Nitrobenzoates and Nitrothiobenzoates with Activity against M. tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
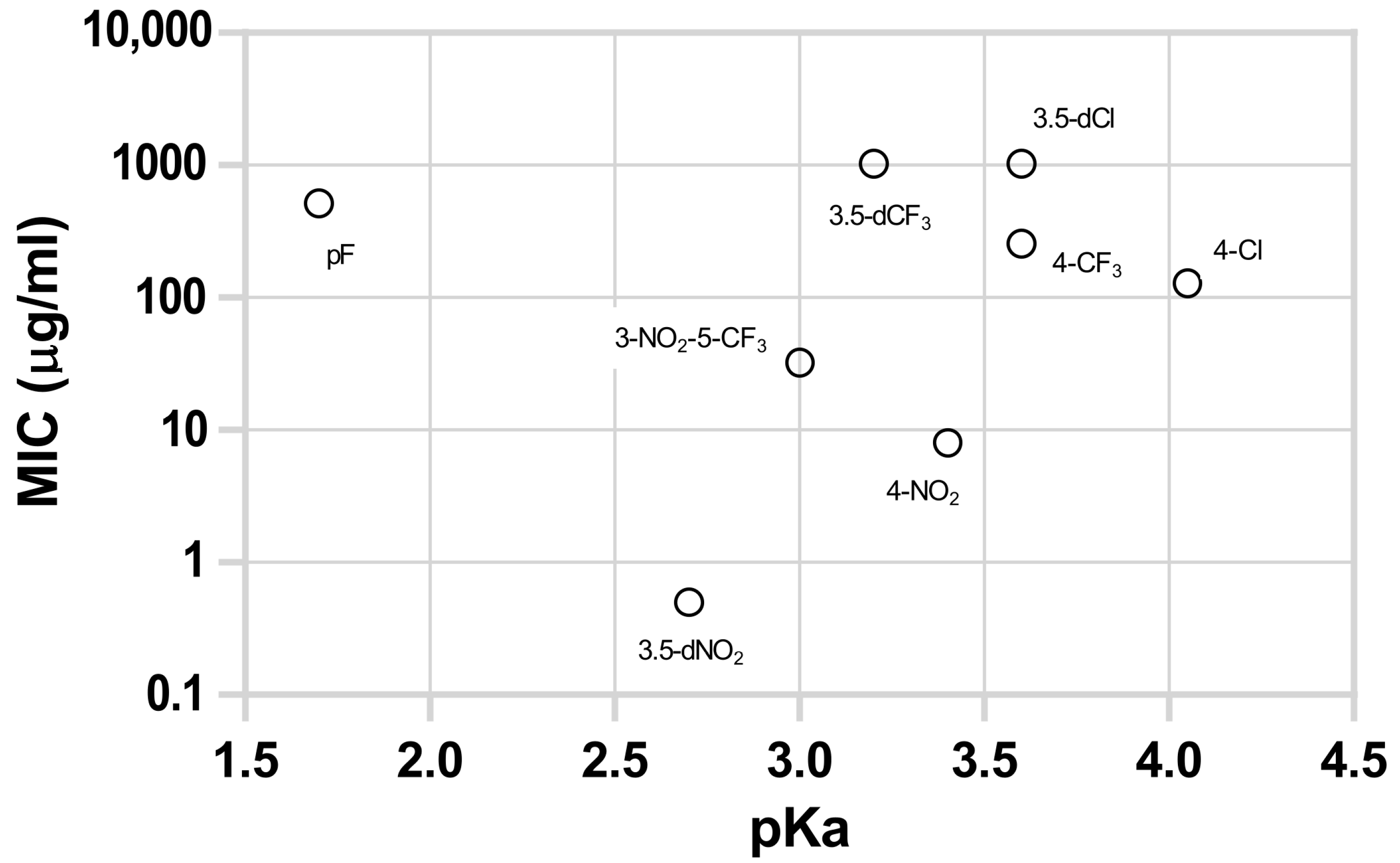
3.1. Activity against M. tuberculosis
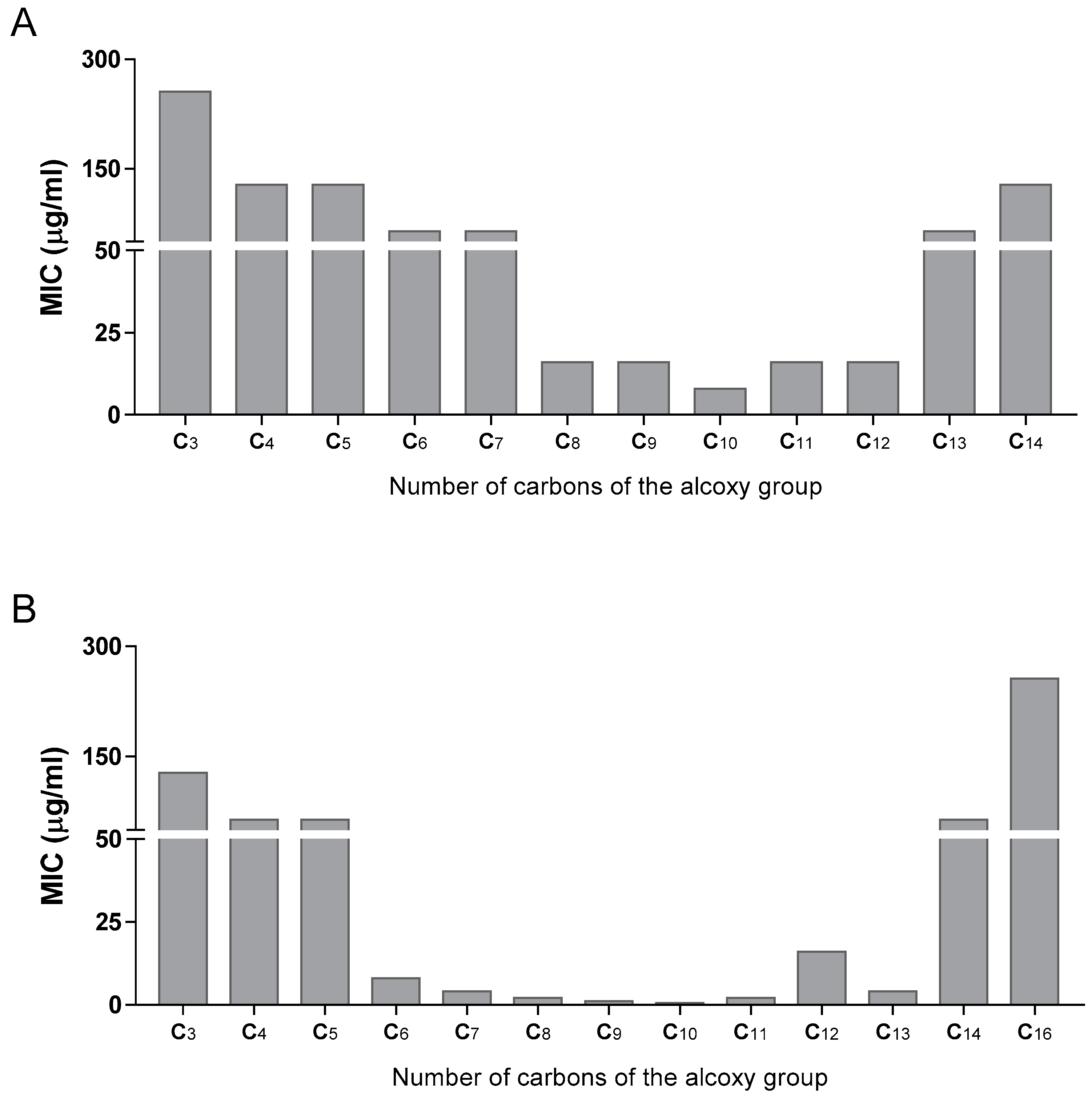
3.1.1. Esters
3.1.2. Thioesters
3.2. Cytotoxicity
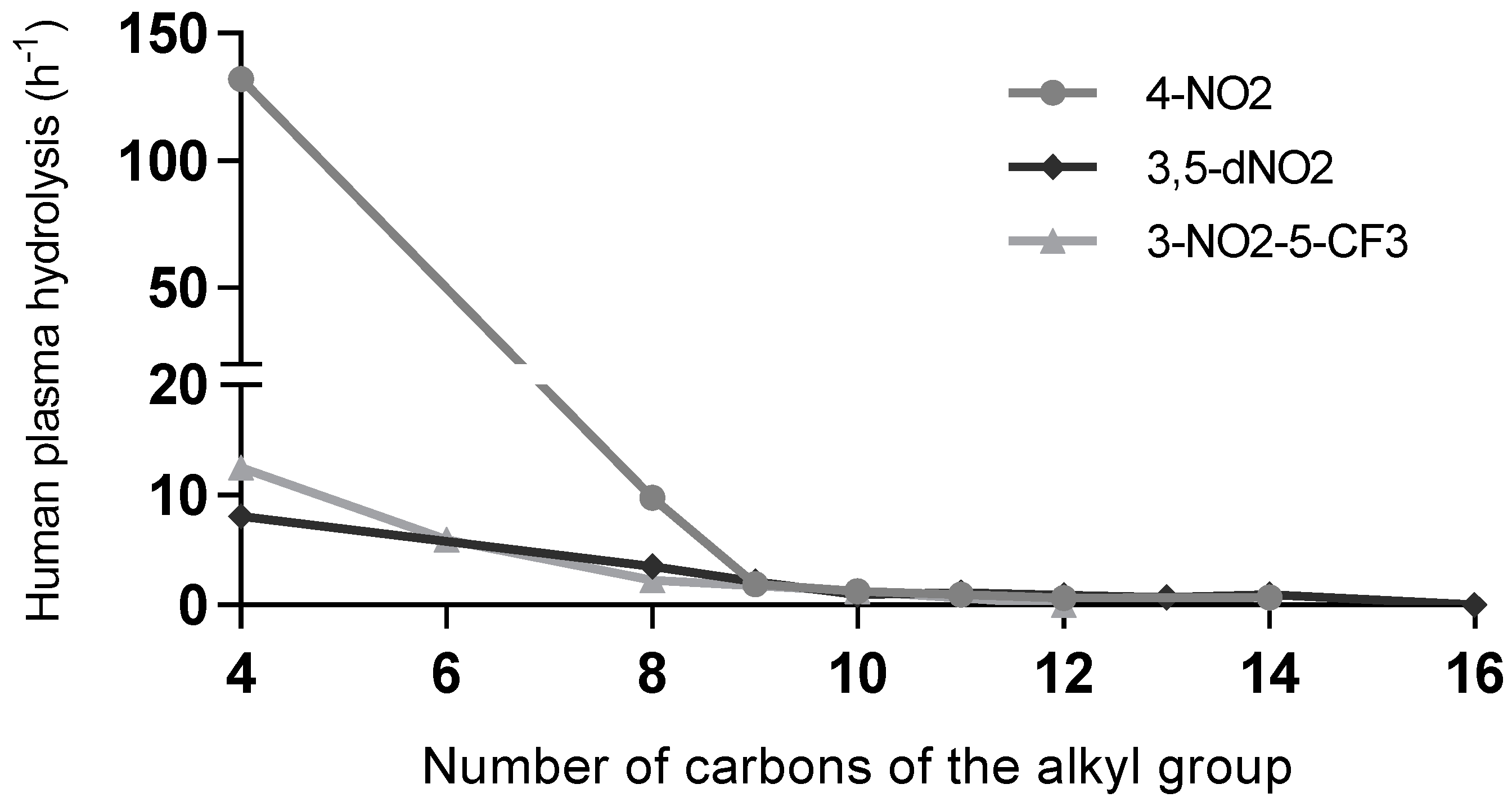
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barberis, I.; Bragazzi, N.L.; Galluzzo, L.; Martini, M. The History of Tuberculosis: From the First Historical Records to the Isolation of Koch’s Bacillus. J. Prev. Med. Hyg. 2017, 58, E9. [Google Scholar]
- Zink, A.R.; Sola, C.; Reischl, U.; Grabner, W.; Rastogi, N.; Wolf, H.; Nerlich, A.G. Characterization of Mycobacterium Tuberculosis Complex DNAs from Egyptian Mummies by Spoligotyping. J. Clin. Microbiol. 2003, 41, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pai, M.; Kasaeva, T.; Swaminathan, S. COVID-19’s Devastating Effect on Tuberculosis Care—A Path to Recovery. N. Engl. J. Med. 2022, 386, 1490–1493. [Google Scholar] [CrossRef]
- Global Tuberculosis Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- Jordao, L.; Bleck, C.K.E.; Mayorga, L.; Griffiths, G.; Anes, E. On the Killing of Mycobacteria by Macrophages. Cell. Microbiol. 2008, 10, 529–548. [Google Scholar] [CrossRef] [PubMed]
- Pethe, K.; Swenson, D.L.; Alonso, S.; Anderson, J.; Wang, C.; Russell, D.G. Isolation of Mycobacterium Tuberculosis Mutants Defective in the Arrest of Phagosome Maturation. Proc. Natl. Acad. Sci. USA 2004, 101, 13642–13647. [Google Scholar] [CrossRef] [Green Version]
- MacGurn, J.A.; Cox, J.S. A Genetic Screen for Mycobacterium Tuberculosis Mutants Defective for Phagosome Maturation Arrest Identifies Components of the ESX-1 Secretion System. Infect. Immun. 2007, 75, 2668–2678. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.S.; Paul, S.; Moreira, A.L.; Appelberg, R.; Rabinovitch, M.; Kaplan, G. Survival of Mycobacterium Avium and Mycobacterium Tuberculosis in Acidified Vacuoles of Murine Macrophages. Infect. Immun. 1999, 67, 3199–3206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, H.; Sun, Z. Susceptibility of Mycobacterium Tuberculosis to Weak Acids. J. Antimicrob. Chemother. 2003, 52, 56–60. [Google Scholar] [CrossRef]
- Gu, P.; Constantino, L.; Zhang, Y. Enhancement of the Antituberculosis Activity of Weak Acids by Inhibitors of Energy Metabolism but Not by Anaerobiosis Suggests That Weak Acids Act Differently from the Front-Line Tuberculosis Drug Pyrazinamide. J. Med. Microbiol. 2008, 57, 1129–1134. [Google Scholar] [CrossRef]
- Valente, E.; Simões, M.F.; Testa, B.; Constantino, L. Development of a Method to Investigate the Hydrolysis of Xenobiotic Esters by a Mycobacterium Smegmatis Homogenate. J. Microbiol. Methods 2011, 85, 98–102. [Google Scholar] [CrossRef]
- Valente, E.; Testa, B.; Constantino, L. Activation of Benzoate Model Prodrugs by Mycobacteria. Comparison with Mammalian Plasma and Liver Hydrolysis. Eur. J. Pharm. Sci. 2021, 162, 105831. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.F.; Valente, E.; Gómez, M.J.R.; Anes, E.; Constantino, L. Lipophilic Pyrazinoic Acid Amide and Ester Prodrugs Stability, Activation and Activity against M. Tuberculosis. Eur. J. Pharm. Sci. 2009, 37, 257–263. [Google Scholar] [CrossRef]
- Pires, D.; Valente, E.; Simões, M.F.; Carmo, N.; Testa, B.; Constantino, L.; Anes, E. Esters of Pyrazinoic Acid Are Active against Pyrazinamide-Resistant Strains of Mycobacterium Tuberculosis and Other Naturally Resistant Mycobacteria in Vitro and Ex Vivo within Macrophages. Antimicrob. Agents Chemother. 2015, 59, 7693–7699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pais, J.P.; Magalhães, M.; Antoniuk, O.; Barbosa, I.; Freire, R.; Pires, D.; Valente, E.; Testa, B.; Anes, E.; Constantino, L. Benzoic Acid Derivatives as Prodrugs for the Treatment of Tuberculosis. Pharmaceuticals 2022, 15, 1118. [Google Scholar] [CrossRef] [PubMed]
- Hirshfield, I.N.; Terzulli, S.; O’Byrne, C. Weak Organic Acids: A Panoply of Effects on Bacteria. Sci. Prog. 2003, 86, 245–269. [Google Scholar] [CrossRef]
- Richter, A.; Rudolph, I.; Möllmann, U.; Voigt, K.; Chung, C.-W.; Singh, O.M.P.; Rees, M.; Mendoza-Losana, A.; Bates, R.; Ballell, L.; et al. Novel Insight into the Reaction of Nitro, Nitroso and Hydroxylamino Benzothiazinones and of Benzoxacinones with Mycobacterium Tuberculosis DprE1. Sci. Rep. 2018, 8, 13473. [Google Scholar] [CrossRef] [Green Version]
- de Jesus Lopes Ribeiro, A.L.; Degiacomi, G.; Ewann, F.; Buroni, S.; Incandela, M.L.; Chiarelli, L.R.; Mori, G.; Kim, J.; Contreras-Dominguez, M.; Park, Y.-S.; et al. Analogous Mechanisms of Resistance to Benzothiazinones and Dinitrobenzamides in Mycobacterium Smegmatis. PLoS ONE 2011, 6, e26675. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Wang, H.; Li, C.; Zhang, J.Z.H.; Ji, C. MolGpka: A Web Server for Small Molecule p K a Prediction Using a Graph-Convolutional Neural Network. J. Chem. Inf. Model. 2021, 61, 3159–3165. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 92nd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781439855126. [Google Scholar]
- Pearce, P.J.; Simkins, R.J.J. Acid Strengths of Some Substituted Picric Acids. Can. J. Chem. 1968, 46, 241–248. [Google Scholar] [CrossRef]
- Jover, J.; Bosque, R.; Sales, J. QSPR Prediction of p K a for Benzoic Acids in Different Solvents. QSAR Comb. Sci. 2008, 27, 563–581. [Google Scholar] [CrossRef]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual Computational Chemistry Laboratory—Design and Description. J. Comput. Aided. Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]
- Nepali, K.; Lee, H.Y.; Liou, J.P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef]
- Wolff, J.; Zhang, M.; Aguilera, D.; Das, C.; Vasquez, H.; Zage, P.; Gopalakrishnan, V. Measuring Cytotoxicity: A New Perspective on LC 50. Anticancer. Res. 2007, 27, 35–38. [Google Scholar]
- Agarwal, A.; Dhole, T.N.; Sharma, Y.K. Evaluation of P-Nitro Benzoic Acid (Pnb) Inhibition Test to Differentiate Mycobacterium Tuberculosis Complex from Non-Tuberculous Mycobacteria Using Microscopic Observation of Drug Susceptibility (MODS) Methodology. Indian J. Tuberc. 2014, 61, 232–235. [Google Scholar] [PubMed]
- Christophe, T.; Jackson, M.; Hee, K.J.; Fenistein, D.; Contreras-Dominguez, M.; Kim, J.; Genovesio, A.; Carralot, J.P.; Ewann, F.; Kim, E.H.; et al. High Content Screening Identifies Decaprenyl-Phosphoribose 2′ Epimerase as a Target for Intracellular Antimycobacterial Inhibitors. PLoS Pathog. 2009, 5, e1000645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Huang, G.; Wang, B.; Lv, K.; Wang, H.; Tao, Z.; Liu, M.; Guo, H.; Lu, Y. Design, Synthesis and Antimycobacterial Activity of 3,5-Dinitrobenzamide Derivatives Containing Fused Ring Moieties. Bioorganic Med. Chem. Lett. 2018, 28, 2945–2948. [Google Scholar] [CrossRef]
- Wang, H.; Lv, K.; Li, X.; Wang, B.; Wang, A.; Tao, Z.; Geng, Y.; Wang, B.; Huang, M.; Liu, M.; et al. Design, Synthesis and Antimycobacterial Activity of Novel Nitrobenzamide Derivatives. Chin. Chem. Lett. 2019, 30, 413–416. [Google Scholar] [CrossRef] [Green Version]
- Mikušová, K.; Huang, H.; Yagi, T.; Holsters, M.; Vereecke, D.; D’Haeze, W.; Scherman, M.S.; Brennan, P.J.; McNeil, M.R.; Crick, D.C. Decaprenylphosphoryl Arabinofuranose, the Donor of the <scp>d</Scp> -Arabinofuranosyl Residues of Mycobacterial Arabinan, Is Formed via a Two-Step Epimerization of Decaprenylphosphoryl Ribose. J. Bacteriol. 2005, 187, 8020–8025. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Driessen, N.N.; Appelmelk, B.J.; Besra, G.S. Lipoarabinomannan and Related Glycoconjugates: Structure, Biogenesis and Role in Mycobacterium Tuberculosis Physiology and Host–Pathogen Interaction. FEMS Microbiol. Rev. 2011, 35, 1126–1157. [Google Scholar] [CrossRef] [Green Version]
- Incandela, M.L.; Perrin, E.; Fondi, M.; de Jesus Lopes Ribeiro, A.L.; Mori, G.; Moiana, A.; Gramegna, M.; Fani, R.; Riccardi, G.; Pasca, M.R. DprE1, a New Taxonomic Marker in Mycobacteria. FEMS Microbiol. Lett. 2013, 348, 66–73. [Google Scholar] [CrossRef]
- Brecik, M.; Centárová, I.; Mukherjee, R.; Kolly, G.S.; Huszár, S.; Bobovská, A.; Kilacsková, E.; Mokošová, V.; Svetlíková, Z.; Šarkan, M.; et al. DprE1 Is a Vulnerable Tuberculosis Drug Target Due to Its Cell Wall Localization. ACS Chem. Biol. 2015, 10, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Amado, P.S.M.; Woodley, C.; Cristiano, M.L.S.; O’Neill, P.M. Recent Advances of DprE1 Inhibitors against Mycobacterium Tuberculosis: Computational Analysis of Physicochemical and ADMET Properties. ACS Omega. 2022, 7, 40659–40681. [Google Scholar] [CrossRef]
- Batt, S.M.; Jabeen, T.; Bhowruth, V.; Quill, L.; Lund, P.A.; Eggeling, L.; Alderwick, L.J.; Fuẗterer, K.; Besra, G.S. Structural Basis of Inhibition of Mycobacterium Tuberculosis DprE1 by Benzothiazinone Inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 11354–11359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piton, J.; Foo, C.S.Y.; Cole, S.T. Structural Studies of Mycobacterium Tuberculosis DprE1 Interacting with Its Inhibitors. Drug Discov. Today 2017, 22, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Chikhale, R.V.; Barmade, M.A.; Murumkar, P.R.; Yadav, M.R. Overview of the Development of DprE1 Inhibitors for Combating the Menace of Tuberculosis. J. Med. Chem. 2018, 61, 8563–8593. [Google Scholar] [CrossRef]
- Degiacomi, G.; Belardinelli, J.M.; Pasca, M.R.; Rossi, E.D.; Riccardi, G.; Chiarelli, L.R. Promiscuous Targets for Antitubercular Drug Discovery: The Paradigm of DprE1 and MmpL3. Appl. Sci. 2020, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Huszár, S.; Chibale, K.; Singh, V. The Quest for the Holy Grail: New Antitubercular Chemical Entities, Targets and Strategies. Drug Discov. Today 2020, 25, 772–780. [Google Scholar] [CrossRef]
- Oh, S.; Trifonov, L.; Yadav, V.D.; Barry, C.E.; Boshoff, H.I. Tuberculosis Drug Discovery: A Decade of Hit Assessment for Defined Targets. Front. Cell. Infect. Microbiol. 2021, 11, 611304. [Google Scholar] [CrossRef]
- Mukherjee, T.; Boshoff, H. Nitroimidazoles for the Treatment of TB: Past, Present and Future NIH Public Access. Futur. Med. Chem 2011, 3, 1427–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudde, S.E.; Upton, A.M.; Lenaerts, A.; Bax, H.I.; De Steenwinkel, J.E.M. Delamanid or Pretomanid? A Solomonic Judgement! J. Antimicrob. Chemother. 2022, 77, 880–902. [Google Scholar] [CrossRef]
- Van den Bossche, A.; Varet, H.; Sury, A.; Sismeiro, O.; Legendre, R.; Coppee, J.Y.; Mathys, V.; Ceyssens, P.J. Transcriptional Profiling of a Laboratory and Clinical Mycobacterium Tuberculosis Strain Suggests Respiratory Poisoning upon Exposure to Delamanid. Tuberculosis 2019, 117, 18–23. [Google Scholar] [CrossRef] [PubMed]

 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | MW (g/mol) | pKa * | LogP ** | MIC (μg/mL) | MBC (μg/mL) |
| Esters | |||||||
| 1 | H | -CH2(CH2)2CH3 | 178.23 | 4.1 | 3.40 | >512 | >512 |
| 2 | -CH2(CH2)4CH3 | 206.29 | 4.51 | 512 | 512 | ||
| 3 | -CH2(CH2)6CH3 | 234.35 | 5.47 | 256 | 512 | ||
| 4 | -CH2(CH2)10CH3 | 290.45 | 7.16 | 256 | 512 | ||
| 5 | 4-Cl | -CH2(CH2)7CH3 | 282.81 | 4.0 | 6.42 | 64 | 256 |
| 6 | -CH(CH3)(CH2)6CH3 | 282.81 | 6.62 | 128 | 512 | ||
| 7 | -CH2(CH2)8CH3 | 296.84 | 6.86 | 128 | 256 | ||
| 8 | -CH2(CH2)9CH3 | 310.86 | 7.30 | 1024 | >1024 | ||
| 9 | -CH2(CH2)10CH3 | 324.89 | 7.70 | >1024 | >1024 | ||
| 10 | -CH2(CH2)12CH3 | 352.94 | 8.52 | >1024 | >1024 | ||
| 11 | -CH2(CH2)14CH3 | 381.00 | 9.25 | 1024 | 1024 | ||
| 12 | 3,5-dCl | -CH2(CH2)7CH3 | 317.25 | 3.6 | 6.87 | >1024 | >1024 |
| 13 | -CH(CH3)(CH2)6CH3 | 317.25 | 7.17 | 1024 | >1024 | ||
| 14 | -CH2(CH2)8CH3 | 331.28 | 7.33 | >1024 | >1024 | ||
| 15 | -CH2(CH2)9CH3 | 345.30 | 7.69 | >1024 | >1024 | ||
| 16 | -CH2(CH2)10CH3 | 359.33 | 8.10 | 1024 | 1024 | ||
| 17 | -CH2(CH2)12CH3 | 387.38 | 8.93 | 1024 | 1024 | ||
| 18 | 4-CF3 | -CH2(CH2)8CH3 | 330.39 | 3.6 | 6.45 | 256 | 1024 |
| 19 | 3,5-dCF3 | -CH2(CH2)8CH3 | 398.39 | 3.2 | 6.46 | >1024 | >1024 |
| 20 | 2,3,4,5,6-pF | -CH2(CH2)8CH3 | 352.35 | 1.7 | 5.42 | 512 | >1024 |
| 21 | 4-NO2 | -CH2CH2CH3 | 209.20 | 3.4 | 2.58 | 256 | 256 |
| 22 | -CH2(CH2)2CH3 | 223.23 | 2.83 | 128 | 256 | ||
| 23 | -CH2(CH2)3CH3 | 237.26 | 3.25 | 128 | 128 | ||
| 24 | -CH2(CH2)4CH3 | 251.28 | 3.73 | 64 | 128 | ||
| 25 | -CH2(CH2)5CH3 | 265.31 | 4.20 | 64 | 128 | ||
| 26 | -CH2(CH2)6CH3 | 279.34 | 4.69 | 16 | 64 | ||
| 27 | -CH2(CH2)7CH3 | 293.36 | 5.22 | 16 | 64 | ||
| 28 | -CH(CH3)(CH2)6CH3 | 293.36 | 5.58 | 16 | 64 | ||
| 29 | -CH2(CH2)8CH3 | 307.39 | 5.63 | 8 | 16 | ||
| 30 | -CH2(CH2)9CH3 | 321.41 | 6.09 | 16 | 64 | ||
| 31 | -CH2(CH2)10CH3 | 335.44 | 6.61 | 16 | 128 | ||
| 32 | -CH2(CH2)11CH3 | 349.47 | 7.07 | 64 | 128 | ||
| 33 | -CH2(CH2)12CH3 | 363.50 | 7.59 | 128 | 512 | ||
| 34 | 3,5-dNO2 | -CH2CH2CH3 | 254.20 | 2.7 | 2.40 | 128 | 256 |
| 35 | -CH2(CH2)2CH3 | 268.23 | 2.77 | 64 | 256 | ||
| 36 | -CH2(CH2)3CH3 | 282.26 | 3.06 | 64 | 128 | ||
| 37 | -CH2(CH2)4CH3 | 296.28 | 3.57 | 8 | 64 | ||
| 38 | -CH2(CH2)5CH3 | 310.31 | 4.05 | 4 | 16 | ||
| 39 | -CH2(CH2)6CH3 | 324.34 | 4.41 | 2 | 8 | ||
| 40 | -CH2(CH2)7CH3 | 338.36 | 4.74 | 1 | 4 | ||
| 41 | -CH(CH3)(CH2)6CH3 | 338.36 | 5.23 | 0.5 | 1 | ||
| 42 | -CH2(CH2)8CH3 | 352.39 | 5.18 | 0.5 | 2 | ||
| 43 | -CH2(CH2)9CH3 | 366.41 | 5.73 | 2 | 4 | ||
| 44 | -CH2(CH2)10CH3 | 380.44 | 6.08 | 16 | 32 | ||
| 45 | -CH2(CH2)11CH3 | 394.47 | 6.33 | 4 | 512 | ||
| 46 | -CH2(CH2)12CH3 | 408.50 | 6.62 | 64 | 256 | ||
| 47 | -CH2(CH2)14CH3 | 436.55 | 7.27 | 256 | 256 | ||
| 48 | 3-NO2-5-CF3 | -CH2(CH2)2CH3 | 291.23 | 3.0 | 3.53 | 256 | 1024 |
| 49 | -CH2(CH2)4CH3 | 319.28 | 4.34 | 64 | 1024 | ||
| 50 | -CH2(CH2)6CH3 | 347.34 | 4.97 | 16 | 64 | ||
| 51 | -CH2(CH2)8CH3 | 375.39 | 5.86 | 32 | 256 | ||
| 52 | -CH2(CH2)10CH3 | 403.44 | 6.79 | >1024 | >1024 | ||
| INH *** | 0.0625 | 0.125 | |||||
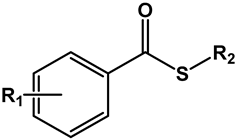 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | MW (g/mol) | pKa * | LogP ** | MIC (μg/mL) | MBC (μg/mL) |
| Thioesters | |||||||
| 53 | H | -CH2(CH2)2CH3 | 194.29 | 4.1 | 3.89 | 512 | >1024 |
| 54 | -CH2(CH2)6CH3 | 250.40 | 5.43 | 32 | 256 | ||
| 55 | -CH2(CH2)10CH3 | 306.50 | 7.07 | 1024 | >1024 | ||
| 56 | 4-NO2 | -CH2(CH2)2CH3 | 239.29 | 3.4 | 3.68 | 64 | 128 |
| 57 | -CH2(CH2)6CH3 | 295.40 | 5.16 | 32 | 128 | ||
| 58 | -CH2(CH2)10CH3 | 351.50 | 6.80 | 32 | 512 | ||
| 59 | 3,5-dNO2 | -CH2(CH2)2CH3 | 284.29 | 2.7 | 2.88 | 128 | 256 |
| 60 | -CH2(CH2)6CH3 | 340.40 | 4.55 | 16 | 64 | ||
| 61 | -CH2(CH2)10CH3 | 396.50 | 6.13 | 8 | 32 | ||
| 62 | 3-NO2-5-CF3 | -CH2(CH2)2CH3 | 307.29 | 3.0 | 3.59 | 128 | 512 |
| 63 | -CH2(CH2)6CH3 | 363.40 | 5.13 | 32 | 128 | ||
| 64 | -CH2(CH2)10CH3 | 419.50 | 6.79 | 128 | 512 | ||
| INH *** | 0.0625 | 0.125 | |||||
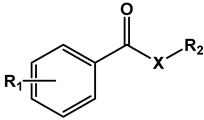 | |||||
|---|---|---|---|---|---|
| Compound | R1 | R2 | PBS (1 Day) | PBS (7 Days) | PBS (14 Days) |
| Esters (X = O) | |||||
| 1 | H | -CH2(CH2)2CH3 | 0 | 1 | 2 |
| 3 | -CH2(CH2)6CH3 | 0 | 0 | 0 | |
| 4 | -CH2(CH2)10CH3 | 0 | 0 | 0 | |
| 22 | 4-NO2 | -CH2(CH2)2CH3 | 0 | 2 | 4 |
| 26 | -CH2(CH2)6CH3 | 0 | 3 | 6 | |
| 31 | -CH2(CH2)10CH3 | 0 | 1 | 1 | |
| 35 | 3,5-dNO2 | -CH2(CH2)2CH3 | 10 | 40 | 65 |
| 39 | -CH2(CH2)6CH3 | 0 | 2 | 3 | |
| 44 | -CH2(CH2)10CH3 | 0 | 1 | 2 | |
| 48 | 3-NO2-5-CF3 | -CH2(CH2)2CH3 | 5 | 35 | 60 |
| 50 | -CH2(CH2)6CH3 | 0 | 3 | 6 | |
| 52 | -CH2(CH2)10CH3 | 1 | 6 | 11 | |
| Thioesters (X = S) | |||||
| 53 | H | -CH2(CH2)2CH3 | 0 | 0 | 0 |
| 54 | -CH2(CH2)6CH3 | 0 | 0 | 1 | |
| 55 | -CH2(CH2)10CH3 | 0 | 0 | 0 | |
| 56 | 4-NO2 | -CH2(CH2)2CH3 | 0 | 2 | 4 |
| 57 | -CH2(CH2)6CH3 | 0 | 0 | 1 | |
| 58 | -CH2(CH2)10CH3 | 0 | 0 | 0 | |
| 59 | 3,5-dNO2 | -CH2(CH2)2CH3 | 3 | 19 | 35 |
| 60 | -CH2(CH2)6CH3 | 0 | 1 | 3 | |
| 61 | -CH2(CH2)10CH3 | 0 | 0 | 1 | |
| 62 | 3-NO2-5-CF3 | -CH2(CH2)2CH3 | 1 | 10 | 18 |
| 63 | -CH2(CH2)6CH3 | 0 | 2 | 3 | |
| 64 | -CH2(CH2)10CH3 | 0 | 0 | 0 | |
| Compound | kobs HP (×100 h−1) | kobs MH (×100 h−1) | Compound | kobs HP (×100 h−1) | kobs MH (×100 h−1) |
|---|---|---|---|---|---|
| 1 | 44.1 ± 6.1 | 356 ± 17 | 40 | 2.140 ± 0.191 | |
| 3 | 2.66 ± 0.23 | 35.5 ± 0.3 | 41 | 0.869 ± 0.019 | n/o |
| 4 | n/o | 0.76 ± 0.02 | 42 | 0.98 ± 0.07 | n/o |
| 5 | 1.66 ± 0.025 | 43 | 1.130 ± 0.061 | ||
| 6 | 0.387 ± 0.025 | 44 | 0.89 ± 0.13 | n/o | |
| 7 | 1.05 ± 0.061 | 45 | 0.724 ± 0.020 | ||
| 8 | 0.899 ± 0.032 | 46 | 0.97 ± 0.23 | n/o | |
| 9 | 0.806 ± 0.010 | 47 | n/o | n/o | |
| 10 | 0.682 ± 0.013 | 48 | 12.5 ± 1.8 | 13.0 ± 1.1 | |
| 11 | n/o | 49 | 5.96 ± 1.28 | 0.67 ± 0.06 | |
| 12 | 1.04 ± 0.030 | 50 | 2.30 ± 0.06 | n/o | |
| 13 | 0.391 ± 0.021 | 51 | 1.26 ± 0.04 | n/o | |
| 14 | 0.796 ± 0.023 | 52 | n/o | n/o | |
| 15 | 0.676 ± 0.034 | Thioesters | |||
| 16 | 0.593 ± 0.026 | 53 | 92.3 ± 1.8 | 417 ± 10 | |
| 17 | n/o | 54 | 14.9 ± 0.9 | 26.9 ± 1.9 | |
| 22 | 132 ± 6.0 | 20.4 ± 3.3 | 55 | 0.60 ± 0.07 | 0.79 ± 0.08 |
| 26 | 9.73 ± 1.2 | 1.80 ± 0.38 | 56 | 34.5 ± 1.3 | 11.3 ± 0.8 |
| 27 | 1.87 ± 0.027 | 57 | 8.08 ± 0.21 | 3.20 ± 0.11 | |
| 28 | 0.800 ± 0.058 | 58 | |||
| 29 | 1.30 ± 0.061 | 59 | 10.3 ± 1.9 | 0.74 ± 0.03 | |
| 30 | 0.939 ± 0.053 | 60 | 11.8 ± 1.9 | 0.79 ± 0.02 | |
| 31 | 0.660 ± 0.10 | 0.88 ± 0.05 | 61 | 2.85 ± 0.21 | 0.38 ± 0.02 |
| 33 | 0.703 ± 0.042 | 62 | 7.86 ± 0.64 | 0.32 ± 0.03 | |
| 35 | 8.08 ± 0.29 | 11.0 ± 0.8 | 63 | 3.47 ± 0.54 | 0.40 ± 0.02 |
| 39 | 3.53 ± 0.44 | n/o | 64 | 0.78 ± 0.01 | 0.15 ± 0.01 |
| Compound | MIC (μg/mL) | LC50 (μg/mL) | T/A | Compound | MIC (μg/mL) | LC50 (μg/mL) | T/A |
|---|---|---|---|---|---|---|---|
| 5 | 64 | NT | NA | 34 | 128 | 571 | 4.46 |
| 6 | 128 | 600 | 4.68 | 35 | 64 | 456 | 7.13 |
| 7 | 128 | 350 | 2.73 | 36 | 64 | 138 | 2.16 |
| 8 | 1024 | 653 | 0.638 | 37 | 8 | 528 | 66.0 |
| 9 | >1024 | 624 | >0.609 | 38 | 4 | 1137 | 284 |
| 10 | >1024 | 461 | >0.450 | 39 | 2 | NT | NA |
| 11 | 1024 | NT | NA | 40 | 1 | 875 | 875 |
| 12 | >1024 | 151 | >0.147 | 41 | 1 | 561 | 561 |
| 13 | 1024 | 345 | 0.337 | 42 | 0.5 | NT | NA |
| 14 | >1024 | 144 | >0.141 | 43 | 2 | 515 | 257 |
| 15 | >1024 | 690 | >0.673 | 44 | 16 | NT | NA |
| 16 | 1024 | 516 | 0.504 | 45 | 4 | 184 | 46.0 |
| 17 | 1024 | 421 | 0.411 | 46 | 64 | NT | NA |
| 21 | 256 | NT | NA | 47 | 256 | NT | NA |
| 22 | 128 | NT | NA | 48 | 256 | 552 | 2.16 |
| 23 | 128 | NT | NA | 49 | 64 | 639 | 9.98 |
| 24 | 64 | 438 | 6.85 | 50 | 16 | NT | NA |
| 25 | 64 | 681 | 10.6 | 51 | 32 | NT | NA |
| 26 | 16 | 869 | 54.3 | 52 | >1024 | NT | NA |
| 27 | 16 | 1217 | 76.1 | 58 | 32 | NT | NA |
| 28 | 16 | 1174 | 73.4 | 61 | 8 | NT | NA |
| 29 | 8 | 103 | 12.8 | 64 | 128 | 513 | 4.00 |
| 30 | 16 | 51.1 | 3.19 | ||||
| 31 | 16 | 623 | 38.9 | ||||
| 32 | 64 | 417 | 6.51 | ||||
| 33 | 128 | 487 | 3.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pais, J.P.; Antoniuk, O.; Freire, R.; Pires, D.; Valente, E.; Anes, E.; Constantino, L. Nitrobenzoates and Nitrothiobenzoates with Activity against M. tuberculosis. Microorganisms 2023, 11, 969. https://doi.org/10.3390/microorganisms11040969
Pais JP, Antoniuk O, Freire R, Pires D, Valente E, Anes E, Constantino L. Nitrobenzoates and Nitrothiobenzoates with Activity against M. tuberculosis. Microorganisms. 2023; 11(4):969. https://doi.org/10.3390/microorganisms11040969
Chicago/Turabian StylePais, João P., Olha Antoniuk, Raquel Freire, David Pires, Emília Valente, Elsa Anes, and Luis Constantino. 2023. "Nitrobenzoates and Nitrothiobenzoates with Activity against M. tuberculosis" Microorganisms 11, no. 4: 969. https://doi.org/10.3390/microorganisms11040969





