International Clones of High Risk of Acinetobacter Baumannii—Definitions, History, Properties and Perspectives
Abstract
:1. Introduction
2. Isolate Typing Methods for A. baumannii
3. International Clone (IC) Definitions, Attributes and Properties
4. Current Situation and Historical Spread of IC1-IC9 Worldwide
4.1. IC1, IC2 and IC3
4.2. IC4 and IC5
4.3. IC6
4.4. IC7
4.5. IC8
4.6. IC9
4.7. IC10 Candidate–CC33
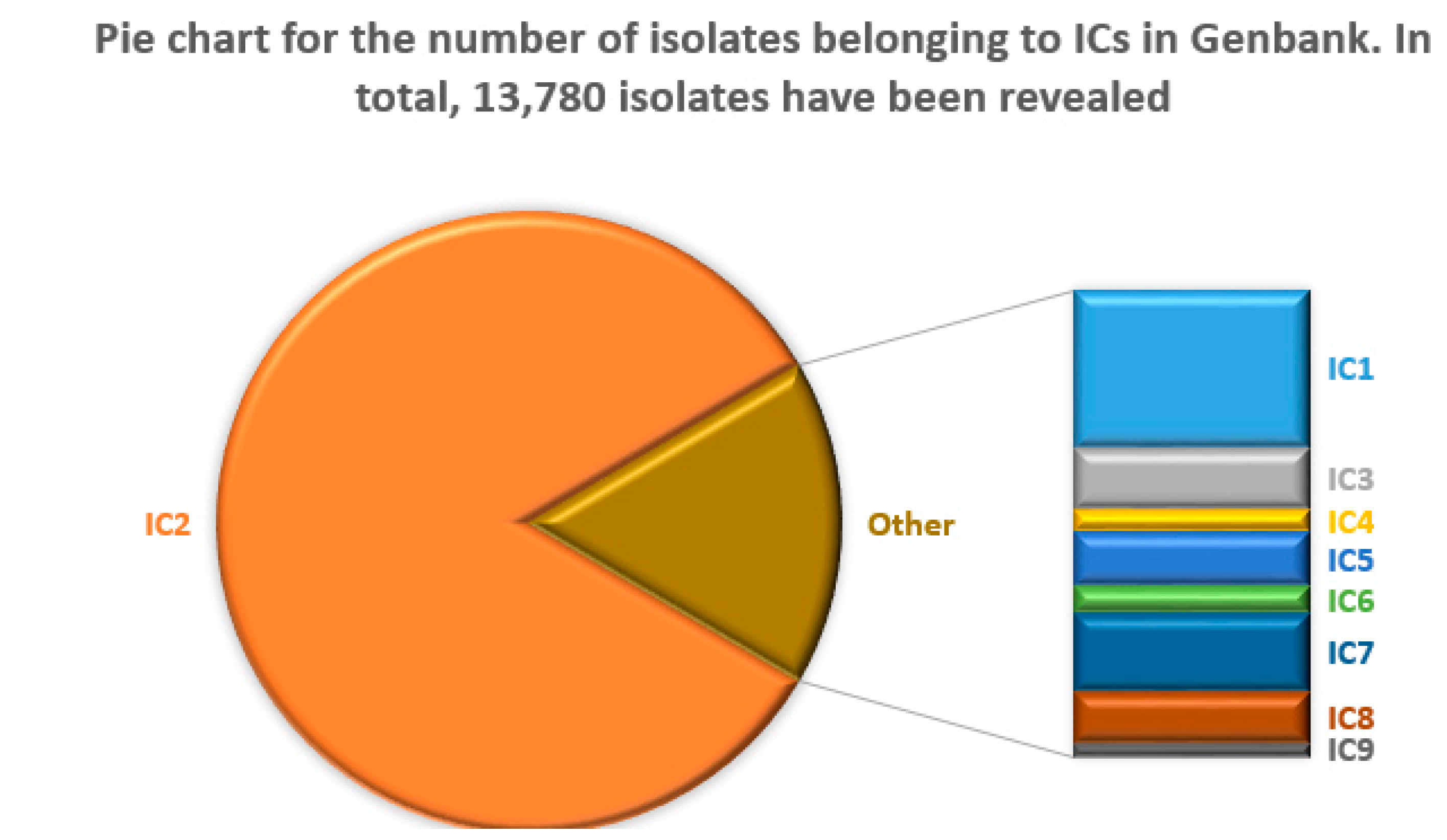
5. Distribution and Characteristics of ICs Available in Genbank
6. Misconceptions, Challenges, and Clarifications of IC Definitions
7. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Nemec, A.; Krizova, L.; Maixnerova, M.; van der Reijden, T.J.; Deschaght, P.; Passet, V.; Vaneechoutte, M.; Brisse, S.; Dijkshoorn, L. Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU). Res. Microbiol. 2011, 162, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Villalon, P.; Ortega, M.; Saez-Nieto, J.A.; Carrasco, G.; Medina-Pascual, M.J.; Garrido, N.; Valdezate, S. Dynamics of a Sporadic Nosocomial Acinetobacter calcoaceticus—Acinetobacter baumannii Complex Population. Front. Microbiol. 2019, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, M.; Behzadi, P.; Scribano, D.; Palamara, A.T.; Ambrosi, C. Acinetobacter baumannii: An Ancient Commensal with Weapons of a Pathogen. Pathogens 2021, 10, 387. [Google Scholar] [CrossRef]
- Kritsotakis, E.I.; Groves-Kozhageldiyeva, A. A systematic review of the global seasonality of infections caused by Acinetobacter species in hospitalized patients. Clin. Microbiol. Infect. 2020, 26, 553–562. [Google Scholar] [CrossRef]
- Towner, K.J. Acinetobacter: An old friend, but a new enemy. J. Hosp. Infect. 2009, 73, 355–363. [Google Scholar] [CrossRef]
- Holt, K.; Kenyon, J.J.; Hamidian, M.; Schultz, M.B.; Pickard, D.J.; Dougan, G.; Hall, R. Five decades of genome evolution in the globally distributed, extensively antibiotic-resistant Acinetobacter baumannii global clone 1. Microb. Genom. 2016, 2, e000052. [Google Scholar] [CrossRef]
- Morris, F.C.; Dexter, C.; Kostoulias, X.; Uddin, M.I.; Peleg, A.Y. The Mechanisms of Disease Caused by Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1601. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Lopez, R.; Solano-Galvez, S.G.; Juarez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padro Alonzo, L.A.; Rivera Resendiz, A.; Muleiro Alvarez, M.; Vega Lopez, E.N.; Franyuti-Kelly, G.; Alvarez-Hernandez, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef]
- Bartal, C.; Rolston, K.V.I.; Nesher, L. Carbapenem-resistant Acinetobacter baumannii: Colonization, Infection and Current Treatment Options. Infect. Dis. Ther. 2022, 11, 683–694. [Google Scholar] [CrossRef]
- Casarotta, E.; Bottari, E.; Vannicola, S.; Giorgetti, R.; Domizi, R.; Carsetti, A.; Damiani, E.; Scorcella, C.; Gabbanelli, V.; Pantanetti, S.; et al. Antibiotic Treatment of Acinetobacter baumannii Superinfection in Patients with SARS-CoV-2 Infection Admitted to Intensive Care Unit: An Observational Retrospective Study. Front. Med. 2022, 9, 910031. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Diancourt, L.; Passet, V.; Nemec, A.; Dijkshoorn, L.; Brisse, S. The population structure of Acinetobacter baumannii: Expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE 2010, 5, e10034. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G. Molecular Epidemiology of Clinical, Animal, and Environmental Isolates of Acinetobacter Baumannii. Available online: https://www.peg-symposien.org/tl_files/symposien/Symposien_2022/Vortraege%20BHS%2022/Higgins%20PEG%202022_for%20pdf.pdf (accessed on 30 March 2023).
- Aedh, A.I.; Al-Swedan, A.D.; Mohammed, A.A.; Alwadai, B.M.; Alyami, A.Y.; Alsaaed, E.A.; Almurdhimah, N.M.; Zaki, M.S.; Othman, A.E.; Hasan, A. Occurrence of Multidrug-Resistant Strains of Acinetobacter spp.: An Emerging Threat for Nosocomial-Borne Infection in Najran Region, KSA. Trop. Med. Infect. Dis. 2023, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Rafei, R.; Osman, M.; Dabboussi, F.; Hamze, M. Update on the epidemiological typing methods for Acinetobacter baumannii. Future Microbiol. 2019, 14, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Thoma, R.; Seneghini, M.; Seiffert, S.N.; Vuichard Gysin, D.; Scanferla, G.; Haller, S.; Flury, D.; Boggian, K.; Kleger, G.R.; Filipovic, M.; et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: Report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob. Resist. Infect. Control 2022, 11, 12. [Google Scholar] [CrossRef]
- Montrucchio, G.; Corcione, S.; Lupia, T.; Shbaklo, N.; Olivieri, C.; Poggioli, M.; Pagni, A.; Colombo, D.; Roasio, A.; Bosso, S.; et al. The Burden of Carbapenem-Resistant Acinetobacter baumannii in ICU COVID-19 Patients: A Regional Experience. J. Clin. Med. 2022, 11, 5208. [Google Scholar] [CrossRef]
- Pustijanac, E.; Hrenovic, J.; Vranic-Ladavac, M.; Mocenic, M.; Karcic, N.; Lazaric Stefanovic, L.; Hrstic, I.; Loncaric, J.; Seruga Music, M.; Drcelic, M.; et al. Dissemination of Clinical Acinetobacter baumannii Isolate to Hospital Environment during the COVID-19 Pandemic. Pathogens 2023, 12, 410. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Yan, R.; Lan, P.; Liu, H.; Fu, Y.; Hua, X.; Jiang, Y.; Zhou, Z.; Yu, Y. Comparing core-genome MLST with PFGE and MLST for cluster analysis of carbapenem-resistant Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2022, 30, 148–151. [Google Scholar] [CrossRef]
- Rafei, R.; Kempf, M.; Eveillard, M.; Dabboussi, F.; Hamze, M.; Joly-Guillou, M.L. Current molecular methods in epidemiological typing of Acinetobacter baumannii. Future Microbiol. 2014, 9, 1179–1194. [Google Scholar] [CrossRef]
- Da Silva, G.; Dijkshoorn, L.; van der Reijden, T.; van Strijen, B.; Duarte, A. Identification of widespread, closely related Acinetobacter baumannii isolates in Portugal as a subgroup of European clone II. Clin. Microbiol. Infect. 2007, 13, 190–195. [Google Scholar] [CrossRef]
- Higgins, P.G.; Dammhayn, C.; Hackel, M.; Seifert, H. Global spread of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2010, 65, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.; Dolzani, L.; Bressan, R.; van der Reijden, T.; van Strijen, B.; Stefanik, D.; Heersma, H.; Dijkshoorn, L. Standardization and interlaboratory reproducibility assessment of pulsed-field gel electrophoresis-generated fingerprints of Acinetobacter baumannii. J. Clin. Microbiol. 2005, 43, 4328–4335. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.F.; Gabriel, S.N.; Valderrey, C.; Kaufmann, M.E.; Pitt, T.L. Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin. Microbiol. Infect. 2007, 13, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Bartual, S.G.; Seifert, H.; Hippler, C.; Luzon, M.A.; Wisplinghoff, H.; Rodriguez-Valera, F. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J. Clin. Microbiol. 2005, 43, 4382–4390. [Google Scholar] [CrossRef]
- Pournaras, S.; Gogou, V.; Giannouli, M.; Dimitroulia, E.; Dafopoulou, K.; Tsakris, A.; Zarrilli, R. Single-locus-sequence-based typing of blaOXA-51-like genes for rapid assignment of Acinetobacter baumannii clinical isolates to international clonal lineages. J. Clin. Microbiol. 2014, 52, 1653–1657. [Google Scholar] [CrossRef]
- Wyres, K.L.; Cahill, S.M.; Holt, K.E.; Hall, R.M.; Kenyon, J.J. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb. Genom. 2020, 6, e000339. [Google Scholar] [CrossRef]
- Higgins, P.G.; Prior, K.; Harmsen, D.; Seifert, H. Development and evaluation of a core genome multilocus typing scheme for whole-genome sequence-based typing of Acinetobacter baumannii. PLoS ONE 2017, 12, e0179228. [Google Scholar] [CrossRef]
- Gaiarsa, S.; Batisti Biffignandi, G.; Esposito, E.P.; Castelli, M.; Jolley, K.A.; Brisse, S.; Sassera, D.; Zarrilli, R. Comparative Analysis of the Two Acinetobacter baumannii Multilocus Sequence Typing (MLST) Schemes. Front. Microbiol. 2019, 10, 930. [Google Scholar] [CrossRef]
- Odih, E.E.; Oaikhena, A.O.; Underwood, A.; Hounmanou, Y.M.G.; Oduyebo, O.O.; Fadeyi, A.; Aboderin, A.O.; Ogunleye, V.O.; Argimon, S.; Akpunonu, V.N.; et al. High Genetic Diversity of Carbapenem-Resistant Acinetobacter baumannii Isolates Recovered in Nigerian Hospitals in 2016 to 2020. mSphere 2023, 8, e0009823. [Google Scholar] [CrossRef]
- Rychert, J. Benefits and Limitations of MALDI-TOF Mass Spectrometry for the Identification of Microorganisms. J. Infect. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Sousa, C.; Botelho, J.; Grosso, F.; Silva, L.; Lopes, J.; Peixe, L. Unsuitability of MALDI-TOF MS to discriminate Acinetobacter baumannii clones under routine experimental conditions. Front. Microbiol. 2015, 6, 481. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Aucken, H.; Gerner-Smidt, P.; Janssen, P.; Kaufmann, M.E.; Garaizar, J.; Ursing, J.; Pitt, T.L. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J. Clin. Microbiol. 1996, 34, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, L.; Ma, Y.; Zhang, Z.; Li, N.; Zhang, F.; Zhao, S. Molecular Epidemiology of Multi-Drug Resistant Acinetobacter baumannii Isolated in Shandong, China. Front. Microbiol. 2016, 7, 1687. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G.; Hujer, A.M.; Hujer, K.M.; Bonomo, R.A.; Seifert, H. Interlaboratory reproducibility of DiversiLab rep-PCR typing and clustering of Acinetobacter baumannii isolates. J. Med. Microbiol. 2012, 61, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Heritier, C.; Poirel, L.; Fournier, P.E.; Claverie, J.M.; Raoult, D.; Nordmann, P. Characterization of the naturally occurring oxacillinase of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2005, 49, 4174–4179. [Google Scholar] [CrossRef]
- Lee, Y.T.; Kuo, S.C.; Chiang, M.C.; Yang, S.P.; Chen, C.P.; Chen, T.L.; Fung, C.P. Emergence of carbapenem-resistant non-baumannii species of Acinetobacter harboring a blaOXA-51-like gene that is intrinsic to A. baumannii. Antimicrob. Agents Chemother. 2012, 56, 1124–1127. [Google Scholar] [CrossRef]
- Brown, S.; Young, H.K.; Amyes, S.G. Characterisation of OXA-51, a novel class D carbapenemase found in genetically unrelated clinical strains of Acinetobacter baumannii from Argentina. Clin. Microbiol. Infect. 2005, 11, 15–23. [Google Scholar] [CrossRef]
- Turton, J.F.; Woodford, N.; Glover, J.; Yarde, S.; Kaufmann, M.E.; Pitt, T.L. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 2006, 44, 2974–2976. [Google Scholar] [CrossRef]
- Rafei, R.; Pailhories, H.; Hamze, M.; Eveillard, M.; Mallat, H.; Dabboussi, F.; Joly-Guillou, M.L.; Kempf, M. Molecular epidemiology of Acinetobacter baumannii in different hospitals in Tripoli, Lebanon using bla(OXA-51-like) sequence based typing. BMC Microbiol. 2015, 15, 103. [Google Scholar] [CrossRef]
- Karah, N.; Khalid, F.; Wai, S.N.; Uhlin, B.E.; Ahmad, I. Molecular epidemiology and antimicrobial resistance features of Acinetobacter baumannii clinical isolates from Pakistan. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Zander, E.; Higgins, P.G.; Fernandez-Gonzalez, A.; Seifert, H. Detection of intrinsic blaOXA-51-like by multiplex PCR on its own is not reliable for the identification of Acinetobacter baumannii. Int. J. Med. Microbiol. 2013, 303, 88–89. [Google Scholar] [CrossRef]
- Maiden, M.C.J.; Bygraves, J.A.; Feil, E.; Morelli, G.; Russell, J.E.; Urwin, R.; Zhang, Q.; Zhou, J.; Zurth, K.; Caugant, D.A.; et al. Multilocus sequence typing: A portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 1998, 95, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Maiden, M.C.; Jansen van Rensburg, M.J.; Bray, J.E.; Earle, S.G.; Ford, S.A.; Jolley, K.A.; McCarthy, N.D. MLST revisited: The gene-by-gene approach to bacterial genomics. Nat. Rev. Microbiol. 2013, 11, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, M.; Nigro, S.J.; Hall, R.M. Problems with the Oxford Multilocus Sequence Typing Scheme for Acinetobacter baumannii: Do Sequence Type 92 (ST92) and ST109 Exist? J. Clin. Microbiol. 2017, 55, 2287–2289. [Google Scholar] [CrossRef]
- Arbatsky, N.P.; Shneider, M.M.; Dmitrenok, A.S.; Popova, A.V.; Shagin, D.A.; Shelenkov, A.A.; Mikhailova, Y.V.; Edelstein, M.V.; Knirel, Y.A. Structure and gene cluster of the K125 capsular polysaccharide from Acinetobacter baumannii MAR13-1452. Int. J. Biol. Macromol. 2018, 117, 1195–1199. [Google Scholar] [CrossRef]
- Cahill, S.M.; Hall, R.M.; Kenyon, J.J. An update to the database for Acinetobacter baumannii capsular polysaccharide locus typing extends the extensive and diverse repertoire of genes found at and outside the K locus. Microb. Genom. 2022, 8, mgen000878. [Google Scholar] [CrossRef]
- Lam, M.M.C.; Wick, R.R.; Judd, L.M.; Holt, K.E.; Wyres, K.L. Kaptive 2.0: Updated capsule and lipopolysaccharide locus typing for the Klebsiella pneumoniae species complex. Microb. Genom. 2022, 8, 000800. [Google Scholar] [CrossRef]
- Tyumentseva, M.; Mikhaylova, Y.; Prelovskaya, A.; Tyumentsev, A.; Petrova, L.; Fomina, V.; Zamyatin, M.; Shelenkov, A.; Akimkin, V. Genomic and Phenotypic Analysis of Multidrug-Resistant Acinetobacter baumannii Clinical Isolates Carrying Different Types of CRISPR/Cas Systems. Pathogens 2021, 10, 205. [Google Scholar] [CrossRef]
- Feijao, P.; Yao, H.T.; Fornika, D.; Gardy, J.; Hsiao, W.; Chauve, C.; Chindelevitch, L. MentaLiST—A fast MLST caller for large MLST schemes. Microb. Genom. 2018, 4, e000146. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Zhang, L.; He, J.; Leptihn, S.; Yu, Y. Population Biology and Epidemiological Studies of Acinetobacter baumannii in the Era of Whole Genome Sequencing: Is the Oxford Scheme Still Appropriate? Front. Microbiol. 2020, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, M.; Nigro, S.J. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb. Genom. 2019, 5, e000306. [Google Scholar] [CrossRef] [PubMed]
- Nodari, C.S.; Cayo, R.; Streling, A.P.; Lei, F.; Wille, J.; Almeida, M.S.; de Paula, A.I.; Pignatari, A.C.C.; Seifert, H.; Higgins, P.G.; et al. Genomic Analysis of Carbapenem-Resistant Acinetobacter baumannii Isolates Belonging to Major Endemic Clones in South America. Front. Microbiol. 2020, 11, 584603. [Google Scholar] [CrossRef] [PubMed]
- Shelenkov, A.; Mikhaylova, Y.; Petrova, L.; Gaidukova, I.; Zamyatin, M.; Akimkin, V. Genomic Characterization of Clinical Acinetobacter baumannii Isolates Obtained from COVID-19 Patients in Russia. Antibiotics 2022, 11, 346. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Awan, F.; Jiang, H.; Zeng, Z.; Lv, W. Molecular Epidemiology and Clone Transmission of Carbapenem-Resistant Acinetobacter baumannii in ICU Rooms. Front. Cell. Infect. Microbiol. 2021, 11, 633817. [Google Scholar] [CrossRef] [PubMed]
- Feil, E.J.; Li, B.C.; Aanensen, D.M.; Hanage, W.P.; Spratt, B.G. eBURST: Inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 2004, 186, 1518–1530. [Google Scholar] [CrossRef]
- Baraniak, A.; Izdebski, R.; Fiett, J.; Sadowy, E.; Adler, A.; Kazma, M.; Salomon, J.; Lawrence, C.; Rossini, A.; Salvia, A.; et al. Comparative population analysis of Klebsiella pneumoniae strains with extended-spectrum beta-lactamases colonizing patients in rehabilitation centers in four countries. Antimicrob. Agents Chemother. 2013, 57, 1992–1997. [Google Scholar] [CrossRef]
- Aguilar-Rodea, P.; Estrada-Javier, E.L.; Jimenez-Rojas, V.; Gomez-Ramirez, U.; Nolasco-Romero, C.G.; Rodea, G.E.; Rodriguez-Espino, B.A.; Mendoza-Elizalde, S.; Arellano, C.; Lopez-Marcelino, B.; et al. New Variants of Pseudomonas aeruginosa High-Risk Clone ST233 Associated with an Outbreak in a Mexican Paediatric Hospital. Microorganisms 2022, 10, 1533. [Google Scholar] [CrossRef]
- Karah, N.; Sundsfjord, A.; Towner, K.; Samuelsen, O. Insights into the global molecular epidemiology of carbapenem non-susceptible clones of Acinetobacter baumannii. Drug Resist. Updates 2012, 15, 237–247. [Google Scholar] [CrossRef]
- Zander, E.; Nemec, A.; Seifert, H.; Higgins, P.G. Association between beta-lactamase-encoding bla(OXA-51) variants and DiversiLab rep-PCR-based typing of Acinetobacter baumannii isolates. J. Clin. Microbiol. 2012, 50, 1900–1904. [Google Scholar] [CrossRef] [PubMed]
- Unger, F.; Eisenberg, T.; Prenger-Berninghoff, E.; Leidner, U.; Semmler, T.; Ewers, C. Imported Pet Reptiles and Their “Blind Passengers”-In-Depth Characterization of 80 Acinetobacter Species Isolates. Microorganisms 2022, 10, 893. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassan, L.; Elbadawi, H.; Osman, E.; Ali, S.; Elhag, K.; Cantillon, D.; Wille, J.; Seifert, H.; Higgins, P.G. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii From Khartoum State, Sudan. Front. Microbiol. 2021, 12, 628736. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, A.; Evans, B.A.; Towner, K.J.; Amyes, S.G. Characterization of epidemiologically unrelated Acinetobacter baumannii isolates from four continents by use of multilocus sequence typing, pulsed-field gel electrophoresis, and sequence-based typing of bla(OXA-51-like) genes. J. Clin. Microbiol. 2010, 48, 2476–2483. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rafei, R.; Hamze, M.; Pailhories, H.; Eveillard, M.; Marsollier, L.; Joly-Guillou, M.L.; Dabboussi, F.; Kempf, M. Extrahuman epidemiology of Acinetobacter baumannii in Lebanon. Appl. Environ. Microbiol. 2015, 81, 2359–2367. [Google Scholar] [CrossRef]
- Müller, C.S.D.; Wille, J.; Hackel, M.; Higgins, P.G.; Seifert, H. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii clinical isolates and identification of the novel international clone IC9: Results from a worldwide surveillance study (2012–2016). In Proceedings of the 29th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, The Netherlands, 13–16 April 2019; p. P0947. [Google Scholar]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-lactamase database (BLDB)—Structure and function. J. Enzym. Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef]
- Villalon, P.; Valdezate, S.; Medina-Pascual, M.J.; Rubio, V.; Vindel, A.; Saez-Nieto, J.A. Clonal diversity of nosocomial epidemic Acinetobacter baumannii strains isolated in Spain. J. Clin. Microbiol. 2011, 49, 875–882. [Google Scholar] [CrossRef]
- Tavares, L.C.B.; de Vasconcellos, F.M.; de Sousa, W.V.; Rocchetti, T.T.; Mondelli, A.L.; Ferreira, A.M.; Montelli, A.C.; Sadatsune, T.; Tiba-Casas, M.R.; Camargo, C.H. Emergence and Persistence of High-Risk Clones Among MDR and XDR A. baumannii at a Brazilian Teaching Hospital. Front. Microbiol. 2018, 9, 2898. [Google Scholar] [CrossRef]
- Simo Tchuinte, P.L.; Rabenandrasana, M.A.N.; Kowalewicz, C.; Andrianoelina, V.H.; Rakotondrasoa, A.; Andrianirina, Z.Z.; Enouf, V.; Ratsima, E.H.; Randrianirina, F.; Collard, J.M. Phenotypic and molecular characterisations of carbapenem-resistant Acinetobacter baumannii strains isolated in Madagascar. Antimicrob. Resist. Infect. Control 2019, 8, 31. [Google Scholar] [CrossRef]
- Van Dessel, H.; Dijkshoorn, L.; van der Reijden, T.; Bakker, N.; Paauw, A.; van den Broek, P.; Verhoef, J.; Brisse, S. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res. Microbiol. 2004, 155, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wohlfarth, E.; Kresken, M.; Higgins, P.G.; Stefanik, D.; Wille, J.; Hafner, D.; Korber-Irrgang, B.; Seifert, H.; Study Group “Antimicrobial Resistance” of the Paul-Ehrlich-Society for Infection Therapy. The evolution of carbapenem resistance determinants and major epidemiological lineages among carbapenem-resistant Acinetobacter baumannii isolates in Germany, 2010–2019. Int. J. Antimicrob. Agents 2022, 60, 106689. [Google Scholar] [CrossRef] [PubMed]
- Zafer, M.M.; Hussein, A.F.A.; Al-Agamy, M.H.; Radwan, H.H.; Hamed, S.M. Genomic Characterization of Extensively Drug-Resistant NDM-Producing Acinetobacter baumannii Clinical Isolates With the Emergence of Novel bla (ADC-257). Front. Microbiol. 2021, 12, 736982. [Google Scholar] [CrossRef]
- Hawkey, J.; Ascher, D.B.; Judd, L.M.; Wick, R.R.; Kostoulias, X.; Cleland, H.; Spelman, D.W.; Padiglione, A.; Peleg, A.Y.; Holt, K.E. Evolution of carbapenem resistance in Acinetobacter baumannii during a prolonged infection. Microb. Genom. 2018, 4, e000165. [Google Scholar] [CrossRef] [PubMed]
- Mayanskiy, N.; Chebotar, I.; Alyabieva, N.; Kryzhanovskaya, O.; Savinova, T.; Turenok, A.; Bocharova, Y.; Lazareva, A.; Polikarpova, S.; Karaseva, O. Emergence of the Uncommon Clone ST944/ST78 Carrying bla(OXA-40-like) and bla(CTX-M-like) Genes Among Carbapenem-Nonsusceptible Acinetobacter baumannii in Moscow, Russia. Microb. Drug Resist. 2017, 23, 864–870. [Google Scholar] [CrossRef]
- Cerezales, M.; Xanthopoulou, K.; Wille, J.; Bustamante, Z.; Seifert, H.; Gallego, L.; Higgins, P.G. Acinetobacter baumannii analysis by core genome multi-locus sequence typing in two hospitals in Bolivia: Endemicity of international clone 7 isolates (CC25). Int. J. Antimicrob. Agents 2019, 53, 844–849. [Google Scholar] [CrossRef]
- Shelenkov, A.; Petrova, L.; Zamyatin, M.; Mikhaylova, Y.; Akimkin, V. Diversity of International High-Risk Clones of Acinetobacter baumannii Revealed in a Russian Multidisciplinary Medical Center during 2017–2019. Antibiotics 2021, 10, 1009. [Google Scholar] [CrossRef]
- Borges Duarte, D.F.; Goncalves Rodrigues, A. Acinetobacter baumannii: Insights towards a comprehensive approach for the prevention of outbreaks in health-care facilities. APMIS 2022, 130, 330–337. [Google Scholar] [CrossRef]
- Rahman, A.; Styczynski, A.; Khaleque, A.; Hossain, S.A.; Sadique, A.; Hossain, A.; Jain, M.; Tabassum, S.N.; Khan, F.; Bhuiyan, M.S.S.; et al. Genomic landscape of prominent XDR Acinetobacter clonal complexes from Dhaka, Bangladesh. BMC Genom. 2022, 23, 802. [Google Scholar] [CrossRef]
- Nowak, J.; Zander, E.; Stefanik, D.; Higgins, P.G.; Roca, I.; Vila, J.; McConnell, M.J.; Cisneros, J.M.; Seifert, H.; MagicBullet Working Group WP4. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J. Antimicrob. Chemother. 2017, 72, 3277–3282. [Google Scholar] [CrossRef]
- Chan, K.W.; Liu, C.Y.; Wong, H.Y.; Chan, W.C.; Wong, K.Y.; Chen, S. Specific Amino Acid Substitutions in OXA-51-Type beta-Lactamase Enhance Catalytic Activity to a Level Comparable to Carbapenemase OXA-23 and OXA-24/40. Int. J. Mol. Sci. 2022, 23, 4496. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Mendes, R.E.; Gales, A.C. Global Epidemiology and Mechanisms of Resistance of Acinetobacter baumannii-calcoaceticus Complex. Clin. Infect. Dis. 2023, 76, S166–S178. [Google Scholar] [CrossRef] [PubMed]
- Klotz, P.; Higgins, P.G.; Schaubmar, A.R.; Failing, K.; Leidner, U.; Seifert, H.; Scheufen, S.; Semmler, T.; Ewers, C. Seasonal Occurrence and Carbapenem Susceptibility of Bovine Acinetobacter baumannii in Germany. Front. Microbiol. 2019, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Levy-Blitchtein, S.; Roca, I.; Plasencia-Rebata, S.; Vicente-Taboada, W.; Velásquez-Pomar, J.; Muñoz, L.; Moreno-Morales, J.; Pons, M.J.; del Valle-Mendoza, J.; Vila, J. Emergence and spread of carbapenem-resistant Acinetobacter baumannii international clones II and III in Lima, Peru. Emerg. Microbes Infect. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Nemec, A.; Dolzani, L.; Brisse, S.; van den Broek, P.; Dijkshoorn, L. Diversity of aminoglycoside-resistance genes and their association with class 1 integrons among strains of pan-European Acinetobacter baumannii clones. J. Med. Microbiol. 2004, 53, 1233–1240. [Google Scholar] [CrossRef]
- Chagas, T.P.; Carvalho, K.R.; de Oliveira Santos, I.C.; Carvalho-Assef, A.P.; Asensi, M.D. Characterization of carbapenem-resistant Acinetobacter baumannii in Brazil (2008–2011): Countrywide spread of OXA-23-producing clones (CC15 and CC79). Diagn. Microbiol. Infect. Dis. 2014, 79, 468–472. [Google Scholar] [CrossRef]
- Cardoso, J.P.; Cayo, R.; Girardello, R.; Gales, A.C. Diversity of mechanisms conferring resistance to beta-lactams among OXA-23-producing Acinetobacter baumannii clones. Diagn. Microbiol. Infect. Dis. 2016, 85, 90–97. [Google Scholar] [CrossRef]
- Rodriguez, C.H.; Balderrama Yarhui, N.; Nastro, M.; Nunez Quezada, T.; Castro Canarte, G.; Magne Ventura, R.; Ugarte Cuba, T.; Valenzuela, N.; Roach, F.; Mota, M.I.; et al. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in South America. J. Med. Microbiol. 2016, 65, 1088–1091. [Google Scholar] [CrossRef]
- Opazo-Capurro, A.; San Martin, I.; Quezada-Aguiluz, M.; Morales-Leon, F.; Dominguez-Yevenes, M.; Lima, C.A.; Esposito, F.; Cerdeira, L.; Bello-Toledo, H.; Lincopan, N.; et al. Evolutionary dynamics of carbapenem-resistant Acinetobacter baumannii circulating in Chilean hospitals. Infect. Genet. Evol. 2019, 73, 93–97. [Google Scholar] [CrossRef]
- Towner, K.J.; Levi, K.; Vlassiadi, M.; Group, A.S. Genetic diversity of carbapenem-resistant isolates of Acinetobacter baumannii in Europe. Clin. Microbiol. Infect. 2008, 14, 161–167. [Google Scholar] [CrossRef]
- Karah, N.; Haldorsen, B.; Hermansen, N.O.; Tveten, Y.; Ragnhildstveit, E.; Skutlaberg, D.H.; Tofteland, S.; Sundsfjord, A.; Samuelsen, O. Emergence of OXA-carbapenemase- and 16S rRNA methylase-producing international clones of Acinetobacter baumannii in Norway. J. Med. Microbiol. 2011, 60, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Picao, R.C.; Adams-Sapper, S.; Riley, L.W.; Moreira, B.M. Association of class 1 and 2 integrons with multidrug-resistant Acinetobacter baumannii international clones and Acinetobacter nosocomialis isolates. Antimicrob. Agents Chemother. 2015, 59, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Martins, A.F.; Machado, A.B.; Barin, J.; Barth, A.L. Carbapenem-susceptible Acinetobacter baumannii carrying the ISAba1 upstream blaOXA-51-like gene in Porto Alegre, southern Brazil. Epidemiol. Infect. 2013, 141, 330–333. [Google Scholar] [CrossRef]
- Romanin, P.; Palermo, R.L.; Cavalini, J.F.; Favaro, L.D.S.; De Paula-Petroli, S.B.; Fernandes, E.V.; Dos Anjos Szczerepa, M.M.; Tognim, M.C.B.; Yamada-Ogatta, S.F.; Carrara-Marroni, F.E.; et al. Multidrug- and Extensively Drug-Resistant Acinetobacter baumannii in a Tertiary Hospital from Brazil: The Importance of Carbapenemase Encoding Genes and Epidemic Clonal Complexes in a 10-Year Study. Microb. Drug Resist. 2019, 25, 1365–1373. [Google Scholar] [CrossRef]
- Krizova, L.; Poirel, L.; Nordmann, P.; Nemec, A. TEM-1 beta-lactamase as a source of resistance to sulbactam in clinical strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 2013, 68, 2786–2791. [Google Scholar] [CrossRef]
- Gerson, S.; Lucassen, K.; Wille, J.; Nodari, C.S.; Stefanik, D.; Nowak, J.; Wille, T.; Betts, J.W.; Roca, I.; Vila, J.; et al. Diversity of amino acid substitutions in PmrCAB associated with colistin resistance in clinical isolates of Acinetobacter baumannii. Int. J. Antimicrob. Agents 2020, 55, 105862. [Google Scholar] [CrossRef] [PubMed]
- Caldart, R.V.; Fonseca, E.L.; Freitas, F.; Rocha, L.; Vicente, A.C. Acinetobacter baumannii infections in Amazon Region driven by extensively drug resistant international clones, 2016–2018. Memórias do Inst. Oswaldo Cruz 2019, 114, e190232. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, M.; Cuccurullo, S.; Crivaro, V.; Di Popolo, A.; Bernardo, M.; Tomasone, F.; Amato, G.; Brisse, S.; Triassi, M.; Utili, R.; et al. Molecular epidemiology of multidrug-resistant Acinetobacter baumannii in a tertiary care hospital in Naples, Italy, shows the emergence of a novel epidemic clone. J. Clin. Microbiol. 2010, 48, 1223–1230. [Google Scholar] [CrossRef]
- Gaiarsa, S.; Bitar, I.; Comandatore, F.; Corbella, M.; Piazza, A.; Scaltriti, E.; Villa, L.; Postiglione, U.; Marone, P.; Nucleo, E.; et al. Can Insertion Sequences Proliferation Influence Genomic Plasticity? Comparative Analysis of Acinetobacter baumannii Sequence Type 78, a Persistent Clone in Italian Hospitals. Front. Microbiol. 2019, 10, 2080. [Google Scholar] [CrossRef]
- Sheck, E.A.; Edelstein, M.V.; Sukhorukova, M.V.; Ivanchik, N.V.; Skleenova, E.Y.; Dekhnich, A.V.; Azizov, I.S.; Kozlov, R.S. Epidemiology and Genetic Diversity of Colistin Nonsusceptible Nosocomial Acinetobacter baumannii Strains from Russia for 2013–2014. Can. J. Infect. Dis. Med. Microbiol. 2017, 2017, 1839190. [Google Scholar] [CrossRef]
- Pfeifer, Y.; Hunfeld, K.P.; Borgmann, S.; Maneg, D.; Blobner, W.; Werner, G.; Higgins, P.G. Carbapenem-resistant Acinetobacter baumannii ST78 with OXA-72 carbapenemase and ESBL gene blaCTX-M-115. J. Antimicrob. Chemother. 2016, 71, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G.; Hagen, R.M.; Podbielski, A.; Frickmann, H.; Warnke, P. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Isolated from War-Injured Patients from the Eastern Ukraine. Antibiotics 2020, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, S.Y.; Nunez, J.C.; Pulido, I.Y.; Gonzalez, E.B.; Valenzuela, E.M.; Reguero, M.T.; Mantilla, J.R.; Arango, A.I.; Bravo, P. Characterisation of carbapenem-resistant Acinetobacter calcoaceticus—A. baumannii complex isolates in a third-level hospital in Bogota, Colombia. Int. J. Antimicrob. Agents 2008, 31, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Jacob, J.J.; Vasudevan, K.; Mathur, P.; Ray, P.; Neeravi, A.; Baskaran, A.; Kirubananthan, A.; Anandan, S.; Biswas, I.; et al. Genomic Characterization of Mobile Genetic Elements Associated With Carbapenem Resistance of Acinetobacter baumannii From India. Front. Microbiol. 2022, 13, 869653. [Google Scholar] [CrossRef]
- Sahl, J.W.; Del Franco, M.; Pournaras, S.; Colman, R.E.; Karah, N.; Dijkshoorn, L.; Zarrilli, R. Phylogenetic and genomic diversity in isolates from the globally distributed Acinetobacter baumannii ST25 lineage. Sci. Rep. 2015, 5, 15188. [Google Scholar] [CrossRef]
- Higgins, P.G.; Kniel, M.; Rojak, S.; Balczun, C.; Rohde, H.; Frickmann, H.; Hagen, R.M. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Strains Isolated at the German Military Field Laboratory in Mazar-e Sharif, Afghanistan. Microorganisms 2021, 9, 2229. [Google Scholar] [CrossRef]
- Seifert, H.; Muller, C.; Stefanik, D.; Higgins, P.G.; Miller, A.; Kresken, M. In vitro activity of sulbactam/durlobactam against global isolates of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2020, 75, 2616–2621. [Google Scholar] [CrossRef]
- Xu, Q.; Hua, X.; He, J.; Zhang, D.; Chen, Q.; Zhang, L.; Loh, B.; Leptihn, S.; Wen, Y.; Higgins, P.G.; et al. The distribution of mutations and hotspots in transcription regulators of resistance-nodulation-cell division efflux pumps in tigecycline non-susceptible Acinetobacter baumannii in China. Int. J. Med. Microbiol. 2020, 310, 151464. [Google Scholar] [CrossRef]
- Chilam, J.; Argimon, S.; Limas, M.T.; Masim, M.L.; Gayeta, J.M.; Lagrada, M.L.; Olorosa, A.M.; Cohen, V.; Hernandez, L.T.; Jeffrey, B.; et al. Genomic surveillance of Acinetobacter baumannii in the Philippines, 2013–2014. West. Pac. Surveill. Response J. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Jacobmeyer, L.; Semmler, T.; Stamm, I.; Ewers, C. Genomic Analysis of Acinetobacter baumannii Isolates Carrying OXA-23 and OXA-58 Genes from Animals Reveals ST1 and ST25 as Major Clonal Lineages. Antibiotics 2022, 11, 1045. [Google Scholar] [CrossRef]
- Wilharm, G.; Skiebe, E.; Higgins, P.G.; Poppel, M.T.; Blaschke, U.; Leser, S.; Heider, C.; Heindorf, M.; Brauner, P.; Jackel, U.; et al. Relatedness of wildlife and livestock avian isolates of the nosocomial pathogen Acinetobacter baumannii to lineages spread in hospitals worldwide. Environ. Microbiol. 2017, 19, 4349–4364. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, K.; Urrutikoetxea-Gutierrez, M.; Vidal-Garcia, M.; Diaz de Tuesta Del Arco, J.L.; Sanchez-Urtaza, S.; Wille, J.; Seifert, H.; Higgins, P.G.; Gallego, L. First Report of New Delhi Metallo-beta-Lactamase-6 (NDM-6) in a Clinical Acinetobacter baumannii Isolate From Northern Spain. Front. Microbiol. 2020, 11, 589253. [Google Scholar] [CrossRef] [PubMed]
- Rafei, R.; Dabboussi, F.; Hamze, M.; Eveillard, M.; Lemarie, C.; Mallat, H.; Rolain, J.M.; Joly-Guillou, M.L.; Kempf, M. First report of blaNDM-1-producing Acinetobacter baumannii isolated in Lebanon from civilians wounded during the Syrian war. Int. J. Infect. Dis. 2014, 21, 21–23. [Google Scholar] [CrossRef]
- Jaidane, N.; Naas, T.; Oueslati, S.; Bernabeu, S.; Boujaafar, N.; Bouallegue, O.; Bonnin, R.A. Whole-genome sequencing of NDM-1-producing ST85 Acinetobacter baumannii isolates from Tunisia. Int. J. Antimicrob. Agents 2018, 52, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamad, A.; Pal, T.; Leskafi, H.; Abbas, H.; Hejles, H.; Alsubikhy, F.; Darwish, D.; Ghazawi, A.; Sonnevend, A. Molecular characterization of clinical and environmental carbapenem resistant Acinetobacter baumannii isolates in a hospital of the Eastern Region of Saudi Arabia. J. Infect. Public Health 2020, 13, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cuenca, F.; Perez-Palacios, P.; Galan-Sanchez, F.; Lopez-Cerero, L.; Lopez-Hernandez, I.; Lopez Rojas, R.; Arca-Suarez, J.; Diaz-de Alba, P.; Rodriguez Iglesias, M.; Pascual, A. First identification of bla(NDM-1) carbapenemase in bla(OXA-94)-producing Acinetobacter baumannii ST85 in Spain. Enfermedades Infecc. Microbiol. Clin. (Engl. Ed.) 2020, 38, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Rafei, R.; Dabboussi, F.; Hamze, M.; Eveillard, M.; Lemarie, C.; Gaultier, M.P.; Mallat, H.; Moghnieh, R.; Husni-Samaha, R.; Joly-Guillou, M.L.; et al. Molecular analysis of Acinetobacter baumannii strains isolated in Lebanon using four different typing methods. PLoS ONE 2014, 9, e115969. [Google Scholar] [CrossRef]
- Shelenkov, A.; Petrova, L.; Mironova, A.; Zamyatin, M.; Akimkin, V.; Mikhaylova, Y. Long-Read Whole Genome Sequencing Elucidates the Mechanisms of Amikacin Resistance in Multidrug-Resistant Klebsiella pneumoniae Isolates Obtained from COVID-19 Patients. Antibiotics 2022, 11, 1364. [Google Scholar] [CrossRef]
- Deng, X.; den Bakker, H.C.; Hendriksen, R.S. Genomic Epidemiology: Whole-Genome-Sequencing-Powered Surveillance and Outbreak Investigation of Foodborne Bacterial Pathogens. Annu. Rev. Food Sci. Technol. 2016, 7, 353–374. [Google Scholar] [CrossRef]
- Russini, V.; Spaziante, M.; Varcasia, B.M.; Diaconu, E.L.; Paolillo, P.; Picone, S.; Brunetti, G.; Mattia, D.; De Carolis, A.; Vairo, F.; et al. A Whole Genome Sequencing-Based Epidemiological Investigation of a Pregnancy-Related Invasive Listeriosis Case in Central Italy. Pathogens 2022, 11, 667. [Google Scholar] [CrossRef]
- Naing, S.Y.; Hordijk, J.; Duim, B.; Broens, E.M.; van der Graaf-van Bloois, L.; Rossen, J.W.; Robben, J.H.; Leendertse, M.; Wagenaar, J.A.; Zomer, A.L. Genomic Investigation of Two Acinetobacter baumannii Outbreaks in a Veterinary Intensive Care Unit in The Netherlands. Pathogens 2022, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zou, S.; Chen, H.; Yu, Y.; Ruan, Z. BacWGSTdb 2.0: A one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2021, 49, D644–D650. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, Y.Y.; Yang, Y.C.; Hsu, C.Y. 5NosoAE: A web server for nosocomial bacterial antibiogram investigation and epidemiology survey. Nucleic Acids Res. 2022, 50, W21–W28. [Google Scholar] [CrossRef] [PubMed]
- Shelenkov, A.; Petrova, L.; Fomina, V.; Zamyatin, M.; Mikhaylova, Y.; Akimkin, V. Multidrug-Resistant Proteus mirabilis Strain with Cointegrate Plasmid. Microorganisms 2020, 8, 1775. [Google Scholar] [CrossRef]
- Shelenkov, A.; Mikhaylova, Y.; Yanushevich, Y.; Samoilov, A.; Petrova, L.; Fomina, V.; Gusarov, V.; Zamyatin, M.; Shagin, D.; Akimkin, V. Molecular Typing, Characterization of Antimicrobial Resistance, Virulence Profiling and Analysis of Whole-Genome Sequence of Clinical Klebsiella pneumoniae Isolates. Antibiotics 2020, 9, 261. [Google Scholar] [CrossRef]
- Khuntayaporn, P.; Kanathum, P.; Houngsaitong, J.; Montakantikul, P.; Thirapanmethee, K.; Chomnawang, M.T. Predominance of international clone 2 multidrug-resistant Acinetobacter baumannii clinical isolates in Thailand: A nationwide study. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Fedrigo, N.H.; Xavier, D.E.; Cerdeira, L.; Fuga, B.; Marini, P.V.B.; Shinohara, D.R.; Carrara-Marroni, F.E.; Lincopan, N.; Tognim, M.C.B. Genomic insights of Acinetobacter baumannii ST374 reveal wide and increasing resistome and virulome. Infect. Genet. Evol. 2022, 97, 105148. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Azizi, O.; Solgi, H.; Fereshteh, S.; Shokouhi, S.; Badmasti, F. Emergence of carbapenem resistant Acinetobacter baumannii clonal complexes CC2 and CC10 among fecal carriages in an educational hospital. Int. J. Environ. Health Res. 2022, 32, 1478–1488. [Google Scholar] [CrossRef]
- Kang, H.M.; Yun, K.W.; Choi, E.H. Molecular epidemiology of Acinetobacter baumannii complex causing invasive infections in Korean children during 2001–2020. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 32. [Google Scholar] [CrossRef]
- Lee, Y.T.; Turton, J.F.; Chen, T.L.; Wu, R.C.; Chang, W.C.; Fung, C.P.; Chen, C.P.; Cho, W.L.; Huang, L.Y.; Siu, L.K. First identification of blaOXA-51-like in non-baumannii Acinetobacter spp. J. Chemother. 2009, 21, 514–520. [Google Scholar] [CrossRef]
- Leski, T.A.; Bangura, U.; Jimmy, D.H.; Ansumana, R.; Lizewski, S.E.; Li, R.W.; Stenger, D.A.; Taitt, C.R.; Vora, G.J. Identification of blaOXA-(5)(1)-like, blaOXA-(5)(8), blaDIM-(1), and blaVIM carbapenemase genes in hospital Enterobacteriaceae isolates from Sierra Leone. J. Clin. Microbiol. 2013, 51, 2435–2438. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Alikhan, N.F.; Carleton, H.A.; Seemann, T.; Keane, J.A.; Katz, L.S. Comparison of classical multi-locus sequence typing software for next-generation sequencing data. Microb. Genom. 2017, 3, e000124. [Google Scholar] [CrossRef] [PubMed]



| Typing Method | Group | Target | Results | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|---|
| Amplified fragment length polymorphism (AFLP) | DNA banding pattern | Whole genome | 50-band pattern | Deep resolution, high discriminatory power (DP) | Laborious, expensive, low inter-reproducibility | [22] |
| Repetitive sequence based-PCR (rep-PCR) | DNA banding pattern | Whole genome | Band pattern | Rapid, high DP | Expensive | [23] |
| Pulsed-field gel electrophoresis (PFGE) | DNA banding pattern | Whole genome | 20-band pattern | High DP | Laborious, low inter-reproducibility | [24] |
| Trilocus sequence-based typing (3-LST) | DNA banding pattern, Allele sequencing | Polymorphism within ompA, csuE and blaOXA-51 | Rapid, easy, public database availability | Inability to recognize certain ICs and non-IC isolates | [25] | |
| Multilocus sequence typing (MLST) | Allele sequencing | Polymorphism within seven housekeeping genes | Sequence type | Portable, reproducible, public database availability | Expensive, limited availability in clinical settings | [13,26] |
| blaOXA-51-like gene | Allele sequencing | Polymorphism within one locus | OXA variant | Cheap, public database availability | Low specificity | [27] |
| Capsule synthesis loci (KL) and lipooligosaccharide outer core loci (OCL) | Allele or whole genome sequencing | Polymorphism within KL and OCL | KL and OCL types | Reproducible, public database availability | Low DP, expensive, limited availability in clinical settings | [28] |
| cgMLST | Whole genome sequencing | Whole genome polymorphism | 2390 gene allelic profile | Rapid, automated, provides a lot of various data, high DP | Expensive, limited availability in clinical settings | [29] |
| core genome Single Nucleotide Polymorphisms (SNP) | Whole genome sequencing | Whole genome polymorphism | SNP-based profile | Rapid, provides a lot of various data, high DP | Expensive, limited availability in clinical settings | [30,31] |
| Isolate ID | cpn60 | fusA | gltA | pyrG | recA | rplB | rpoB | ST |
|---|---|---|---|---|---|---|---|---|
| Exp1 | 3 | 29 | 30 | 1 | 9 | 1 | 4 | 17 |
| Pas * | Oxf | Major OXA-51 Variant | Minor OXA-51 Variants ** | |
|---|---|---|---|---|
| IC1 | CC1 | CC231 | OXA-69 | OXA-92, OXA-107, OXA-110, OXA-112 |
| IC2 | CC2 | CC208, CC218, CC281 | OXA-66 | OXA-82, OXA-83, OXA-84, OXA-109, OXA-172, OXA-201, OXA-202 |
| IC3 | CC3 | CC106 | OXA-71 | OXA-113 |
| IC4 | CC15 | CC103 | OXA-51 | OXA-98 |
| IC5 | CC79 | CC205 | OXA-65 | - |
| IC6 | CC78 | CC944 | OXA-90 | OXA-200 |
| IC7 | CC25 | CC229 | OXA-64 | - |
| IC8 | CC10 | CC447 | OXA-68 | OXA-128, OXA-144 |
| IC9 | CC464 | CC1078 | OXA-94 | - |
| IC | Pasteur Scheme Sequence Types (ST) | OXA-51 Variants |
|---|---|---|
| IC1 | 1, 19, 20, 81, 94, 315, 460, 623, 717, 734, 986, 1090, 1106 | OXA-69, OXA-92, OXA-107, OXA-110, OXA-112 |
| IC2 | 2, 45, 187, 195, 414, 492, 570, 571, 577, 600, 604, 664, 745, 823, 1537, 1550, 1555, 1579 | OXA-66, OXA-82, OXA-83, OXA-84, OXA-109, OXA-172, OXA-201, OXA-202 |
| IC3 | 3, 124, 229, 500, 1822 | OXA-71, OXA-113 |
| IC4 | 15, 238 | OXA-51, OXA-98 |
| IC5 | 79, 156, 175, 422, 730, 1163, 1196 | OXA-65 |
| IC6 | 78 | OXA-90, OXA-200 |
| IC7 | 25, 113, 619, 945, 1487 | OXA-64 |
| IC8 | 10, 23, 82, 575, 613, 1512 | OXA-68, OXA-128, OXA-144 |
| IC9 | 6, 85, 464 | OXA-94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelenkov, A.; Akimkin, V.; Mikhaylova, Y. International Clones of High Risk of Acinetobacter Baumannii—Definitions, History, Properties and Perspectives. Microorganisms 2023, 11, 2115. https://doi.org/10.3390/microorganisms11082115
Shelenkov A, Akimkin V, Mikhaylova Y. International Clones of High Risk of Acinetobacter Baumannii—Definitions, History, Properties and Perspectives. Microorganisms. 2023; 11(8):2115. https://doi.org/10.3390/microorganisms11082115
Chicago/Turabian StyleShelenkov, Andrey, Vasiliy Akimkin, and Yulia Mikhaylova. 2023. "International Clones of High Risk of Acinetobacter Baumannii—Definitions, History, Properties and Perspectives" Microorganisms 11, no. 8: 2115. https://doi.org/10.3390/microorganisms11082115





