Crop Productivity Boosters: Native Mycorrhizal Fungi from an Old-Growth Grassland Benefits Tomato (Solanum lycopersicum) and Pepper (Capsicum annuum) Varieties in Organically Farmed Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seedling Germination and Transplanting
2.2. Experimental Design
2.3. Nutrient and Pest Amendments for Both Tomatoes and Peppers
2.4. Inoculation Treatments for Both Tomatoes and Peppers
2.5. Statistical Analysis
3. Results
3.1. Peppers
3.2. Tomatoes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gosling, P.; Shepherd, M. Long-term changes in soil fertility in organic arable farming systems in England, with particular reference to phosphorus and potassium. Agric. Ecosyst. Environ. 2005, 105, 425–432. [Google Scholar] [CrossRef]
- Clark, M.S.; Horwath, W.R.; Shennan, C.; Scow, K.M. Changes in soil chemical properties resulting from organic and low-input farming practices. Agron. J. 1998, 90, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Oehl, F.; Sieverding, E.; Mäder, P.; Dubois, D.; Ineichen, K.; Boller, T.; Wiemken, A. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 2004, 138, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Read, D.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems–a journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Hetrick, B.; Wilson, G.; Cox, T. Mycorrhizal dependence of modern wheat cultivars and ancestors: A synthesis. Can. J. Bot. 1993, 71, 512–518. [Google Scholar] [CrossRef]
- Jun, D.J.; Allen, E.B. Physiological responses of 6 wheatgrass cultivars to mycorrhizae. J. Range Manag. 1991, 44, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Lehmann, A.; Zheng, W.; You, Z.; Rillig, M.C. Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol. 2019, 222, 543–555. [Google Scholar] [CrossRef]
- Vejsadova, H.; Siblikova, D.; Gryndler, M.; Simon, T.; Miksik, I. Influence of inoculation with Bradyrhizobium japonicum and Glomus claroideum on seed yield of soybean under greenhouse and field conditions. J. Plant Nutr. 1993, 16, 619–629. [Google Scholar] [CrossRef]
- Koziol, L.; Crews, T.E.; Bever, J.D. Benefits of native mycorrhizal amendments to perennial agroecosystems increases with field inoculation density. Agronomy 2019, 9, 353. [Google Scholar] [CrossRef] [Green Version]
- Davies, F.; Potter, J.; Linderman, R. Drought resistance of mycorrhizal pepper plants independent of leaf P concentration-response in gas exchange and water relations. Physiol. Plant. 1993, 87, 45–53. [Google Scholar] [CrossRef]
- Abbott, L.; Robson, A. Factors influencing the occurrence of vesicular-arbuscular mycorrhizas. Agric. Ecosyst. Environ. 1991, 35, 121–150. [Google Scholar] [CrossRef]
- Jasper, D.; Abbott, L.; Robson, A. The effect of soil disturbance on vesicular—Arbuscular mycorrhizal fungi in soils from different vegetation types. New Phytol. 1991, 118, 471–476. [Google Scholar] [CrossRef]
- Ryan, M.; Chilvers, G.; Dumaresq, D. Colonisation of wheat by VA-mycorrhizal fungi was found to be higher on a farm managed in an organic manner than on a conventional neighbour. Plant Soil 1994, 160, 33–40. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mäder, P.; Boller, T.; Wiemken, A. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 2003, 69, 2816–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrino, E.; Bedini, S.; Avio, L.; Bonari, E.; Giovannetti, M. Field inoculation effectiveness of native and exotic arbuscular mycorrhizal fungi in a Mediterranean agricultural soil. Soil Biol. Biochem. 2011, 43, 367–376. [Google Scholar] [CrossRef]
- Schüßler, A.; Krüger, C.; Urgiles, N. Phylogenetically diverse AM fungi from Ecuador strongly improve seedling growth of native potential crop trees. Mycorrhiza 2016, 26, 199–207. [Google Scholar] [CrossRef]
- Affokpon, A.; Coyne, D.L.; Lawouin, L.; Tossou, C.; Dossou Agbèdè, R.; Coosemans, J. Effectiveness of native West African arbuscular mycorrhizal fungi in protecting vegetable crops against root-knot nematodes. Biol. Fertil. Soils 2011, 47, 207–217. [Google Scholar] [CrossRef]
- Castillo, C.; Sotomayor, L.; Ortiz, C.; Leonelli, G.; Borie, F.; Rubio, R. Effect of arbuscular mycorrhizal fungi on an ecological crop of chili peppers (Capsicum annuum L.). Chil. J. Agric. Res. 2009, 69, 79–87. [Google Scholar]
- Kouadio, A.N.M.-S.; Nandjui, J.; Krou, S.M.; Séry, D.J.-M.; Nelson, P.N.; Zézé, A. A native arbuscular mycorrhizal fungus inoculant outcompetes an exotic commercial species under two contrasting yam field conditions. Rhizosphere 2017, 4, 112–118. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Bowker, M.A.; Wilson, J.A.; Miller, R.M. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Gehring, C.A.; Wilson, G.W.; Miller, R.; Flores-Rentería, L.; Johnson, N.C. Patterns of diversity and adaptation in Glomeromycota from three prairie grasslands. Mol. Ecol. 2013, 22, 2573–2587. [Google Scholar] [CrossRef] [PubMed]
- Ortas, I. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crops Res. 2012, 125, 35–48. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Mayerhofer, T. What is this thing called organic?—How organic farming is codified in regulations. Food Policy 2017, 68, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Koziol, L.; Rieseberg, L.H.; Kane, N.; Bever, J.D. Reduced drought tolerance during domestication and the evolution of weediness results from tolerance-growth trade-offs. Evolution 2012, 66, 3803–3814. [Google Scholar] [CrossRef]
- McKenna, T.P.; Koziol, L.; Bever, J.D.; Crews, T.E.; Sikes, B.A. Abiotic and biotic context dependency of perennial crop yield. PLoS ONE 2020, 15, e0234546. [Google Scholar] [CrossRef]
- Felföldi, Z.; Vidican, R.; Stoian, V.; Roman, I.A.; Sestras, A.F.; Rusu, T.; Sestras, R.E. Arbuscular Mycorrhizal Fungi and Fertilization Influence Yield, Growth and Root Colonization of Different Tomato Genotype. Plants 2022, 11, 1743. [Google Scholar] [CrossRef]
- Sensoy, S.; Demir, S.; Turkmen, O.; Erdinc, C.; Savur, O.B. Responses of some different pepper (Capsicum annuum L.) genotypes to inoculation with two different arbuscular mycorrhizal fungi. Sci. Hortic. 2007, 113, 92–95. [Google Scholar] [CrossRef]
- Koziol, L.; McKenna, T.P.; Crews, T.E.; Bever, J.D. Native arbuscular mycorrhizal fungi promote native grassland diversity and suppress weeds 4 years following inoculation. Restor. Ecol. 2022, 31, e13772. [Google Scholar] [CrossRef]
- Koziol, L.; Schultz, P.A.; Parsons, S.; Bever, J.D. Native mycorrhizal fungi improve milkweed growth, latex, and establishment while some commercial fungi may inhibit them. Ecosphere 2022, 13, e4052. [Google Scholar] [CrossRef]
- OMRI Organic Materials Review Institute (OMRI) Products List 2023. Available online: https://www.omri.org/omri-list (accessed on 25 July 2023).
- Wang, G.; Schultz, P.; Tipton, A.; Zhang, J.; Zhang, F.; Bever, J.D. Soil microbiome mediates positive plant diversity-productivity relationships in late successional grassland species. Ecol. Lett. 2019, 22, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.; Crews, T.E.; Bever, J.D. Native plant abundance, diversity, and richness increases in prairie restoration with field inoculation density of native mycorrhizal amendments. Restor. Ecol. 2020, 28 (Suppl. S4), S373–S380. [Google Scholar] [CrossRef]
- Koziol, L.; McKenna, T.P.; Bever, J.D. Native Microbes Amplify Native Seedling Establishment and Diversity While Inhibiting a Non-Native Grass. Plants 2023, 12, 1184. [Google Scholar] [CrossRef]
- SAS. SAS/ACCESS® 9.4 Interface to ADABAS: Reference; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Tipton, A.G.; Nelsen, D.; Koziol, L.; Duell, E.B.; House, G.; Wilson, G.W.; Schultz, P.A.; Bever, J.D. Arbuscular Mycorrhizal Fungi Taxa Show Variable Patterns of Micro-Scale Dispersal in Prairie Restorations. Front. Microbiol. 2022, 13, 827293. [Google Scholar] [CrossRef]
- Verbruggen, E.; Röling, W.F.; Gamper, H.A.; Kowalchuk, G.A.; Verhoef, H.A.; van der Heijden, M.G. Positive effects of organic farming on below-ground mutualists: Large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol. 2010, 186, 968–979. [Google Scholar] [CrossRef]
- House, G.L.; Bever, J.D. Disturbance reduces the differentiation of mycorrhizal fungal communities in grasslands along a precipitation gradient. Ecol. Appl. 2018, 28, 736–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottshall, C.B.; Cooper, M.; Emery, S.M. Activity, diversity and function of arbuscular mycorrhizae vary with changes in agricultural management intensity. Agric. Ecosyst. Environ. 2017, 241, 142–149. [Google Scholar] [CrossRef]
- Verbruggen, E.; Toby Kiers, E. Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol. Appl. 2010, 3, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef] [Green Version]
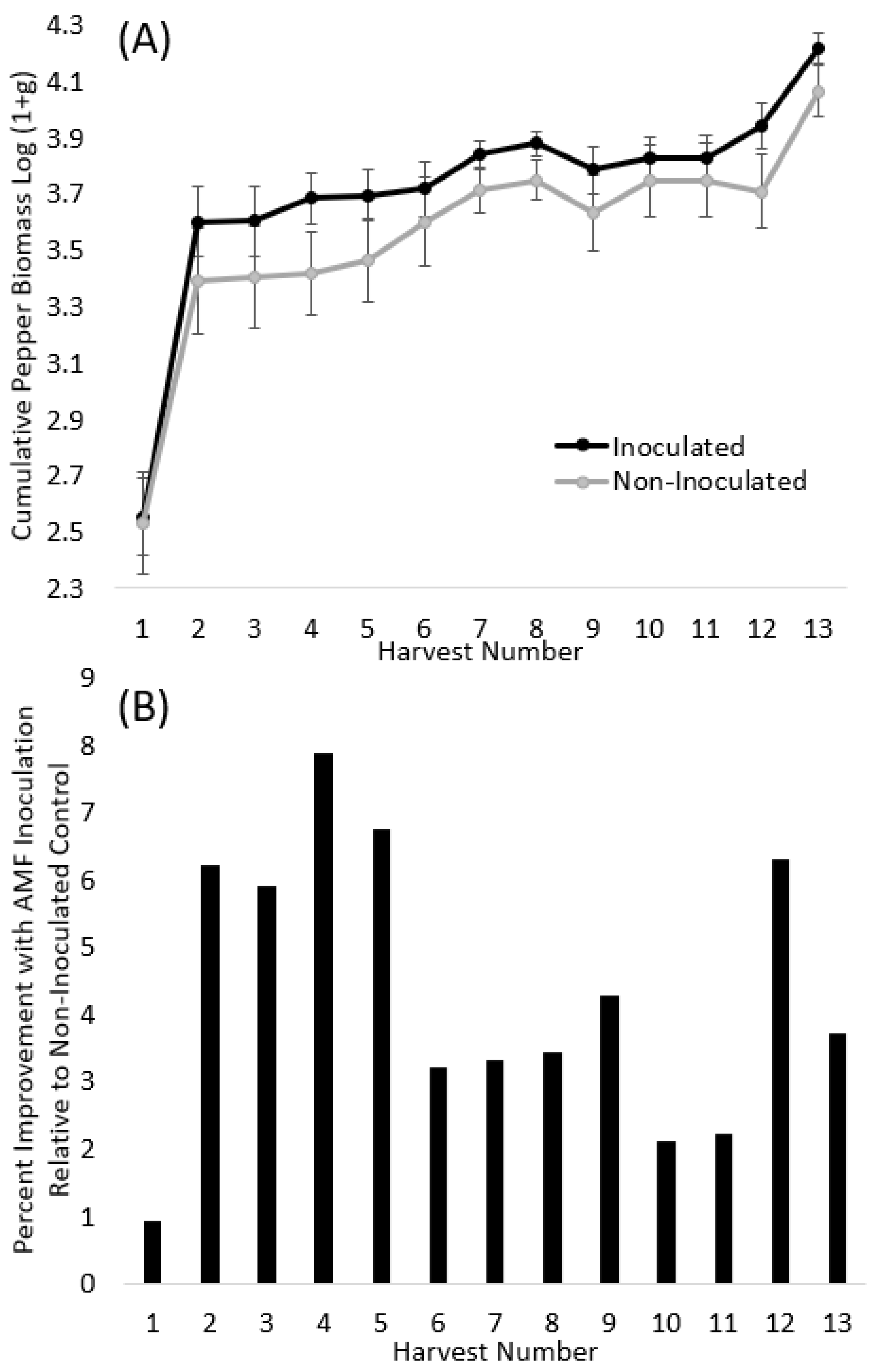
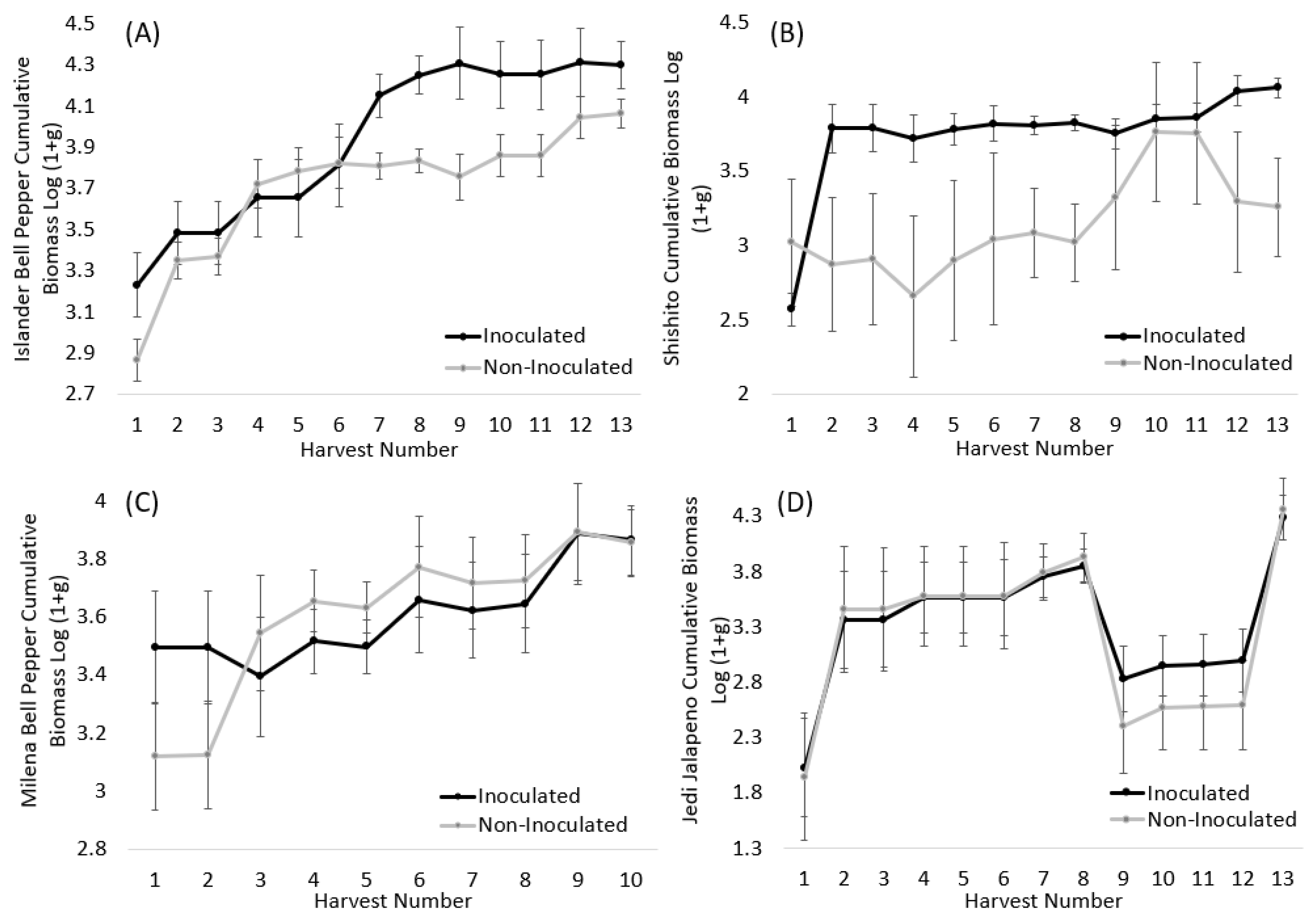
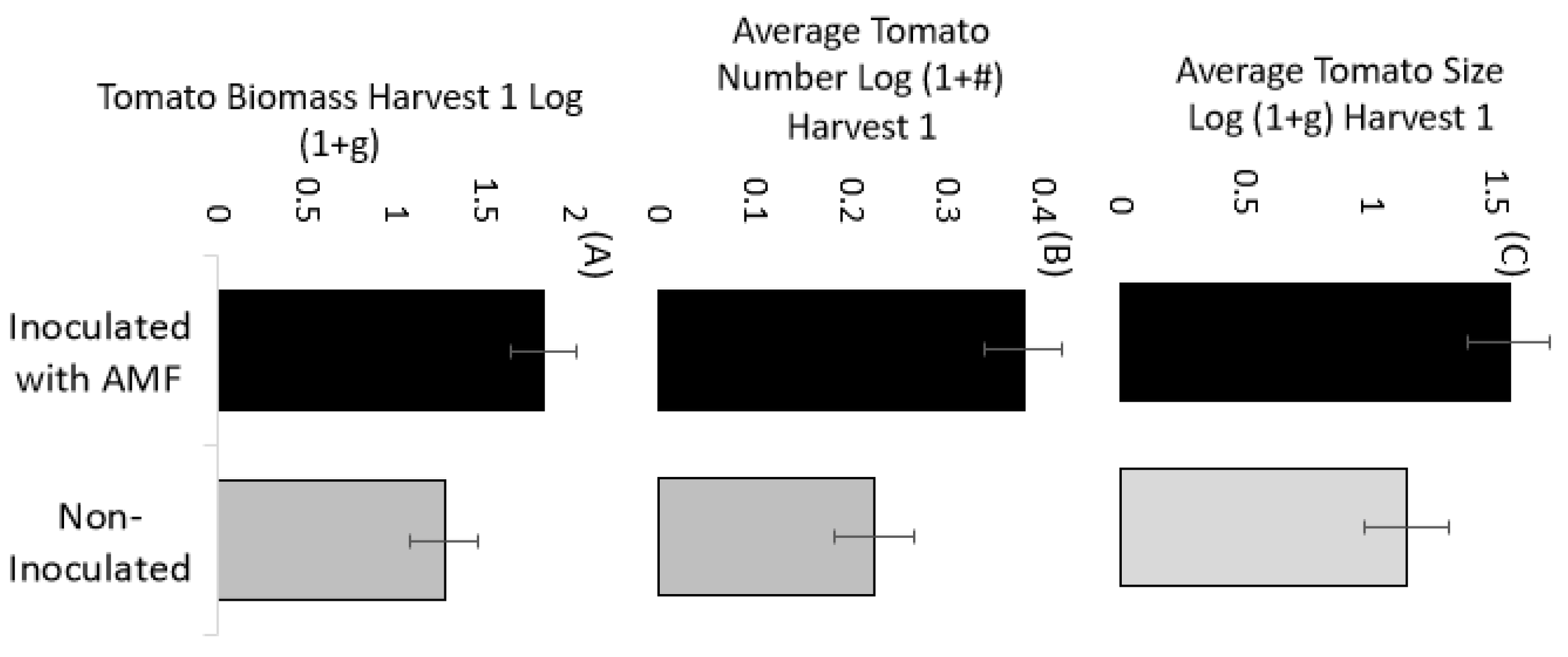
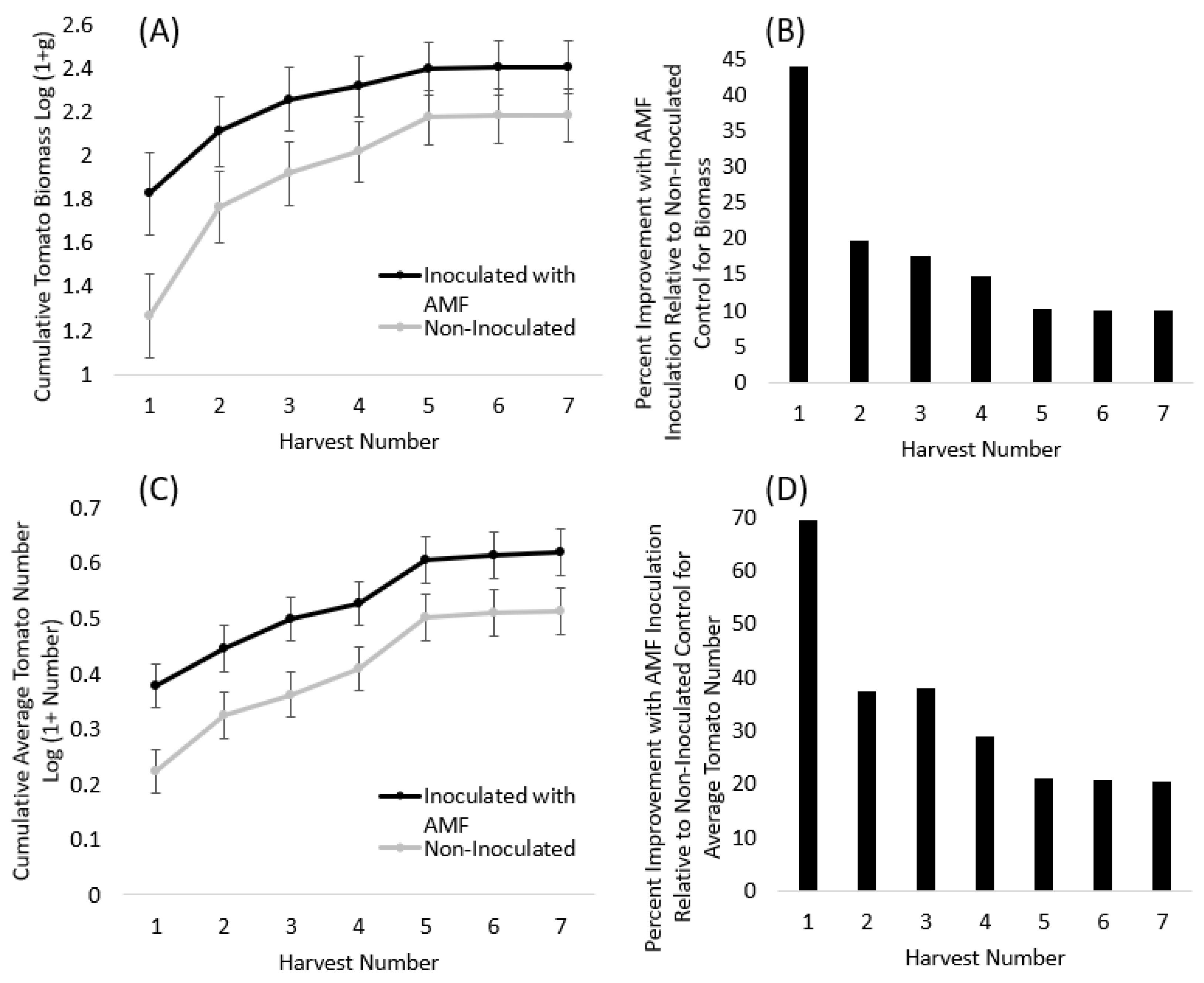
| Harvest Number | Pepper Harvest Date | Tomato Harvest Date |
|---|---|---|
| 1 | 28 June 2021 | 14 August 2021 |
| 2 | 15 July 2021 | 24 August 2021 |
| 3 | 27 July 2021 | 31 August 2021 |
| 4 | 28 July 2021 | 7 September 2021 |
| 5 | 15 August 2021 | 14 September 2021 |
| 6 | 21 August 2021 | 21 September 2021 |
| 7 | 26 August 2021 | 27 September 2021 |
| 8 | 10 September 2021 | |
| 9 | 25 September 2021 | |
| 10 | 28 September 2021 | |
| 11 | 7 October 2021 | |
| 12 | 12 October 2021 | |
| 13 | 20 October 2021 |
| Predictors | N | D | Harvest 1 | Harvest 2 | Harvest 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||||||
| Initial Height × Variety | 4 | 11 | 1.72 | 0.216 | 0.8 | 0.55 | 0.76 | 0.573 | ||||
| Initial Leaf × Variety | 4 | 11 | 0.57 | 0.69 | 1.32 | 0.323 | 1.27 | 0.339 | ||||
| Soil Treatment | 1 | 11 | 0.03 | 0.875 | 1.82 | 0.204 | 1.71 | 0.218 | ||||
| Variety | 3 | 11 | 0.99 | 0.432 | 1.36 | 0.306 | 1.33 | 0.314 | ||||
| Soil Treatment × Variety | 3 | 11 | 1.96 | 0.179 | 1.78 | 0.208 | 1.66 | 0.234 | ||||
| Predictors | N | D | Harvest 4 | Harvest 5 | Harvest 6 | Harvest 7 | Harvest 8 | |||||
| F | p | F | p | F | p | F | p | F | p | |||
| Initial Height × Variety | 5 | 14 | 0.96 | 0.474 | 0.75 | 0.599 | 0.52 | 0.76 | 1.78 | 0.183 | 3.22 | 0.038 |
| Initial Leaf × Variety | 5 | 14 | 1.97 | 0.146 | 1.78 | 0.182 | 0.66 | 0.657 | 3.01 | 0.047 | 5.41 | 0.006 |
| Soil Treatment | 1 | 14 | 4.3 | 0.057 | 3.27 | 0.092 | 0.7 | 0.418 | 2.99 | 0.106 | 4.19 | 0.06 |
| Variety | 4 | 14 | 3.33 | 0.041 | 3.06 | 0.052 | 0.69 | 0.608 | 3.64 | 0.031 | 5.97 | 0.005 |
| Soil Treatment × Variety | 4 | 14 | 3.61 | 0.032 | 3.43 | 0.038 | 0.72 | 0.591 | 3.31 | 0.042 | 4.03 | 0.022 |
| Predictors | N | D | Harvest 9 | Harvest 10 | Harvest 11 | Harvest 12 | Harvest 13 | |||||
| F | p | F | p | F | p | F | p | F | p | |||
| Initial Height × Variety | 5 | 14 | 2.36 | 0.094 | 2.02 | 0.139 | 2.07 | 0.131 | 2.43 | 0.087 | 2.75 | 0.062 |
| Initial Leaf × Variety | 5 | 14 | 4.16 | 0.016 | 3.59 | 0.027 | 3.34 | 0.034 | 3.7 | 0.024 | 3.21 | 0.039 |
| Soil Treatment | 1 | 14 | 1.82 | 0.199 | 0.49 | 0.493 | 0.53 | 0.478 | 4.25 | 0.058 | 3.65 | 0.077 |
| Variety | 4 | 14 | 4.67 | 0.013 | 4.16 | 0.02 | 3.93 | 0.024 | 4.45 | 0.016 | 6.61 | 0.003 |
| Soil Treatment × Variety | 4 | 14 | 3.25 | 0.044 | 2.4 | 0.1 | 2.17 | 0.126 | 2.55 | 0.086 | 1.75 | 0.195 |
| Predictors | Harvest 1 | Harvest 2 | Harvest 3 | Harvest 4 | Harvest 5 | Harvest 6 | Harvest 7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) Total Tomato Biomass | N | D | F | p | F | p | F | p | F | p | F | p | F | p | F | p |
| Initial Height × Variety | 5 | 86 | 1.38 | 0.239 | 2.53 | 0.035 | 0.88 | 0.496 | 0.42 | 0.836 | 0.41 | 0.840 | 0.4 | 0.845 | 0.4 | 0.849 |
| Initial Leaf × Variety | 5 | 86 | 1.4 | 0.231 | 1.51 | 0.196 | 2 | 0.087 | 2.21 | 0.061 | 2.19 | 0.063 | 2.2 | 0.062 | 2.19 | 0.062 |
| Variety | 4 | 19 | 2.17 | 0.111 | 3.04 | 0.043 | 2.33 | 0.093 | 0.93 | 0.465 | 0.58 | 0.681 | 0.58 | 0.682 | 0.57 | 0.687 |
| Soil Treatment | 1 | 19 | 5.44 | 0.031 | 2.84 | 0.108 | 3.37 | 0.082 | 2.75 | 0.114 | 1.9 | 0.185 | 1.86 | 0.188 | 1.86 | 0.189 |
| Soil Treatment × Variety | 4 | 19 | 0.92 | 0.472 | 0.32 | 0.858 | 0.13 | 0.971 | 0.08 | 0.988 | 0.23 | 0.917 | 0.23 | 0.917 | 0.23 | 0.918 |
| (B) Total Tomato Number | N | D | F | p | F | p | F | p | F | p | F | p | F | p | F | p |
| Initial Height × Variety | 5 | 86 | 1.17 | 0.328 | 1.69 | 0.145 | 0.97 | 0.438 | 0.98 | 0.435 | 0.56 | 0.733 | 0.51 | 0.771 | 0.5 | 0.779 |
| Initial Leaf × Variety | 5 | 86 | 1.18 | 0.327 | 1.39 | 0.236 | 1.73 | 0.135 | 2.5 | 0.036 | 1.24 | 0.299 | 1.41 | 0.229 | 1.4 | 0.233 |
| Variety | 4 | 19 | 1.82 | 0.167 | 2.66 | 0.064 | 2.27 | 0.099 | 2.41 | 0.085 | 1.08 | 0.394 | 1.18 | 0.352 | 1.16 | 0.360 |
| Soil Treatment | 1 | 19 | 9.25 | 0.007 | 5.28 | 0.033 | 7.36 | 0.014 | 5.29 | 0.033 | 3.72 | 0.069 | 3.54 | 0.075 | 3.52 | 0.076 |
| Soil Treatment × Variety | 4 | 19 | 1.27 | 0.318 | 0.77 | 0.558 | 0.22 | 0.922 | 0.29 | 0.880 | 0.49 | 0.741 | 0.48 | 0.753 | 0.46 | 0.766 |
| (C) Average Tomato Size | N | D | F | p | F | p | F | p | F | p | F | p | F | p | F | p |
| Initial Height × Variety | 5 | 86 | 1.31 | 0.266 | 2.43 | 0.042 | 0.64 | 0.673 | 0.38 | 0.864 | 0.71 | 0.616 | 0.68 | 0.641 | 0.68 | 0.639 |
| Initial Leaf × Variety | 5 | 86 | 1.37 | 0.245 | 1.42 | 0.226 | 1.92 | 0.099 | 1.89 | 0.104 | 2.17 | 0.064 | 2.14 | 0.068 | 2.14 | 0.068 |
| Variety | 4 | 19 | 2.06 | 0.127 | 2.64 | 0.066 | 1.79 | 0.172 | 0.36 | 0.836 | 0.35 | 0.844 | 0.31 | 0.865 | 0.32 | 0.862 |
| Soil Treatment | 1 | 19 | 3.73 | 0.068 | 1.58 | 0.224 | 1.32 | 0.265 | 1.28 | 0.273 | 0.71 | 0.411 | 0.69 | 0.417 | 0.69 | 0.418 |
| Soil Treatment × Variety | 4 | 19 | 1.07 | 0.399 | 0.59 | 0.673 | 0.31 | 0.865 | 0.28 | 0.886 | 0.48 | 0.747 | 0.47 | 0.754 | 0.48 | 0.751 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koziol, L.; Bever, J.D. Crop Productivity Boosters: Native Mycorrhizal Fungi from an Old-Growth Grassland Benefits Tomato (Solanum lycopersicum) and Pepper (Capsicum annuum) Varieties in Organically Farmed Soils. Microorganisms 2023, 11, 2012. https://doi.org/10.3390/microorganisms11082012
Koziol L, Bever JD. Crop Productivity Boosters: Native Mycorrhizal Fungi from an Old-Growth Grassland Benefits Tomato (Solanum lycopersicum) and Pepper (Capsicum annuum) Varieties in Organically Farmed Soils. Microorganisms. 2023; 11(8):2012. https://doi.org/10.3390/microorganisms11082012
Chicago/Turabian StyleKoziol, Liz, and James D. Bever. 2023. "Crop Productivity Boosters: Native Mycorrhizal Fungi from an Old-Growth Grassland Benefits Tomato (Solanum lycopersicum) and Pepper (Capsicum annuum) Varieties in Organically Farmed Soils" Microorganisms 11, no. 8: 2012. https://doi.org/10.3390/microorganisms11082012





