The Tripartite Lichen Ricasolia virens: Involvement of Cyanobacteria and Bacteria in Its Morphogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens Used for Anatomical and Molecular Studies
2.2. Revised Samples in Different Herbaria
2.3. Molecular Studies
2.3.1. DNA Extraction, Amplification and Sequencing
2.3.2. Phylogenetic Analyses
2.4. Anatomical and Ultrastructural Analyses
2.4.1. Fixing of Samples
2.4.2. Observations under Light Microscopy
2.4.3. Observations under TEM
2.4.4. Observations under LTSEM (Cryo-SEM)
3. Results
3.1. Phycobiont Identification
3.2. Mycobiont Identification
3.3. Cyanobacterial Identification
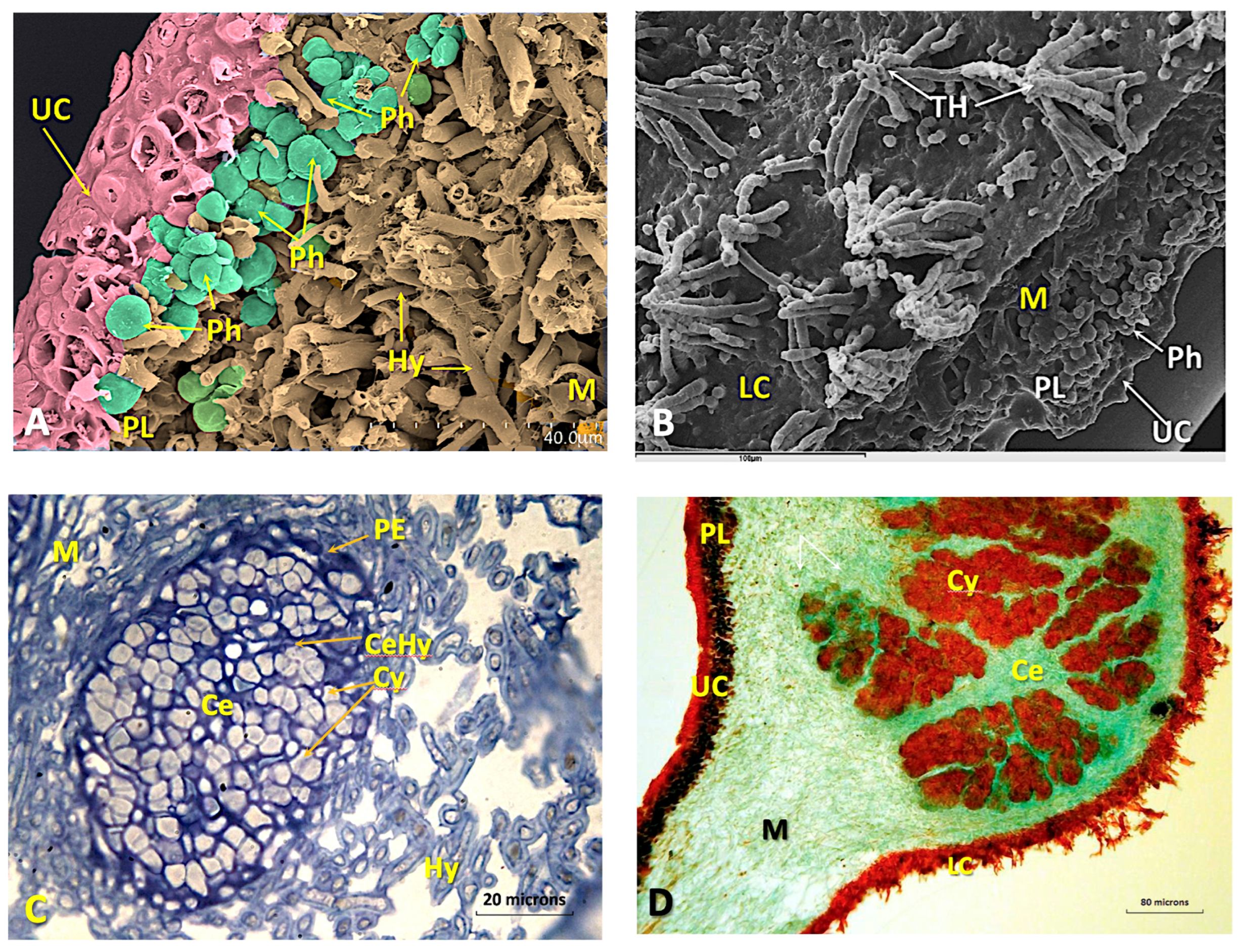
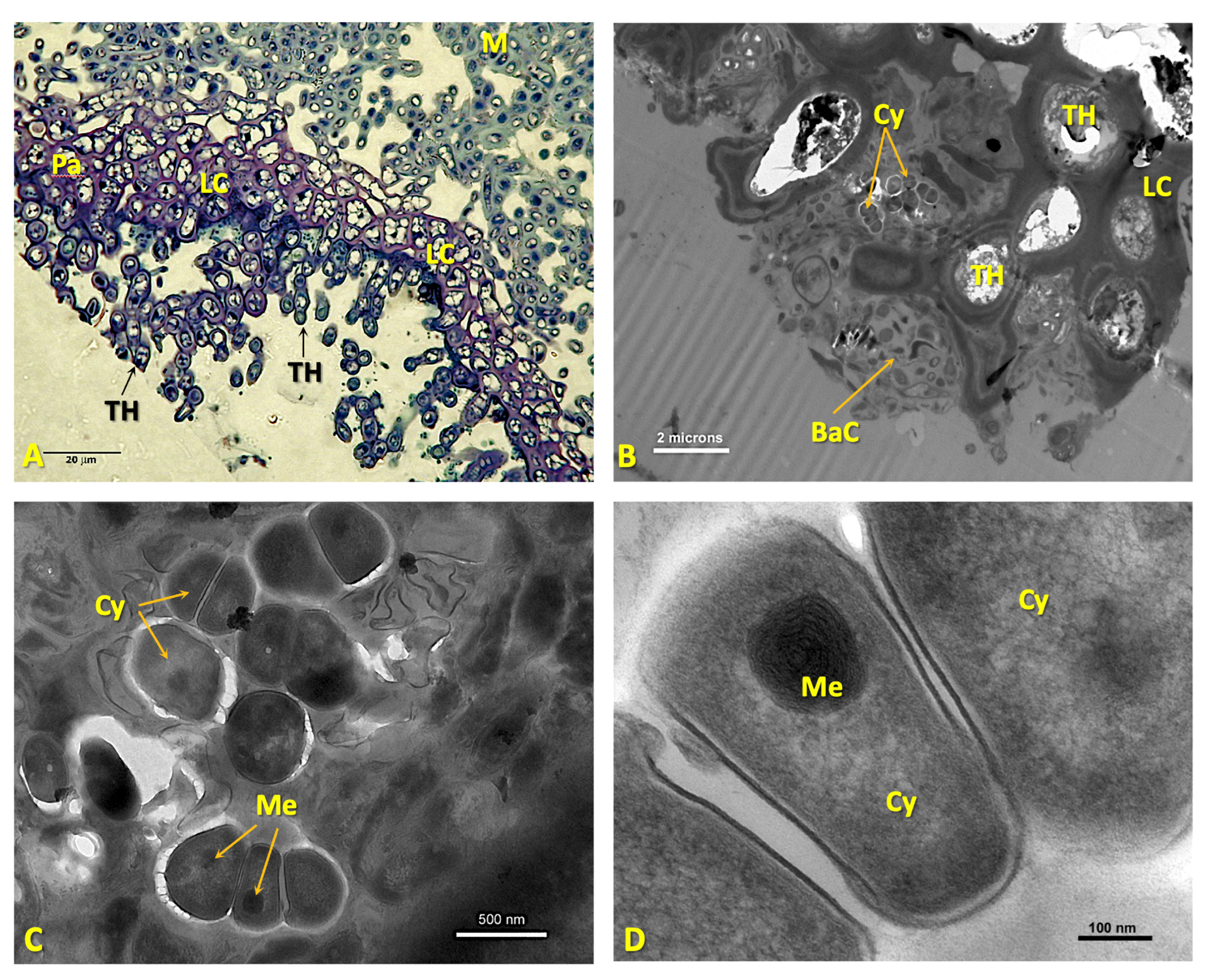
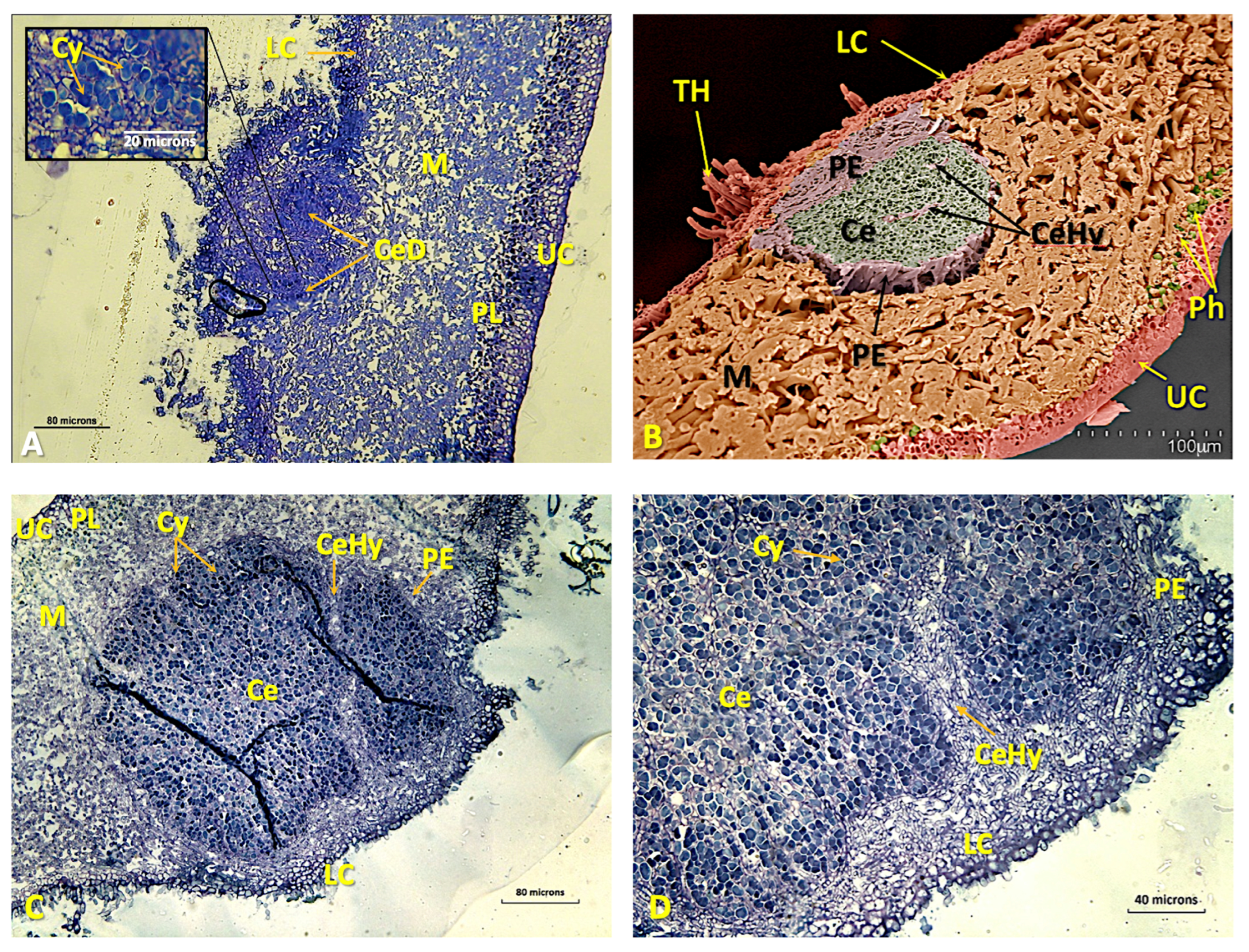
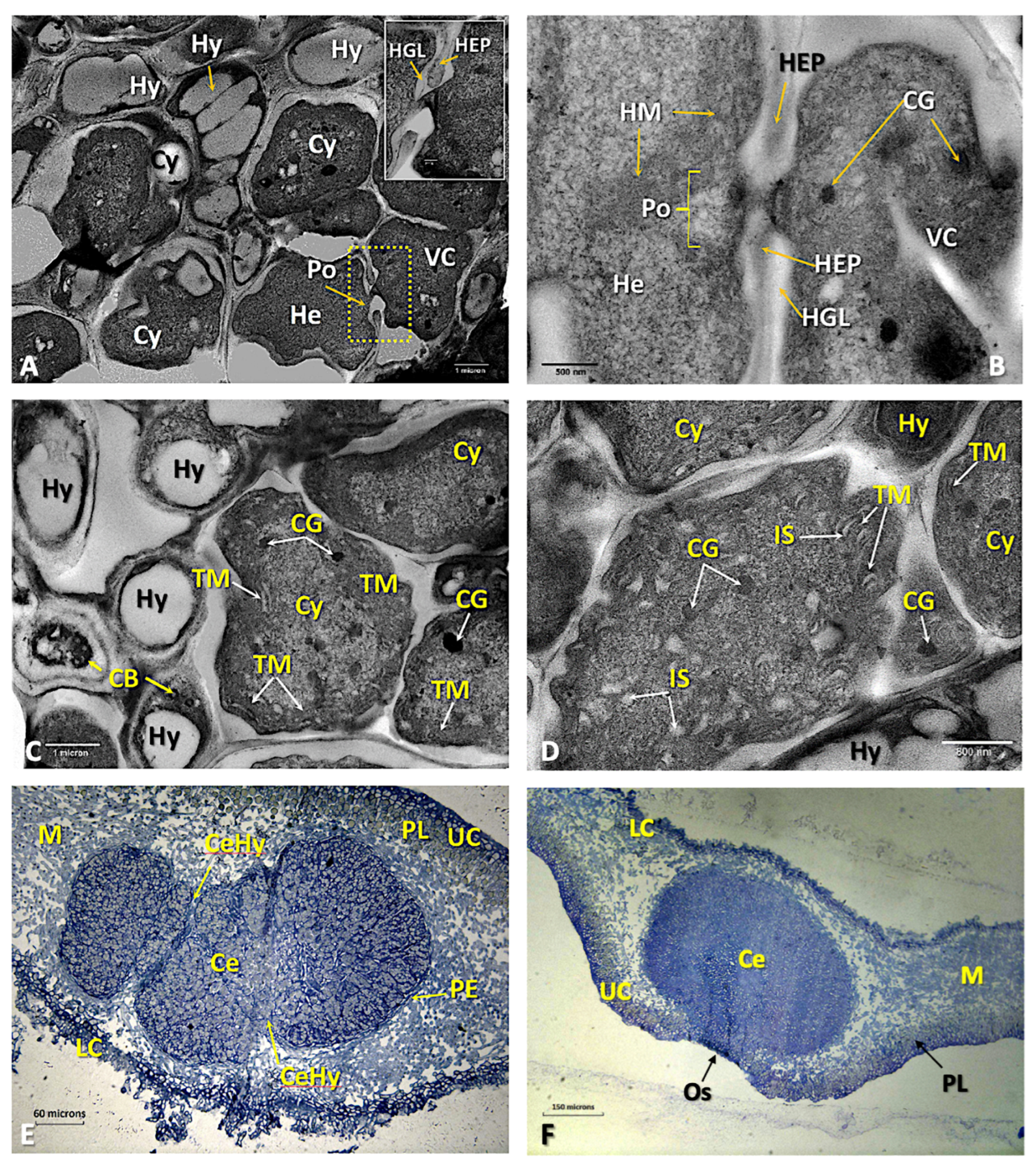
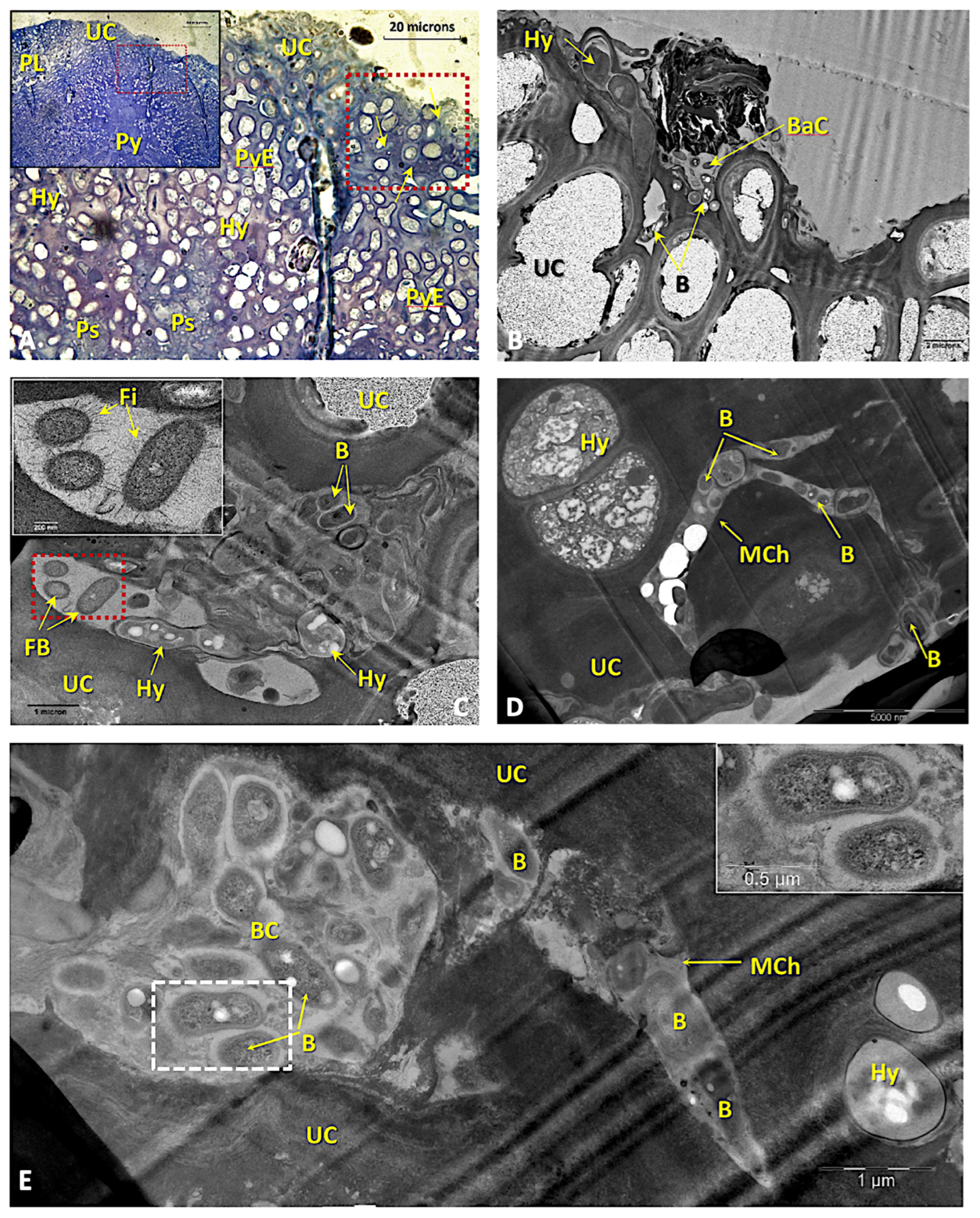
3.4. Anatomical and Ultrastructural Studies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L.; Honegger, R. The Lichen Thallus: A Symbiotic Phenotype of Nutritionally Specialized Fungi and Its Response to Gall Producers. Syst. Assoc. Spec. 1994, 49, 77. [Google Scholar]
- Margulis, L.; Barreno, E. Looking at Lichens. Bioscience 2003, 53, 776–778. [Google Scholar] [CrossRef]
- Chapman, M.J.; Margulis, L. Morphogenesis by Symbiogenesis. Int. Microbiol. 1998, 1, 319–326. [Google Scholar] [PubMed]
- Guerrero, R.; Margulis, L.; Berlanga, M. Symbiogenesis: The Holobiont as a Unit of Evolution. Int. Microbiol. 2013, 16, 133–143. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Grube, M. Lichens Redefined as Complex Ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef]
- Aschenbrenner, I.A.; Cardinale, M.; Berg, G.; Grube, M. Microbial Cargo: Do Bacteria on Symbiotic Propagules Reinforce the Microbiome of Lichens? Environ. Microbiol. 2014, 16, 3743–3752. [Google Scholar] [CrossRef]
- Cernava, T.; Berg, G.; Grube, M. High Life Expectancy of Bacteria on Lichens. Microb. Ecol. 2016, 72, 510–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, M.; Grube, M.; Schiefelbein, U.; Zühlke, D.; Bernhardt, J.; Riedel, K. The Lichens’ Microbiota, Still a Mystery? Front. Microbiol. 2021, 12, 714. [Google Scholar] [CrossRef]
- Barreno, E. Life Is Symbiosis. In Once upon a Time Lynn Margulis: A Portrait of Lynn Margulis by Colleagues and Friends; Chica, C., Ed.; Septimus: Barcelona, Spain, 2013; pp. 56–60. [Google Scholar]
- Margulis, L. Symbiosis in Cell Evolution, 2nd ed.; Freeman: New York, NY, USA, 1993. [Google Scholar]
- Winter, G. Lichenologische Notizen: Cephalodien von Sticta Und Solorina. Flora 1877, 60, 177–203. [Google Scholar]
- Forsell, K.B.J. Lichenologische Untersuchungen: Ueber Die Cephalodien. Flora 1884, 67, 79–84. [Google Scholar]
- Jordan, W.P. The Internal Cephalodia of the Genus Lobaria. Bryologist 1970, 73, 669. [Google Scholar] [CrossRef]
- Henssen, A.; Jahns, H.M.; Santesson, J. Lichenes, Eine Einfuhrung in Die Flechtenkunde; Georg Thieme Verlag: Stuttgart, Germany, 1974. [Google Scholar]
- Cornejo, C.; Scheidegger, C. New Morphological Aspects of Cephalodium Formation in the Lichen Lobaria pulmonaria (Lecanorales, Ascomycota). Lichenologist 2013, 45, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Moreau, M.F. Recherches Sur Les Lichens de La Famille Des Stictacées. Ann. Des Sci. Nat. Dixième Série 1921, 3, 297–374. [Google Scholar]
- Kaule, A. Die Cephalodien Der Flechten. Flora Oder Allg. Bot. Ztg. 1931, 126, 1–44. [Google Scholar] [CrossRef]
- Cardinale, M.; Vieira De Castro, J.; Müller, H.; Berg, G.; Grube, M. In Situ Analysis of the Bacterial Community Associated with the Reindeer Lichen Cladonia arbuscula Reveals Predominance of Alphaproteobacteria. FEMS Microbiol. Ecol. 2008, 66, 63–71. [Google Scholar] [CrossRef]
- Grube, M.; Cardinale, M.; De Castro, J.V.; Müller, H.; Berg, G. Species-Specific Structural and Functional Diversity of Bacterial Communities in Lichen Symbioses. ISME J. 2009, 3, 1105–1115. [Google Scholar] [CrossRef] [Green Version]
- Bates, S.T.; Cropsey, G.W.G.; Caporaso, J.G.; Knight, R.; Fierer, N. Bacterial Communities Associated with the Lichen Symbiosis. Appl. Environ. Microbiol. 2011, 77, 1309–1314. [Google Scholar] [CrossRef] [Green Version]
- Tønsberg, T.; Blom, H.H.; Goffinet, B.; Holtan-Hartwig, J.; Lindblom, L. The Cyanomorph of Ricasolia virens Comb. Nov. (Lobariaceae, Lichenized Ascomycetes). Opusc. Philolichenum 2016, 15, 12–21. [Google Scholar]
- Barreno, E.; Pérez-Ortega, S. Líquenes de La Reserva Natural Integral de Muniellos, Asturias; Cuadernos de Medio Ambiente. Serie Naturaleza 5; Consejería de Medio Ambiente, Ordenación del Territorio e Infraestructuras del Principado de Asturias & KRK Ediciones: Oviedo, Spain, 2003; p. 555. Available online: https://www.uv.es/barreno/Liquenes_Muniellos (accessed on 1 May 2023).
- Dal Grande, F.; Beck, A.; Cornejo, C.; Singh, G.; Cheenacharoen, S.; Nelsen, M.P.; Scheidegger, C. Molecular Phylogeny and Symbiotic Selectivity of the Green Algal Genus Dictyochloropsis s.l. (Trebouxiophyceae): A Polyphyletic and Widespread Group Forming Photobiont-Mediated Guilds in the Lichen Family Lobariaceae. New Phytol. 2014, 202, 455–470. [Google Scholar] [CrossRef]
- NRLWG. The National Red List Working Group. Available online: https://archive.nationalredlist.org/ (accessed on 1 May 2023).
- Scheidegger, C.; Clerc, P. Liste Rouge Des Espèces Menacées En Suisse: Lichens Épiphytes et Terricoles; Office Fédéral de L’Environnement, des Forêts et du Paysage, Ed.; OFEFP—Série: L’environnement Pratique; Institut Fédéral de Recherches WSL: Birmensdorf, Switzerland; Conservatoire et Jardin Botaniques de la Ville de Genève, CJBG: Berne, Switzerland, 2002. [Google Scholar]
- Nascimbene, J.; Nimis, P.L.; Ravera, S. Evaluating the Conservation Status of Epiphytic Lichens of Italy: A Red List. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2013, 147, 898–904. [Google Scholar] [CrossRef]
- Roux, C. Catalogue Des Lichens et Champignons Lichénicoles de France Métropolitaine, 3rd ed.; Association française de lichénologie (AFL): Fontainebleau, France, 2020. [Google Scholar]
- Nimis, P.L.; Martellos, S. Towards a Digital Key to the Lichens of Italy. Symbiosis 2020, 82, 149–155. [Google Scholar] [CrossRef]
- Škaloud, P.; Friedl, T.; Hallmann, C.; Beck, A.; Dal Grande, F. Taxonomic Revision and Species Delimitation of Coccoid Green Algae Currently Assigned to the Genus Dictyochloropsis (Trebouxiophyceae, Chlorophyta). J. Phycol. 2016, 52, 599–617. [Google Scholar] [CrossRef]
- Barreno, E.; Reig Armiñana, J.; García-Breijo, F.J.; Álvarez-Aspra, J.S. Lobaria Virens (With.) J.R. Laundon, Liquen Amenazado En Europa, Bioindicador Del Estado de Conservación de Los Bosques, En La Sierra Del Sueve (Asturias). Boletín Cienc. Nat. RIDEA 2006, 50, 343–354. [Google Scholar]
- Schumm, F. Die Flechtengattung Lobaria Auf Madeira. Herzogia 2003, 16, 91–112. [Google Scholar]
- Sanders, W.B.; Masumoto, H. Lichen Algae: The Photosynthetic Partners in Lichen Symbioses. Lichenologist 2021, 53, 347–393. [Google Scholar] [CrossRef]
- Laundon, J.R. The Typification of Withering’s Neglected Lichens. Lichenologist 1984, 16, 211–239. [Google Scholar] [CrossRef]
- Pérez-Ortega, S.; Barreno, E. La Reserva Integral de Muniellos (Asturias) Como Ejemplo de Alta Diversidad Liquénica y de Estrategias Para Conservación En Espacios Naturales. BIDEA (Bol. Ciencias Nat. RI-DEA). In Actas del I Congreso de Estudios Asturianos 2006; Comisión de Ciencias de la Naturaleza y Tecnología: Oviedo, Spain, 2006; pp. 189–218. [Google Scholar]
- Elvebakk, A. Lepidocollema polyphyllinum (Pannariaceae) from the Solomon Islands: Cephalodium-like Structure with Two Different Nostoc Symbionts in Dimorphous Thalli. Lichenologist 2016, 48, 339–341. [Google Scholar] [CrossRef]
- Cardinale, M.; Puglia, A.M.; Grube, M. Molecular Analysis of Lichen-Associated Bacterial Communities. FEMS Microbiol. Ecol. 2006, 57, 484–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodkinson, B.P.; Gottel, N.R.; Schadt, C.W.; Lutzoni, F. Photoautotrophic Symbiont and Geography Are Major Factors Affecting Highly Structured and Diverse Bacterial Communities in the Lichen Microbiome. Environ. Microbiol. 2012, 14, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Mark, K.; Laanisto, L.; Bueno, C.G.; Niinemets, Ü.; Keller, C.; Scheidegger, C. Contrasting Co-Occurrence Patterns of Photobiont and Cystobasidiomycete Yeast Associated with Common Epiphytic Lichen Species. New Phytol. 2020, 227, 1362–1375. [Google Scholar] [CrossRef]
- Molins, A.; García-Breijo, F.J.; Reig-Armiñana, J.; Del Campo, E.M.; Casano, L.; Barreno, E. Coexistence of Different Intrathalline Symbiotic Algae and Bacterial Biofilms in the Foliose Canarian Lichen Parmotrema pseudotinctorum. Vieraea 2013, 41, 349–370. [Google Scholar] [CrossRef]
- Grube, M.; Cernava, T.; Soh, J.; Fuchs, S.; Aschenbrenner, I.; Lassek, C.; Wegner, U.; Becher, D.; Riedel, K.; Sensen, C.W.; et al. Exploring Functional Contexts of Symbiotic Sustain within Lichen-Associated Bacteria by Comparative Omics. ISME J. 2014, 9, 412–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eymann, C.; Lassek, C.; Wegner, U.; Bernhardt, J.; Fritsch, O.A.; Fuchs, S.; Otto, A.; Albrecht, D.; Schiefelbein, U.; Cernava, T.; et al. Symbiotic Interplay of Fungi, Algae, and Bacteria within the Lung Lichen Lobaria pulmonaria L. Hoffm. as Assessed by State-of-the-Art Metaproteomics. J. Proteome Res. 2017, 16, 2160–2173. [Google Scholar] [CrossRef] [PubMed]
- Nazem-Bokaee, H.; Hom, E.F.Y.; Warden, A.C.; Mathews, S.; Gueidan, C. Towards a Systems Biology Approach to Understanding the Lichen Symbiosis: Opportunities and Challenges of Implementing Network Modelling. Front. Microbiol. 2021, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Berg, G. Microbial Consortia of Bacteria and Fungi with Focus on the Lichen Symbiosis. Fungal Biol. Rev. 2009, 23, 72–85. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Chemical Mediators at the Bacterial-Fungal Interface. Annu. Rev. Microbiol. 2020, 74, 267–290. [Google Scholar] [CrossRef]
- Carr, E.C.; Harris, S.D.; Herr, J.R.; Riekhof, W.R. Lichens and Biofilms: Common Collective Growth Imparts Similar Developmental Strategies. Algal Res. 2021, 54, 102217. [Google Scholar] [CrossRef]
- Honegger, R. Long-Term in Vitro Preservation of the Symbiotic Phenotype of Lichen-Forming Fungi and Their Photobionts: The Impact of Different Modes of Storage on Their Viability. In lnternational Conference on Lichen Conservation Biology; Licons, A., Ed.; Swiss Federal lnstitute for Forest, Snow and Landscape Research: Birmensdorf, Switzerland, 1999; pp. 1–19. [Google Scholar]
- Molins, A.; Moya, P.; García-Breijo, F.J.; Reig-Armiñana, J.; Barreno, E. A Multi-Tool Approach to Assess Microalgal Diversity in Lichens: Isolation, Sanger Sequencing, HTS and Ultrastructural Correlations. Lichenologist 2018, 50, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.E.; Miadlikowska, J.; Higgins, K.L.; Sarvate, S.D.; Gugger, P.; Way, A.; Hofstetter, V.; Kauff, F.; Lutzoni, F. A Phylogenetic Estimation of Trophic Transition Networks for Ascomycetous Fungi: Are Lichens Cradles of Symbiotrophic Fungal Diversification? Syst. Biol. 2009, 58, 283–297. [Google Scholar] [CrossRef] [Green Version]
- Moya, P.; Molins, A.; Martinez-Alberola, F.; Muggia, L.; Barreno, E. Unexpected Associated Microalgal Diversity in the Lichen Ramalina farinacea Is Uncovered by Pyrosequencing Analyses. PLoS ONE 2017, 12, e0175091. [Google Scholar] [CrossRef] [Green Version]
- del Campo, E.M.; Gimeno, J.; De Nova, J.P.G.; Casano, L.M.; Gasulla, F.; García-Breijo, F.; Armiñana, J.R.; Rodríguez, E. South European populations of Ramalina farinacea (L) Ach share different Trebouxia algae. Bibl. Lichenol. 2010, 105, 247–256. [Google Scholar]
- Piercey-Normore, M.D.; Depriest, P.T. Algal Switching among Lichen Symbioses. Am. J. Bot. 2001, 88, 1490–1498. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Frazão, B.; Martins, R.; Vasconcelos, V. Are Known Cyanotoxins Involved in the Toxicity of Picoplanktonic and Filamentous North Atlantic Marine Cyanobacteria? Mar. Drugs 2010, 8, 1908–1919. [Google Scholar] [CrossRef] [Green Version]
- Turner, S.; Pryer, K.M.; Miao, V.P.W.; Palmer, J.D. Investigating Deep Phylogenetic Relationships among Cyanobacteria and Plastids by Small Subunit RRNA Sequence Analysis. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A Rapid Bootstrap Algorithm for the RAxML Web Servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A. FigTree; Institute of Evolutionary Biology, Ashworth Laboratories: Edinburgh, Scotland, 2014. [Google Scholar]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the 2010 Gateway Computing Environments Workshop, GCE 2010, New Orleans, LA, USA, 14 November 2010. [Google Scholar] [CrossRef] [Green Version]
- Karnovsky, M.J. A Formaldehyde-Glutaraldehyde Fixative of High Osmolality for Use in Electron Microscopy. J. Cell Biol. 1965, 27, 1A–149A. [Google Scholar]
- Molins, A.; Moya, P.; García-Breijo, F.J.; Reig-Armiñana, J.; Barreno, E. Molecular and Morphological Diversity of Trebouxia Microalgae in Sphaerothallioid Circinaria spp. Lichens. J. Phycol. 2018, 54, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Reig-Armiñana, J.; Calatayud, V.; Cerveró, J.; García-Breijo, F.J.; Ibars, A.; Sanz, M.J. Effects of Ozone on the Foliar Histology of the Mastic Plant (Pistacia lentiscus L.). Environ. Pollut. 2004, 132, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, C.; Derr, C.; Dillman, K. Ricasolia amplissima (Lobariaceae): One Species, Three Genotypes and a New Taxon from South-Eastern Alaska. Lichenologist 2017, 49, 579–596. [Google Scholar] [CrossRef]
- Simon, A.; Goffinet, B.; Wang, L.S.; Spribille, T.; Goward, T.; Pystina, T.; Semenova, N.; Stepanov, N.V.; Moncada, B.; Lücking, R.; et al. Global Phylogeny and Taxonomic Reassessment of the Lichen Genus Dendriscosticta (Ascomycota: Peltigerales). Taxon 2022, 71, 256–287. [Google Scholar] [CrossRef]
- Magain, N.; Sérusiaux, E. Do Photobiont Switch and Cephalodia Emancipation Act as Evolutionary Drivers in the Lichen Symbiosis? A Case Study in the Pannariaceae (Peltigerales). PLoS ONE 2014, 9, e89876. [Google Scholar] [CrossRef] [Green Version]
- Rikkinen, J. Symbiotic Cyanobacteria in Lichens. In Algal Cyanobacteria Symbioses; World Scientific: London, UK, 2017; pp. 147–167. [Google Scholar] [CrossRef] [Green Version]
- Almer, J.; Resl, P.; Gudmundsson, H.; Warshan, D.; Andrésson, Ó.S.; Werth, S. Symbiont-Specific Responses to Environmental Cues in a Threesome Lichen Symbiosis. Mol. Ecol. 2022, 32, 1045–1061. [Google Scholar] [CrossRef]
- Green, T.G.A.; Budel, B.; Heber, U.; Meyer, A.; Zellner, H.; Lange, O.L. Differences in Photosynthetic Performance between Cyanobacterial and Green Algal Components of Lichen Photosymbiodemes Measured in the Field. New Phytol. 1993, 125, 723–731. [Google Scholar] [CrossRef]
- Gupta, M.M.; Richardson, D.H.S. Editorial: Anthropogenic Impacts on Symbiotic Systems. Symbiosis 2021, 84, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; McMullin, R.T.; Tripp, E.A.; Lendemer, J.C. Lichen Conservation in North America: A Review of Current Practices and Research in Canada and the United States. Biodivers Conserv. 2019, 28, 3103–3138. [Google Scholar] [CrossRef]
- Allen, J.L.; Scheidegger, C. Short Communication: Co-Occurring Lobaria pulmonaria and Ricasolia quercizans Share Green Algal Photobionts: Consequences for Conservation. Bryologist 2022, 125, 219–221. [Google Scholar] [CrossRef]
- Scheidegger, C.; Stofer, S.; Senn-Irlet, B. Gefährdete Arten. In Waldbericht 2015. Zustand und Nutzung des Schweizer Waldes; Rigling, A., Schaffer, H.P., Eds.; Bundesamt für Umwelt BAFU: Ittigen, Switzerland; Eidg. Forschungsanstalt für Wald, Schnee und Landschaft WSL: Zurich, Switzerland, 2015; pp. 86–87. [Google Scholar]
- Letrouit-Galinou, M. Etudes Sur Le Lobaria laetevirens (Lght.) Zahlbr. (Discolichen, Stictacée). I: Le Thalle, Les Apothécies, Les Asques. Le Botaniste 1971, 54, 190–234. [Google Scholar]
- Letrouit-galinou, M. Etudes Sur Le Lobaria laetevirens (Lght.) Zahlbr. (Discolichen, Stictacée). II: Le Développement Des Pycnides. Bull. Société Bot. Fr. 1972, 1, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Jordan, W.P. The Genus Lobaria in North America North of Mexico. Bryologist 1973, 76, 225. [Google Scholar] [CrossRef]
- Paulsrud, P.; Rikkinen, J.; Lindblad, P. Cyanobiont Specificity in Some Nostoc-Containing Lichens and in a Peltigera aphthosa Photosymbiodeme. New Phytol. 1998, 139, 517–524. [Google Scholar] [CrossRef]
- Paulsrud, P.; Rikkinen, J.; Lindblad, P. Spatial Patterns of Photobiont Diversity in Some Nostoc-Containing Lichens. New Phytol. 2000, 146, 291–299. [Google Scholar] [CrossRef]
- Rikkinen, J.; Oksanen, I.; Lohtander, K. Lichen Guilds Share Related Cyanobacterial Symbionts. Science 2002, 297, 357. [Google Scholar] [CrossRef] [Green Version]
- Summerfield, T.C.; Galloway, D.J.; Eaton-Rye, J.J. Species of Cyanolichens from Pseudocyphellaria with Indistinguishable ITS Sequences Have Different Photobionts. New Phytol. 2002, 155, 121–129. [Google Scholar] [CrossRef]
- Stenroos, S.; Högnabba, F.; Myllys, L.; Hyvönen, J.; Thell, A. High Selectivity in Symbiotic Associations of Lichenized Ascomycetes and Cyanobacteria. Cladistics 2006, 22, 230–238. [Google Scholar] [CrossRef]
- O’Brien, H.E.; Miadlikowska, J.; Lutzoni, F. Assessing Population Structure and Host Specialization in Lichenized Cyanobacteria. New Phytol. 2013, 198, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Myllys, L.; Stenroos, S.; Thell, A.; Kuusinen, M. High Cyanobiont Selectivity of Epiphytic Lichens in Old Growth Boreal Forest of Finland. New Phytol. 2007, 173, 621–629. [Google Scholar] [CrossRef]
- Moya, P.; Molins, A.; Chiva, S.; Bastida, J.; Barreno, E. Symbiotic Microalgal Diversity within Lichenicolous Lichens and Crustose Hosts on Iberian Peninsula Gypsum Biocrusts. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Molins, A.; Moya, P.; Muggia, L.; Barreno, E. Thallus Growth Stage and Geographic Origin Shape Microalgal Diversity in Ramalina farinacea Lichen Holobionts. J. Phycol. 2021, 57, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Widmer, I.; Dal Grande, F.; Excoffier, L.; Holderegger, R.; Keller, C.; Mikryukov, V.S.; Scheidegger, C. European Phylogeography of the Epiphytic Lichen Fungus Lobaria pulmonaria and Its Green Algal Symbiont. Mol. Ecol. 2012, 21, 5827–5844. [Google Scholar] [CrossRef]
- Werth, S.; Scheidegger, C. Congruent Genetic Structure in the Lichen-Forming Fungus Lobaria pulmonaria and Its Green-Algal Photobiont. Mol. Plant Microbe Interact 2012, 25, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Muggia, L.; Vancurová, L.; Škaloud, P.; Peksa, O.; Wedin, M.; Grube, M. The Symbiotic Playground of Lichen Thalli—A Highly Flexible Photobiont Association in Rock-Inhabiting Lichens. FEMS Microbiol. Ecol. 2013, 85, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Muggia, L.; Candotto-Carniel, F.; Grube, M. The Lichen Photobiont Trebouxia: Towards and Appreciation of Species Diversity and Molecular Studies. In Algal and Cyanobacteria Symbioses; World Scientific (Europe): London, UK, 2017; pp. 111–146. [Google Scholar] [CrossRef]
- Muggia, L.; Leavitt, S.; Barreno, E. The Hidden Diversity of Lichenised Trebouxiophyceae (Chlorophyta). Phycologia 2018, 57, 503–524. [Google Scholar] [CrossRef] [Green Version]
- Casano, L.M.; Del Campo, E.M.; García-Breijo, F.J.; Reig-Armiñana, J.; Gasulla, F.; Del Hoyo, A.; Guéra, A.; Barreno, E. Two Trebouxia Algae with Different Physiological Performances Are Ever-Present in Lichen Thalli of Ramalina farinacea. Coexistence versus Competition? Environ. Microbiol. 2011, 13, 806–818. [Google Scholar] [CrossRef]
- Lerch, M.; Nadyeina, O.; Scheidegger, C. Genetic Structure of Lobaria pulmonaria in the Alps as a Result of Post-Glacial Recolonization History. Herzogia 2018, 31 Pt 1, 650–665. [Google Scholar] [CrossRef]
- Cannon, P.; Magain, N.; Sérusiaux, E.; Yahr, R.; Coppins, B.; Sanderson, N.; Simkin, J. Peltigerales: Peltigeraceae, Including the Genera Crocodia, Lobaria, Lobarina, Nephroma, Peltigera, Pseudocyphellaria, Ricasolia, Solorina and Sticta. Revis. Br. Ir. Lichens 2021, 20, 1–34. [Google Scholar]
- Galun, M.; Kardish, N. Lectins as Determinants of Symbiotic Specificity in Lichens. Cryptogam. Bot. 2013, 5, 144–148. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201301507218 (accessed on 1 December 2022).
- Legaz, M.E.; Fontaniella, B.; Millanes, A.M.; Vicente, C. Secreted Arginases from Phylogenetically Farrelated Lichen Species Act as Cross-Recognition Factors for Two Different Algal Cells. Eur. J. Cell Biol. 2004, 83, 435–446. [Google Scholar] [CrossRef]
- Feoktistov, A.S.; Kitashov, A.V.; Lobakova, E.S. The Characterization of Lectins from the Thripartite Lichen Peltigera aphthosa (L.) Willd. Mosc. Univ. Biol. Sci. Bull. 2009, 64, 23–27. [Google Scholar] [CrossRef]
- Díaz, E.M.; Vicente-Manzanares, M.; Sacristan, M.; Vicente, C.; Legaz, M.E. Fungal Lectin of Peltigera canina Induces Chemotropism of Compatible Nostoc Cells by Constriction-Relaxation Pulses of Cyanobiont Cytoskeleton. Plant Signal Behav. 2011, 6, 1525–1536. [Google Scholar] [CrossRef] [Green Version]
- Rikkinen, J. Cyanobacteria in Terrestrial Symbiotic Systems. In Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects; Springer: Cham, Switzerland, 2017; pp. 243–294. [Google Scholar] [CrossRef] [Green Version]
- Jüriado, I.; Kaasalainen, U.; Jylhä, M.; Rikkinen, J. Relationships between Mycobiont Identity, Photobiont Specificity and Ecological Preferences in the Lichen Genus Peltigera (Ascomycota) in Estonia (Northeastern Europe). Fungal Ecol. 2019, 39, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Lehr, H.; Galun, M.; Ott, S.; Jahns, H.; Fleminger, G. Cephalodia of the Lichen Peltigera aphthosa (L.) Willd. Specific Recognition of the Compatible Photobiont. Symbiosis 2000, 29, 357–365. [Google Scholar]
- Paulsrud, P.; Rikkinen, J.; Lindblad, P. Field Investigations on Cyanobacterial Specificity in Peltigera aphthosa. New Phytol. 2001, 152, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.S.; Miao, V.P.W.; Andrésson, Ó.S. LEC-2, a Highly Variable Lectin in the Lichen Peltigera membranacea. Symbiosis 2012, 58, 91. [Google Scholar] [CrossRef] [Green Version]
- Poelt, J.; Mayrhofer, H. Über Cyanotrophie Bei Flechten. Plant Syst. Evol. 1988, 158, 265–281. [Google Scholar] [CrossRef]
- Gerasimova, J.V.; Urbanavichene, I.N.; Urbanavichus, G.P.; Beck, A. Morphological and Phylogenetic Analyses of Toniniopsis subincompta s. Lat. (Ramalinaceae, Lecanorales) in Eurasia. Lichenologist 2021, 53, 171–183. [Google Scholar] [CrossRef]
- Stocker-Wörgötter, E. Resynthesis of Photosymbiodemes. In Protocols in Lichenology; Springer: Berlin/Heidelberg, Germany, 2002; pp. 47–60. [Google Scholar] [CrossRef]
- Fávaro, A.; do Nascimento, A.G.; de Freitas Coelho, F. Urban Environmental Influences on Heterocyst investment in Leptogium cyanescens (Collemataceae). Nova Hedwig. 2021, 113, 259–277. [Google Scholar] [CrossRef]
- Kershaw, K.A.; Millbank, J.W. Nitrogen Metabolism in Lichens. II. The Partition of Cephalodial-Fixed Nitrogen between the Mycobiont and Phycobionts of Peltigera Aphthosa. New Phytol. 2013, 69, 75–79. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201301186583 (accessed on 2 December 2022).
- Millbank, J.W.; Kershaw, K.A. Nitrogen Metabolism In Lichens: Iii. Nitrogen Fixation By Internal Cephalodia In Lobaria pulmonaria. New Phytol. 1970, 69, 595–597. [Google Scholar] [CrossRef]
- Antoine, M.E. An Ecophysiological Approach to Quantifying Nitrogen Fixation by Lobaria oregana. Bryologist 2004, 107, 82–87. [Google Scholar] [CrossRef]
- Pentecost, A. Estimates of Abundance and Biomass of Cephalodia and Their Relationship to Nitrogen Deposition in Some British Populations of Lobaria pulmonaria (L.) Hoffm. Lichenologist 2021, 53, 335–339. [Google Scholar] [CrossRef]
- Crittenden, P.D.; Ellis, C.J.; Smith, R.I.; Wanek, W.; Thornton, B. Loss of Nitrogen Fixing Capacity in a Montane Lichen Is Linked to Increased Nitrogen Deposition. J. Ecol. 2022, 111, 280–299. [Google Scholar] [CrossRef]
- Rai, A.N.; Bergman, B. Cyanolichens. Biol. Environ. Proceed. R. Ir. Acad. 2002, 102B, 19–22. [Google Scholar] [CrossRef]
- Kaasalainen, U.; Jokela, J.; Fewer, D.P.; Sivonen, K.; Rikkinen, J. Microcystin Production in the Tripartite Cyanolichen Peltigera leucophlebia. Mol. Plant Microbe Interact 2009, 22, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Kaasalainen, U.; Tuovinen, V.; Mwachala, G.; Pellikka, P.; Rikkinen, J. Complex Interaction Networks Among Cyanolichens of a Tropical Biodiversity Hotspot. Front. Microbiol. 2021, 12, 1246. [Google Scholar] [CrossRef] [PubMed]
- Lawrey, J.D. Biological Role of Lichen Substances. Bryologist 1986, 89, 111. [Google Scholar] [CrossRef]
- Büdel, B.; Scheidegger, C. Thallus Morphology and Anatomy. In Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 40–68. [Google Scholar] [CrossRef]
- Schneider, K.; Resl, P.; Spribille, T. Escape from the Cryptic Species Trap: Lichen Evolution on Both Sides of a Cyanobacterial Acquisition Event. Mol. Ecol. 2016, 25, 3453–3468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheidegger, C. As Thick as Three in a Bed. Mol. Ecol. 2016, 25, 3261–3263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, T.G.A.; Horstmann, J.; Bonnett, H.; Wilkins, A.; Silvester, W.B. Nitrogen Fixation by Members of the Stictaceae (Lichenes) of New Zealand on JSTOR. New Phytol. 1980, 84, 339–348. Available online: https://www.jstor.org/stable/2431729 (accessed on 1 December 2022). [CrossRef]
- Guzman, G.; Quilhot, W.; Galloway, D.J. Decomposition of Species of Pseudocyphellaria and Sticta in a Southern Chilean Forest*. Lichenologist 1990, 22, 325–331. [Google Scholar] [CrossRef]
- Knowles, R.D.; Pastor, J.; Biesboer, D.D. Increased Soil Nitrogen Associated with Dinitrogen-Fixing, Terricolous Lichens of the Genus Peltigera in Northern Minnesota. Oikos 2006, 114, 37–48. [Google Scholar] [CrossRef]
- Benner, J.W.; Conroy, S.; Lunch, C.K.; Toyoda, N.; Vitousek, P.M. Phosphorus Fertilization Increases the Abundance and Nitrogenase Activity of the Cyanolichen Pseudocyphellaria Crocata in Hawaiian Montane Forests. Biotropica 2007, 39, 400–405. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Goward, T.; Pypker, T. Canopy Settings Shape Elemental Composition of the Epiphytic Lichen Lobaria pulmonaria in Unmanaged Conifer Forests. Ecol. Indic. 2020, 113, 106294. [Google Scholar] [CrossRef]
- Van Langenhove, L.; Depaepe, T.; Verryckt, L.T.; Fuchslueger, L.; Donald, J.; Leroy, C.; Krishna Moorthy, S.M.; Gargallo-Garriga, A.; Ellwood, M.D.F.; Verbeeck, H.; et al. Comparable Canopy and Soil Free-Living Nitrogen Fixation Rates in a Lowland Tropical Forest. Sci. Total Environ. 2021, 754, 142202. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.; Fernández Otárola, M. Bryophyte and Lichen Biomass and Nitrogen Fixation in a High Elevation Cloud Forest in Cerro de La Muerte, Costa Rica. Oecologia 2021, 195, 489–497. [Google Scholar] [CrossRef]
- Hodkinson, B.P.; Allen, J.L.; Forrest, L.L.; Goffinet, B.; Sérusiaux, E.; Andrésson, Ó.S.; Miao, V.; Bellenger, J.P.; Lutzoni, F. Lichen-Symbiotic Cyanobacteria Associated with Peltigera Have an Alternative Vanadium-Dependent Nitrogen Fixation System. Eur. J. Phycol. 2014, 49, 11–19. [Google Scholar] [CrossRef]
- Rikkinen, J. Cyanolichens. Biodivers Conserv. 2015, 24, 973–993. [Google Scholar] [CrossRef]
- Zúñiga, C.; Leiva, D.; Carú, M.; Orlando, J. Substrates of Peltigera Lichens as a Potential Source of Cyanobionts. Microb. Ecol. 2017, 74, 561–569. [Google Scholar] [CrossRef]
- Gagunashvili, A.N.; Andrésson, Ó.S. Distinctive Characters of Nostoc Genomes in Cyanolichens. BMC Genom. 2018, 19, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Vančurová, L.; Muggia, L.; Peksa, O.; Řídká, T.; Škaloud, P. The Complexity of Symbiotic Interactions Influences the Ecological Amplitude of the Host: A Case Study in Stereocaulon (Lichenized Ascomycota). Mol. Ecol. 2018, 27, 3016–3033. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.; Yaneva, G.; Potoroko, I.; Ivanova, D.G. Contribution of Cyanotoxins to the Ecotoxicological Role of Lichens. Toxins 2021, 13, 321. [Google Scholar] [CrossRef]
- Jiang, D.F.; Wang, H.Y.; Si, H.L.; Zhao, L.; Liu, C.P.; Zhang, H. Isolation and Culture of Lichen Bacteriobionts. Lichenologist 2017, 49, 175–181. [Google Scholar] [CrossRef]
- Spribille, T.; Tagirdzhanova, G.; Goyette, S.; Tuovinen, V.; Case, R.; Zandberg, W.F. 3D Biofilms: In Search of the Polysaccharides Holding Together Lichen Symbioses. FEMS Microbiol. Lett. 2020, 367, fnaa023. [Google Scholar] [CrossRef] [Green Version]
- Figás-Segura, A.; Sargas, C.; Lacasta, C.; Barreno, E.; Biosca, E.G. Understanding Bacterial Nutrient Supply in Lichens from Iberian and Canarian Lichen Spe-Cies: Symbiosis Sustainability and Environmental Stress Tolerance. In Proceedings of the 8th IAL Symposium. IAL8 Lichens in Deep Time, Helsinki, Finland, 1–5 August 2016; p. 85. [Google Scholar]
- Figàs Segura, À. Bacterial Communities Associated with the Lichen Ramalina farinacea (L.) Ach.: Composition, Biodiversity and Biotechnological Potential. Ph.D. Thesis, Universitat de València, Valencia, Spain, 2017. [Google Scholar]
- Gimeno-Molina, B. Caracterización Biotecnológica de Bacterias Asociadas al Liquen Parmotrema Pseudotinctorum; Universitat de Valéncia: Valencia, Spain, 2017. [Google Scholar]
- Muggia, L.; Klug, B.; Berg, G.; Grube, M. Localization of Bacteria in Lichens from Alpine Soil Crusts by Fluorescence in Situ Hybridization. Appl. Soil Ecol. 2013, 68, 20–25. [Google Scholar] [CrossRef]
- Ott, S. The Development of Regenerative Thallus Structures in Lichens. Bot. J. Linn. Soc. 1993, 113, 61–76. [Google Scholar] [CrossRef]
- Garg, N.; Zeng, Y.; Edlund, A.; Melnik, A.V.; Sanchez, L.M.; Mohimani, H.; Gurevich, A.; Miao, V.; Schiffler, S.; Lim, Y.W.; et al. Spatial Molecular Architecture of the Microbial Community of a Peltigera Lichen. mSystems 2016, 1, e00139-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noël, A.; Garnier, A.; Clément, M.; Rouaud, I.; Sauvager, A.; Bousarghin, L.; Vásquez-Ocmín, P.; Maciuk, A.; Tomasi, S. Lichen-Associated Bacteria Transform Antibacterial Usnic Acid to Products of Lower Antibiotic Activity. Phytochemistry 2021, 181, 112535. [Google Scholar] [CrossRef] [PubMed]
- Pankratov, T.A.; Grouzdev, D.S.; Patutina, E.O.; Kolganova, T.V.; Suzina, N.E.; Berestovskaya, J.J. Lichenibacterium Ramalinae Gen. Nov, Sp. Nov., Lichenibacterium minor Sp. Nov., the First Endophytic, Beta-Carotene Producing Bacterial Representatives from Lichen Thalli and the Proposal of the New Family Lichenibacteriaceae within the Order Rhizobiales. Antonie Van Leeuwenhoek 2020, 113, 477–489. [Google Scholar] [CrossRef]
- Schneider, T.; Schmid, E.; de Castro, J.V.; Cardinale, M.; Eberl, L.; Grube, M.; Berg, G.; Riedel, K. Structure and Function of the Symbiosis Partners of the Lung Lichen (Lobaria pulmonaria L. Hoffm.) Analyzed by Metaproteomics. Proteomics 2011, 11, 2752–2756. [Google Scholar] [CrossRef]
- Cardinale, M.; Grube, M.; Castro, J.V.; Müller, H.; Berg, G. Bacterial Taxa Associated with the Lung Lichen Lobaria pulmonaria Are Differentially Shaped by Geography and Habitat. FEMS Microb. Lett. 2012, 329, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Erlacher, A.; Cernava, T.; Cardinale, M.; Soh, J.; Sensen, C.W.; Grube, M.; Berg, G. Rhizobiales as Functional and Endosymbiontic Members in the Lichen Symbiosis of Lobaria pulmonaria L. Front. Microbiol. 2015, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavarria-Pizarro, T.; Resl, P.; Kuhl-Nagel, T.; Janjic, A.; Fernandez Mendoza, F.; Werth, S. Antibiotic-Induced Treatments Reveal Stress-Responsive Gene Expression in the Endangered Lichen Lobaria pulmonaria. J. Fungi 2022, 8, 625. [Google Scholar] [CrossRef]
- Dal Grande, F.; Widmer, I.; Beck, A.; Scheidegger, C. Microsatellite Markers for Dictyochloropsis Reticulata (Trebouxiophyceae), the Symbiotic Alga of the Lichen Lobaria pulmonaria (L.). Conserv. Genet. 2010, 11, 1147–1149. [Google Scholar] [CrossRef] [Green Version]
- Nadyeina, O.; Cornejo, C.; Boluda, C.G.; Myllys, L.; Rico, V.J.; Crespo, A.; Scheidegger, C. Characterization of Microsatellite Loci in Lichen-Forming Fungi of Bryoria Section Implexae (Parmeliaceae). Appl. Plant Sci. 2014, 2, 1400037. [Google Scholar] [CrossRef]
- Álvarez-Aspra, J.S.; Barreno, E. Development of a Predictive Bioinformatic Model to Estimate Populations of the Endangered Lichen Ricasolia virens, in the Sierra Del Sueve (Asturias). In Boletín de Ciencias y Tecnología; Real Instituto de Estudios Asturianos: Asturias, Spain, 2023; Volume 57. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Breijo, F.J.; Molins, A.; Reig-Armiñana, J.; Barreno, E. The Tripartite Lichen Ricasolia virens: Involvement of Cyanobacteria and Bacteria in Its Morphogenesis. Microorganisms 2023, 11, 1517. https://doi.org/10.3390/microorganisms11061517
García-Breijo FJ, Molins A, Reig-Armiñana J, Barreno E. The Tripartite Lichen Ricasolia virens: Involvement of Cyanobacteria and Bacteria in Its Morphogenesis. Microorganisms. 2023; 11(6):1517. https://doi.org/10.3390/microorganisms11061517
Chicago/Turabian StyleGarcía-Breijo, Francisco J., Arantzazu Molins, José Reig-Armiñana, and Eva Barreno. 2023. "The Tripartite Lichen Ricasolia virens: Involvement of Cyanobacteria and Bacteria in Its Morphogenesis" Microorganisms 11, no. 6: 1517. https://doi.org/10.3390/microorganisms11061517






