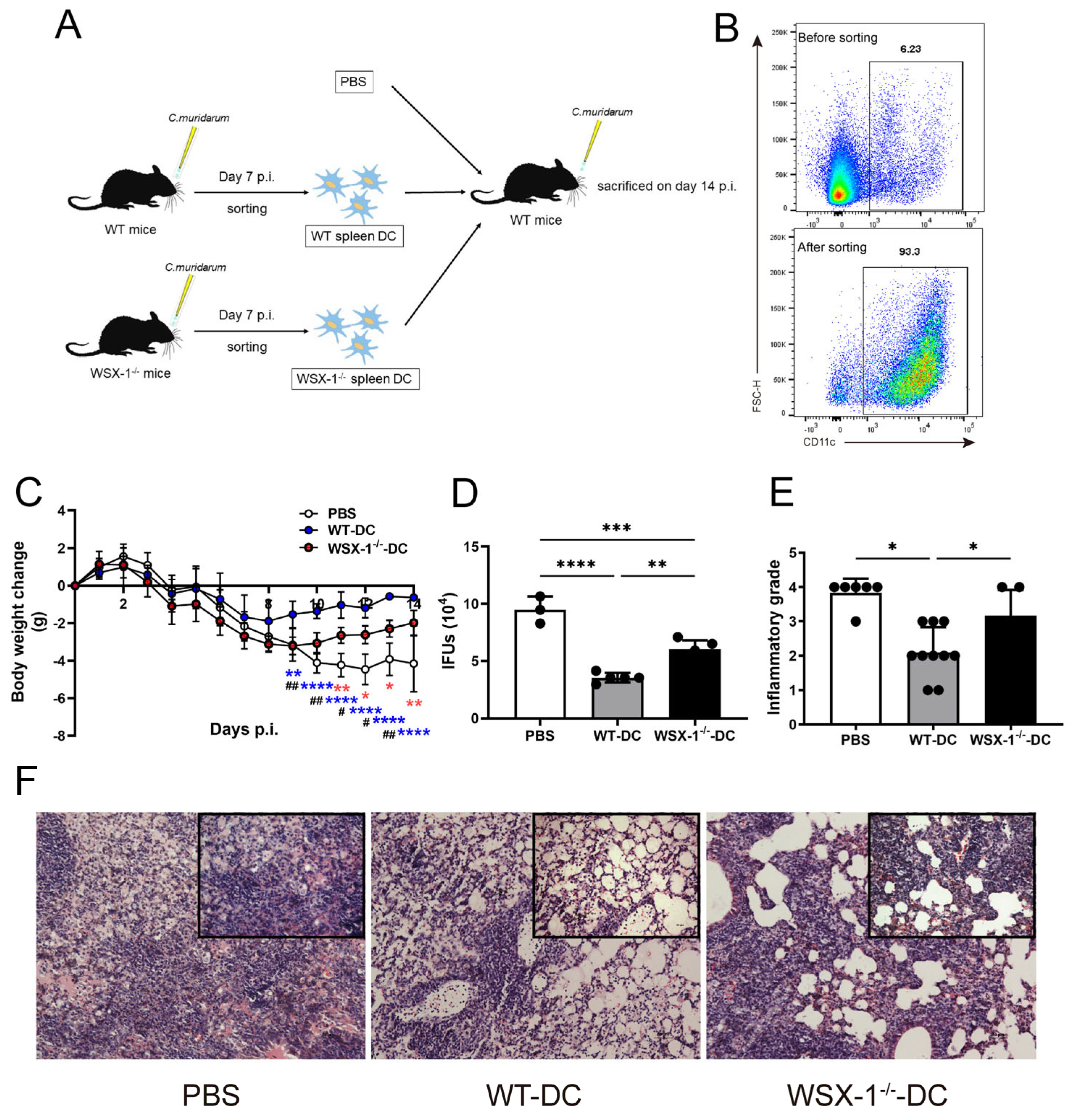
3.2. Adoptive Transfer of WSX-1−/− DC Reduces Protection against C. muridarum Respiratory Infection
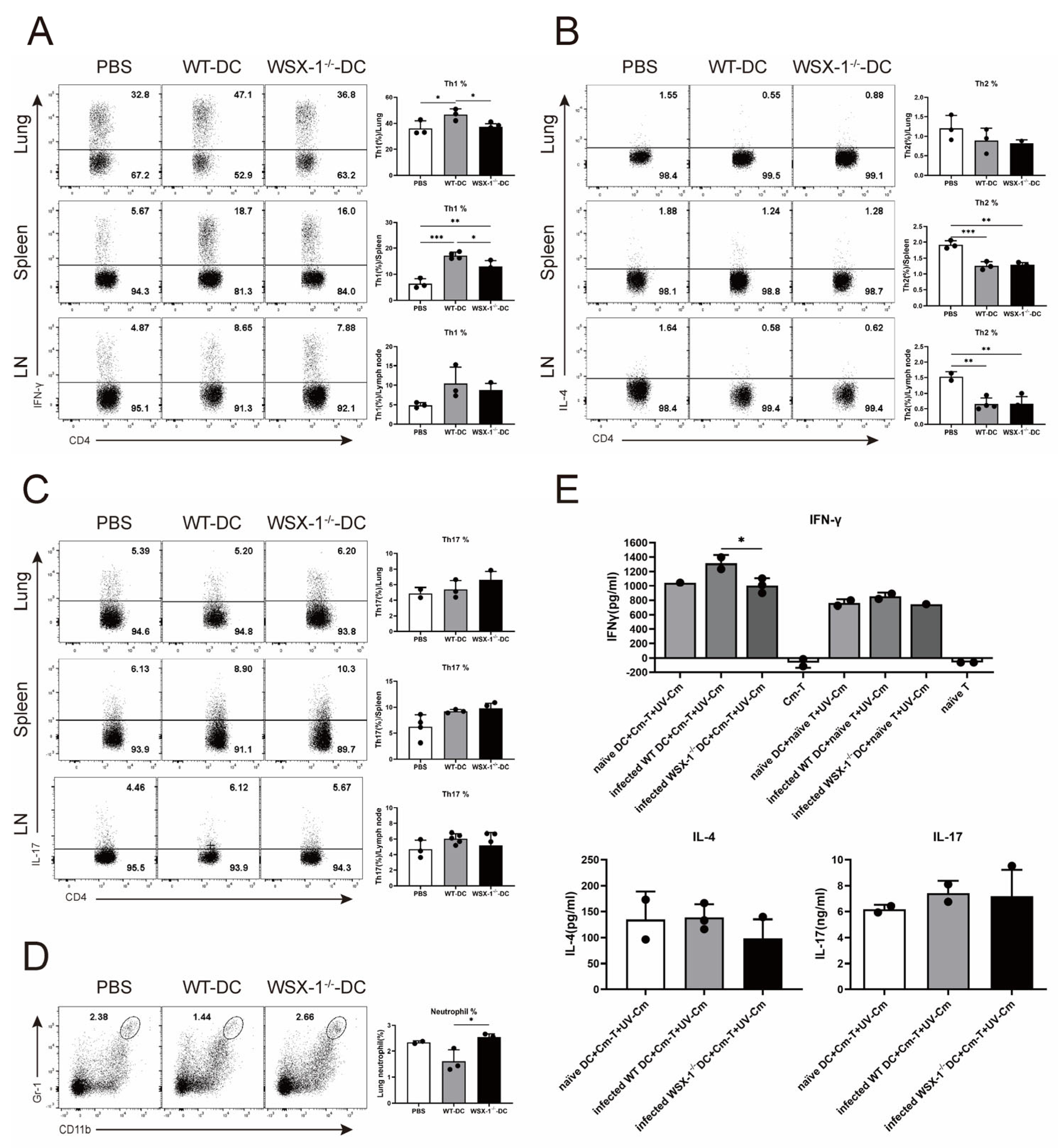
3.3. Adoptive Transfer of WSX-1−/− DC Reduces Th1 Responses following C. muridarum Respiratory Infection
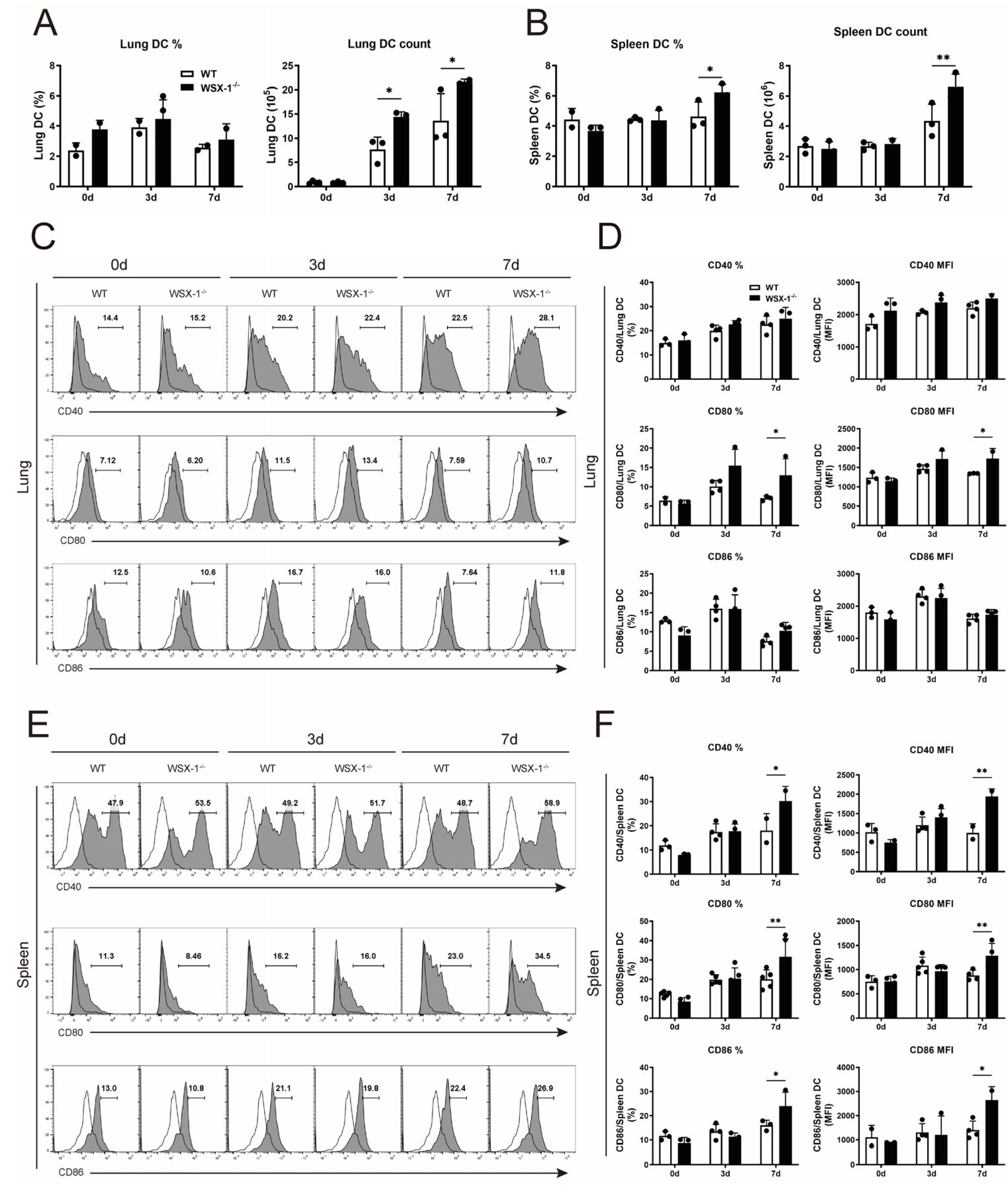
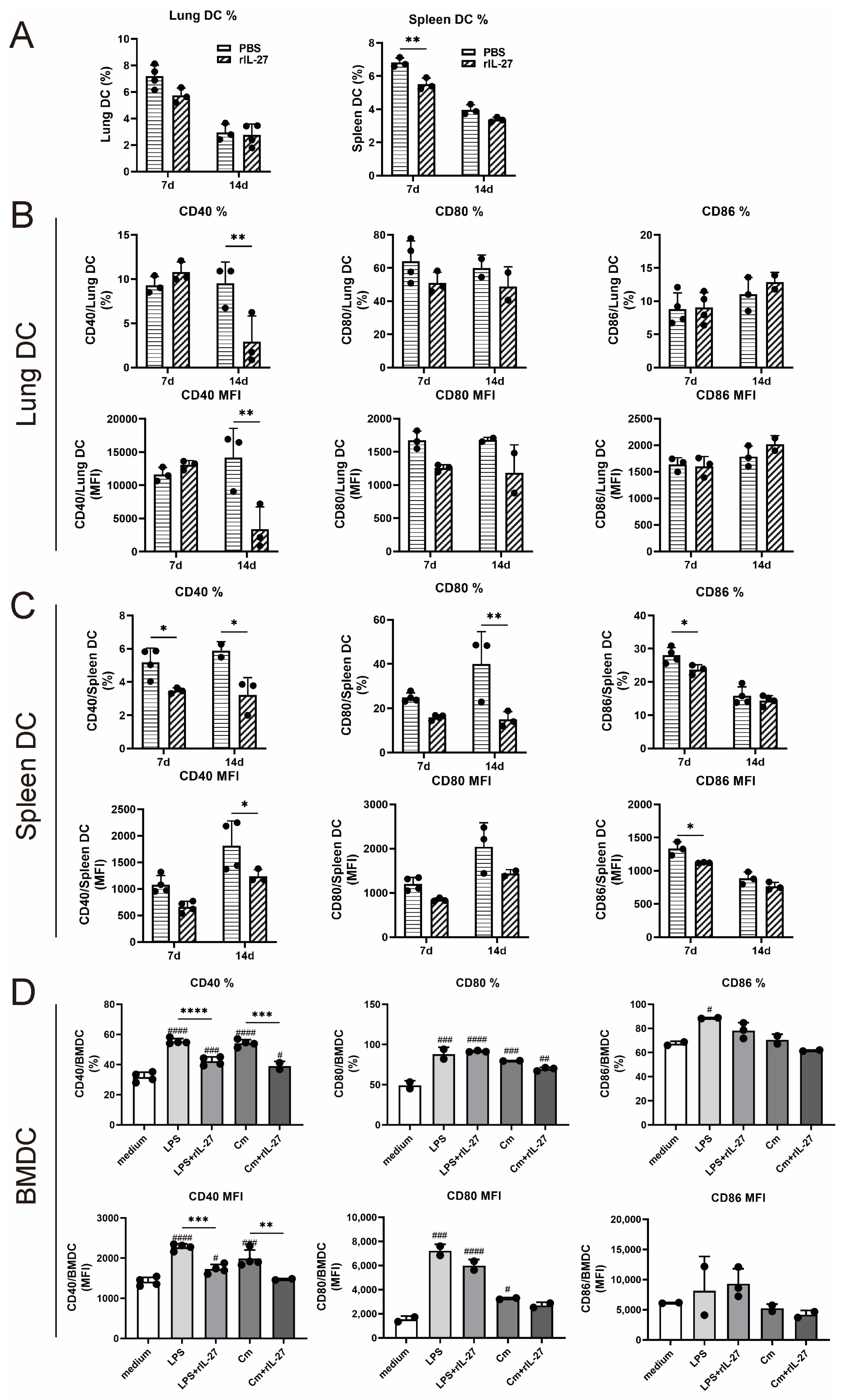
3.4. Deficiency of WSX-1 Promotes the Accumulation and Phenotypic Maturation of DCs following Chlamydial Respiratory Infection
3.5. rIL-27 Treatment Suppresses the Accumulation and Phenotypic Maturation of DC following Chlamydial Respiratory Infection
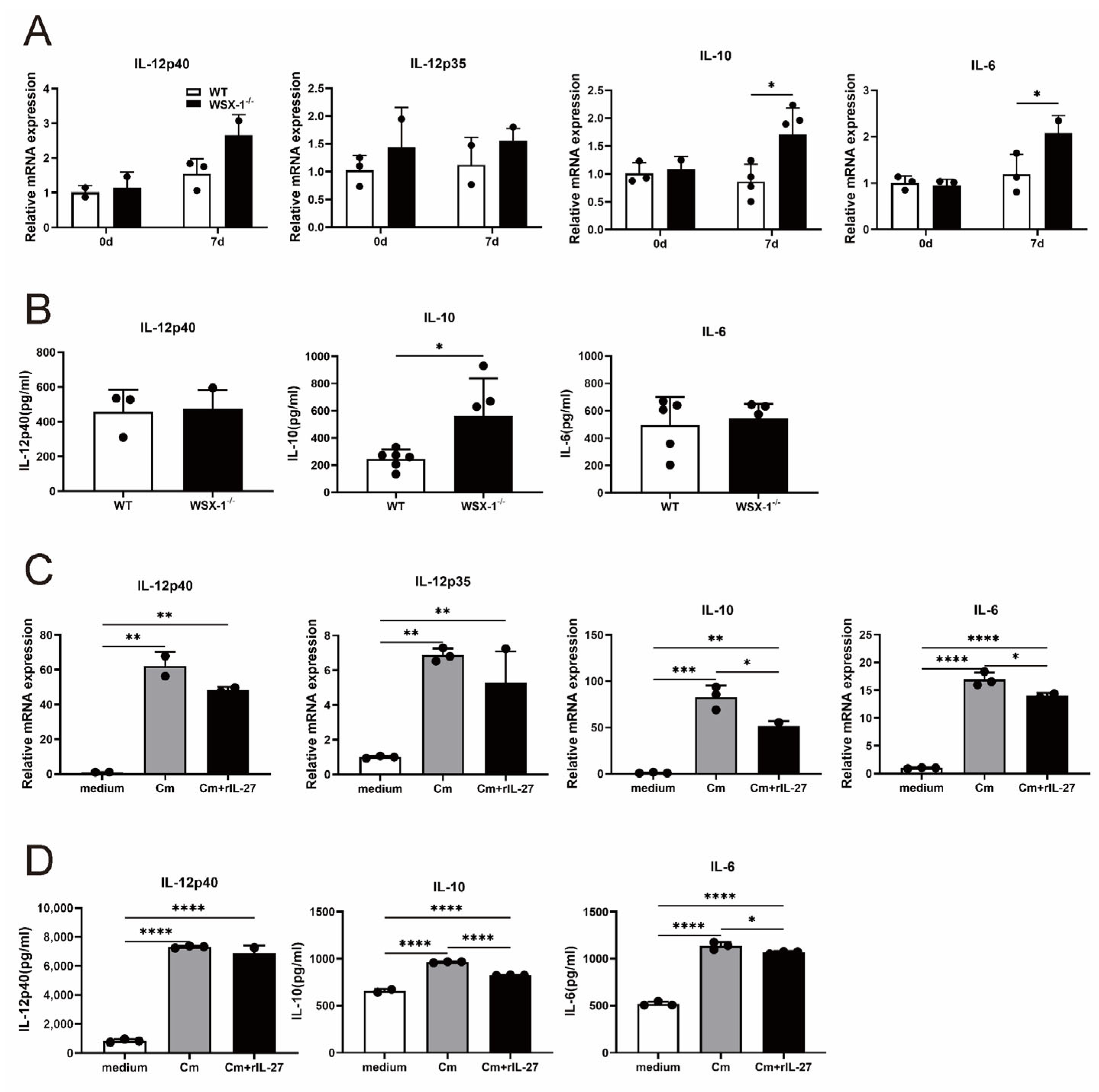
3.6. IL-27 Signaling Inhibits IL-10 Production in DCs following C. muridarum Respiratory Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murthy, A.K.; Li, W.; Ramsey, K.H. Immunopathogenesis of Chlamydial Infections. Curr. Top. Microbiol. Immunol. 2018, 412, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Klockner, A.; Buhl, H.; Viollier, P.; Henrichfreise, B. Deconstructing the Chlamydial Cell Wall. Curr. Top. Microbiol. Immunol. 2018, 412, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Cheng, J.; Gao, X.; Joyee, A.G.; Fan, Y.; Wang, S.; Jiao, L.; Yao, Z.; Yang, X. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J. Immunol. 2009, 183, 5886–5895. [Google Scholar] [CrossRef] [PubMed]
- Ziklo, N.; Huston, W.M.; Hocking, J.S.; Timms, P. Chlamydia trachomatis Genital Tract Infections: When Host Immune Response and the Microbiome Collide. Trends Microbiol. 2016, 24, 750–765. [Google Scholar] [CrossRef]
- De Clercq, E.; Kalmar, I.; Vanrompay, D. Animal models for studying female genital tract infection with Chlamydia trachomatis. Infect. Immun. 2013, 81, 3060–3067. [Google Scholar] [CrossRef]
- Giebel, A.M.; Hu, S.; Rajaram, K.; Finethy, R.; Toh, E.; Brothwell, J.A.; Morrison, S.G.; Suchland, R.J.; Stein, B.D.; Coers, J.; et al. Genetic Screen in Chlamydia muridarum Reveals Role for an Interferon-Induced Host Cell Death Program in Antimicrobial Inclusion Rupture. mBio 2019, 10, e00385-19. [Google Scholar] [CrossRef]
- Roan, N.R.; Starnbach, M.N. Immune-mediated control of Chlamydia infection. Cell Microbiol. 2008, 10, 9–19. [Google Scholar] [CrossRef]
- Rottenberg, M.E.; Gigliotti-Rothfuchs, A.; Wigzell, H. The role of IFN-gamma in the outcome of chlamydial infection. Curr. Opin. Immunol. 2002, 14, 444–451. [Google Scholar] [CrossRef]
- Zha, X.; Yang, S.; Niu, W.; Tan, L.; Xu, Y.; Zeng, J.; Tang, Y.; Sun, L.; Pang, G.; Qiao, S.; et al. IL-27/IL-27R Mediates Protective Immunity against Chlamydial Infection by Suppressing Excessive Th17 Responses and Reducing Neutrophil Inflammation. J. Immunol. 2021, 206, 2160–2169. [Google Scholar] [CrossRef]
- Qian, C.; Cao, X. Dendritic cells in the regulation of immunity and inflammation. Semin. Immunol. 2018, 35, 3–11. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Rey-Ladino, J.; Jiang, X.; Gabel, B.R.; Shen, C.; Brunham, R.C. Survival of Chlamydia muridarum within dendritic cells. Infect. Immun. 2007, 75, 3707–3714. [Google Scholar] [CrossRef]
- Rey-Ladino, J.; Koochesfahani, K.M.; Zaharik, M.L.; Shen, C.; Brunham, R.C. A live and inactivated Chlamydia trachomatis mouse pneumonitis strain induces the maturation of dendritic cells that are phenotypically and immunologically distinct. Infect. Immun. 2005, 73, 1568–1577. [Google Scholar] [CrossRef]
- Bilenki, L.; Wang, S.; Yang, J.; Fan, Y.; Jiao, L.; Joyee, A.G.; Han, X.; Yang, X. Adoptive transfer of CD8alpha+ dendritic cells (DC) isolated from mice infected with Chlamydia muridarum are more potent in inducing protective immunity than CD8alpha- DC. J. Immunol. 2006, 177, 7067–7075. [Google Scholar] [CrossRef]
- Shekhar, S.; Peng, Y.; Wang, S.; Yang, X. CD103+ lung dendritic cells (LDCs) induce stronger Th1/Th17 immunity to a bacterial lung infection than CD11b(hi) LDCs. Cell Mol. Immunol. 2018, 15, 377–387. [Google Scholar] [CrossRef]
- Vignali, D.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef]
- Hunter, C.A.; Kastelein, R. Interleukin-27: Balancing protective and pathological immunity. Immunity 2012, 37, 960–969. [Google Scholar] [CrossRef]
- Morita, Y.; Masters, E.A.; Schwarz, E.M.; Muthukrishnan, G. Interleukin-27 and Its Diverse Effects on Bacterial Infections. Front. Immunol. 2021, 12, 678515. [Google Scholar] [CrossRef]
- Yoshida, H.; Hunter, C.A. The immunobiology of interleukin-27. Annu. Rev. Immunol. 2015, 33, 417–443. [Google Scholar] [CrossRef]
- Harker, J.A.; Wong, K.A.; Dallari, S.; Bao, P.; Dolgoter, A.; Jo, Y.; Wehrens, E.J.; Macal, M.; Zuniga, E.I. Interleukin-27R Signaling Mediates Early Viral Containment and Impacts Innate and Adaptive Immunity after Chronic Lymphocytic Choriomeningitis Virus Infection. J. Virol. 2018, 92, e02196-17. [Google Scholar] [CrossRef]
- Cao, J.; Wang, D.; Xu, F.; Gong, Y.; Wang, H.; Song, Z.; Li, D.; Zhang, H.; Li, D.; Zhang, L.; et al. Activation of IL-27 signalling promotes development of postinfluenza pneumococcal pneumonia. EMBO Mol. Med. 2014, 6, 120–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, J.; Li, C.; Zhang, L. IL-27/IL-27 Receptor Signaling Provides Protection in Clostridium difficile-Induced Colitis. J. Infect. Dis. 2018, 217, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Lee, B.; Scheller, E.V.; Mandalapu, S.; Enelow, R.I.; Kolls, J.K.; Alcorn, J.F. The role of IL-27 in susceptibility to post-influenza Staphylococcus aureus pneumonia. Respir. Res. 2015, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Peng, Y.; Gao, X.; Joyee, A.G.; Wang, S.; Bai, H.; Zhao, L.; Yang, J.; Yang, X. NK cells modulate the lung dendritic cell-mediated Th1/Th17 immunity during intracellular bacterial infection. Eur. J. Immunol. 2015, 45, 2810–2820. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Sun, L.; Pang, G.; Zha, X.; Niu, W.; Tan, L.; Zhang, H.; Bai, H. Chlamydia muridarum infection induces CD4+ T cells apoptosis via PI3K/AKT signal pathway. Pathog. Dis. 2019, 77, ftz029. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.L.; Feilzer, K.; Caldwell, H.D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 1997, 158, 3344–3352. [Google Scholar] [CrossRef]
- Steinman, R.M.; Idoyaga, J. Features of the dendritic cell lineage. Immunol. Rev. 2010, 234, 5–17. [Google Scholar] [CrossRef]
- Reis e Sousa, C. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006, 6, 476–483. [Google Scholar] [CrossRef]
- Steinman, R.M. Decisions about dendritic cells: Past, present, and future. Annu. Rev. Immunol. 2012, 30, 1–22. [Google Scholar] [CrossRef]
- Fujii, S.; Liu, K.; Smith, C.; Bonito, A.J.; Steinman, R.M. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 2004, 199, 1607–1618. [Google Scholar] [CrossRef]
- Ugur, M.; Mueller, S.N. T cell and dendritic cell interactions in lymphoid organs: More than just being in the right place at the right time. Immunol. Rev. 2019, 289, 115–128. [Google Scholar] [CrossRef]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, J. CD4 T Helper Cell Subsets and Related Human Immunological Disorders. Int. J. Mol. Sci. 2020, 21, 8011. [Google Scholar] [CrossRef]
- Perona-Wright, G.; Jenkins, S.J.; Crawford, A.; Gray, D.; Pearce, E.J.; MacDonald, A.S. Distinct sources and targets of IL-10 during dendritic cell-driven Th1 and Th2 responses in vivo. Eur. J. Immunol. 2006, 36, 2367–2375. [Google Scholar] [CrossRef]
- Rasquinha, M.T.; Sur, M.; Lasrado, N.; Reddy, J. IL-10 as a Th2 Cytokine: Differences between Mice and Humans. J. Immunol. 2021, 207, 2205–2215. [Google Scholar] [CrossRef]
- Cribas, E.S.; Denny, J.E.; Maslanka, J.R.; Abt, M.C. Loss of Interleukin-10 (IL-10) Signaling Promotes IL-22-Dependent Host Defenses against Acute Clostridioides difficile Infection. Infect. Immunol. 2021, 89, e00730-20. [Google Scholar] [CrossRef]
- Clark, S.E.; Schmidt, R.L.; Aguilera, E.R.; Lenz, L.L. IL-10-producing NK cells exacerbate sublethal Streptococcus pneumoniae infection in the lung. Transl. Res. 2020, 226, 70–82. [Google Scholar] [CrossRef]
- Ferreira, C.M.; Barbosa, A.M.; Barreira-Silva, P.; Silvestre, R.; Cunha, C.; Carvalho, A.; Rodrigues, F.; Correia-Neves, M.; Castro, A.G.; Torrado, E. Early IL-10 promotes vasculature-associated CD4+ T cells unable to control Mycobacterium tuberculosis infection. JCI Insight 2021, 6, e150060. [Google Scholar] [CrossRef]
- Wynn, T.A.; Cheever, A.W.; Williams, M.E.; Hieny, S.; Caspar, P.; Kühn, R.; Müller, W.; Sher, A. IL-10 regulates liver pathology in acute murine Schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J. Immunol. 1998, 160, 4473–4480. [Google Scholar] [CrossRef]
- Yang, X.; Gartner, J.; Zhu, L.; Wang, S.; Brunham, R.C. IL-10 gene knockout mice show enhanced Th1-like protective immunity and absent granuloma formation following Chlamydia trachomatis lung infection. J. Immunol. 1999, 162, 1010–1017. [Google Scholar] [CrossRef]
- Jiao, L.; Gao, X.; Joyee, A.G.; Zhao, L.; Qiu, H.; Yang, M.; Fan, Y.; Wang, S.; Yang, X. NK cells promote type 1 T cell immunity through modulating the function of dendritic cells during intracellular bacterial infection. J. Immunol. 2011, 187, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014, 33, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Steinman, R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Hilligan, K.L.; Ronchese, F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol. Immunol. 2020, 17, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Curato, C.; Bernshtein, B.; Zupancic, E.; Dufner, A.; Jaitin, D.; Giladi, A.; David, E.; Chappell-Maor, L.; Leshkowitz, D.; Knobeloch, K.P.; et al. DC Respond to Cognate T Cell Interaction in the Antigen-Challenged Lymph Node. Front. Immunol. 2019, 10, 863. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Yang, S.; Tuo, Y.; Zha, X.; Sun, R.; Lu, T.; Zhang, H.; Tan, L.; Qiao, S.; Bai, H. IL-27 Signaling Promotes Th1 Response by Downregulating IL-10 Production in DCs during Chlamydial Respiratory Infection. Microorganisms 2023, 11, 604. https://doi.org/10.3390/microorganisms11030604
Zeng J, Yang S, Tuo Y, Zha X, Sun R, Lu T, Zhang H, Tan L, Qiao S, Bai H. IL-27 Signaling Promotes Th1 Response by Downregulating IL-10 Production in DCs during Chlamydial Respiratory Infection. Microorganisms. 2023; 11(3):604. https://doi.org/10.3390/microorganisms11030604
Chicago/Turabian StyleZeng, Jiajia, Shuaini Yang, Yuqing Tuo, Xiaoyu Zha, Ruoyuan Sun, Tingsha Lu, Hong Zhang, Lu Tan, Sai Qiao, and Hong Bai. 2023. "IL-27 Signaling Promotes Th1 Response by Downregulating IL-10 Production in DCs during Chlamydial Respiratory Infection" Microorganisms 11, no. 3: 604. https://doi.org/10.3390/microorganisms11030604




