Influence of Source Materials, Concentration, Gastric Digestion, and Encapsulation on the Bioactive Response of Brassicaceae-Derived Samples against Helicobacter pylori
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Cauliflower-Derived Plasma Membrane Purification
2.3. Sample Preparation and Gastric In Vitro Digestion Process
2.4. Isothiocyanates Identification in the Gastric Digested Treatments
2.5. Growth and Culture Conditions of H. pylori
2.6. Cell Culture Conditions
2.7. Cell Viability
2.8. Determination of the Antibacterial Activity of the Brassicaceae-Derived Samples against H. pylori
2.9. Determination of the Antioxidant Activity of the Brassicaceae-Derived Samples against Intracellular Reactive Oxygen Species (ROS) Production by AGS Cells
2.10. Determination of the Anti-Inflammatory Activity of the Brassicaceae-Derived Samples
2.11. Statistical Analysis
3. Results
3.1. Raw Samples and Digestates Characterization
3.2. Antibacterial Activity of Brassicaceae-Derived Samples and Digestates against H. pylori
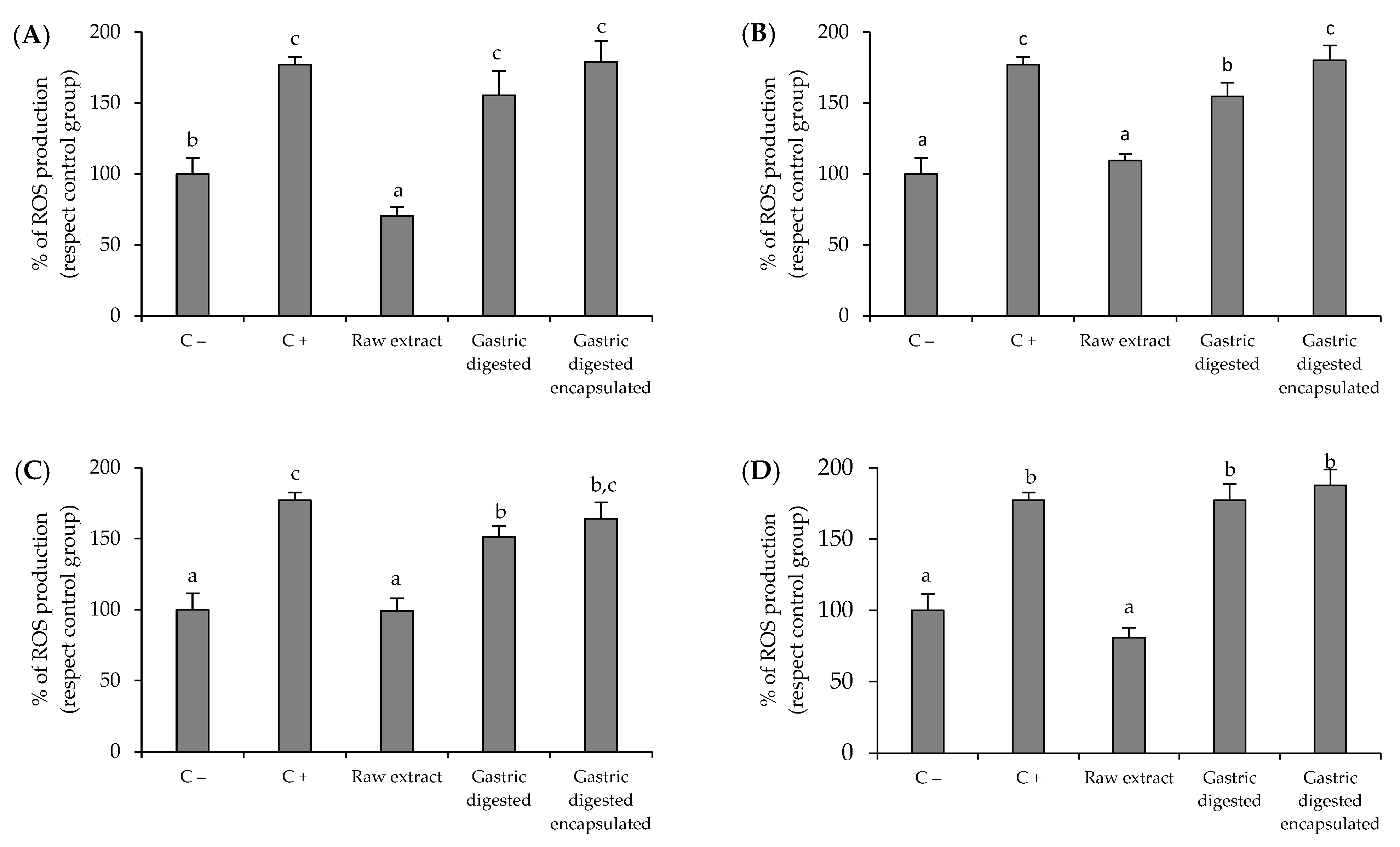
3.3. Antioxidant Activity of Brassicaceae-Derived Samples and Digestates against Intracellular Reactive Oxygen Species (ROS) Production by AGS Cells
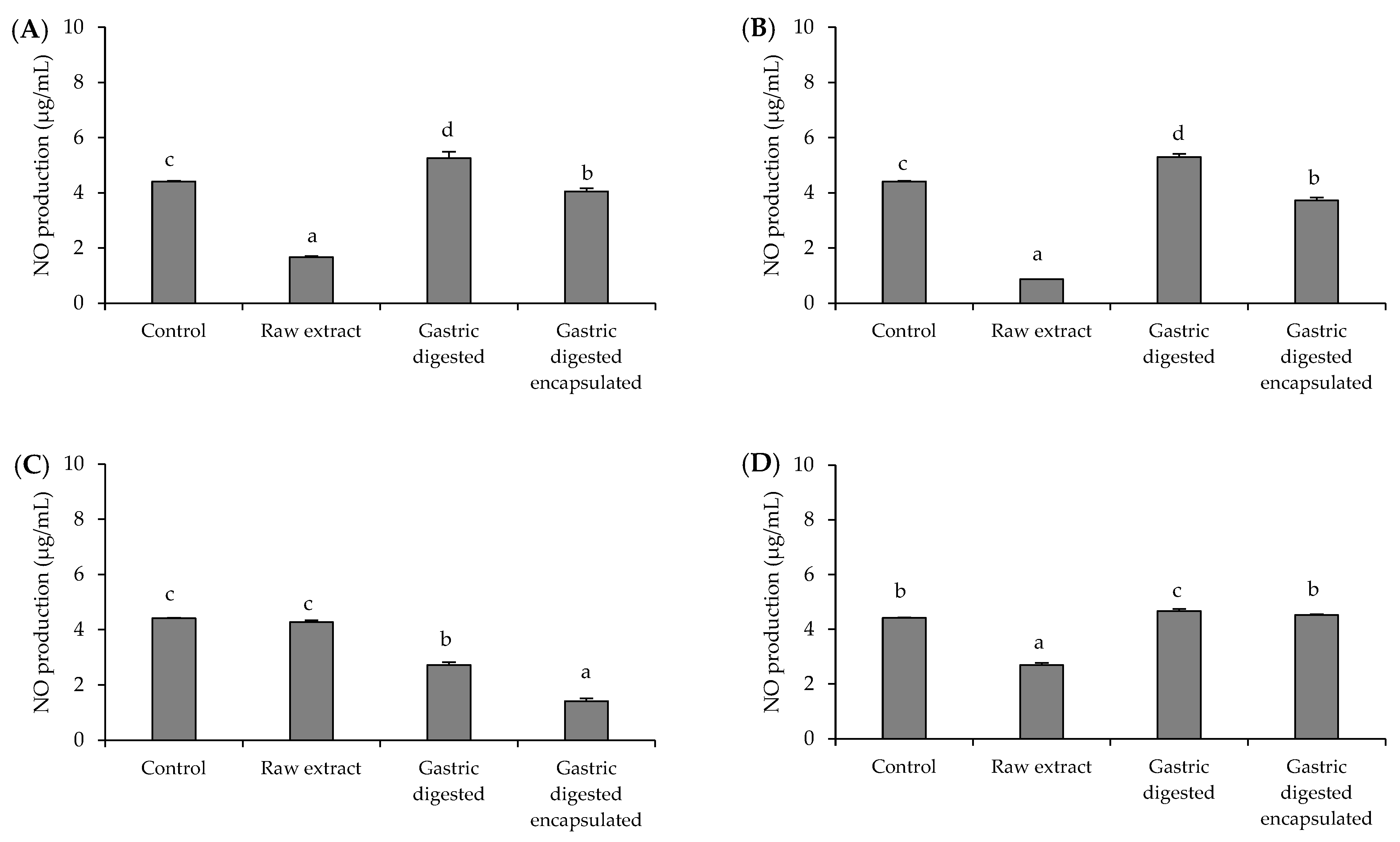
3.4. Anti-Inflammatory Activity of Brassicaceae-Derived Samples and Digestates
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y. How to eliminate gastric cancer-related death worldwide? Nat. Rev. Clin. Oncol. 2018, 15, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Zhao, H.P.; Yu, Y.; Wang, J.H.; Guo, L.; Liu, J.Y.; Pu, J.; Lv, J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Flores-Treviño, S.; Mendoza-Olazarán, S.; Bocanegra-Ibarias, P.; Maldonado-Garza, H.J.; Garza-González, E. Helicobacter pylori drug resistance: Therapy changes and challenges. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Kasahun, G.G.; Demoz, G.T.; Desta, D.M. Primary resistance pattern of Helicobacter pylori to antibiotics in adult population: A systematic review. Infect. Drug Resist. 2020, 13, 1567–1573. [Google Scholar] [CrossRef]
- Mladenova, I. Epidemiology of Helicobacter pylori Resistance to Antibiotics (A Narrative Review). Antibiotics 2023, 12, 1184. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, S.; Gan, R.-Y.; Li, H.-B. Natural products for the prevention and management of Helicobacter pylori infection. Compr. Rev. Food Sci. Food Saf. 2018, 17, 937–952. [Google Scholar] [CrossRef]
- Takeuchi, H.; Trang, V.T.; Morimoto, N.; Nishida, Y.; Matsumura, Y.; Sugiura, T. Natural products and food components with anti-Helicobacter pylori activities. World J. Gastroenterol. 2014, 20, 8971–8978. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Shams-Ardakani, M.; Foroumadi, A. Medicinal plants in the treatment of Helicobacter pylori infections. Pharm. Biol. 2015, 53, 939–960. [Google Scholar] [CrossRef] [PubMed]
- Abou Baker, D. Plants against Helicobacter pylori to combat resistance: An ethnopharmacological review. Biotechnol. Rep. 2020, 26, e00470. [Google Scholar] [CrossRef]
- Abdelshafeek, K.A.; El-Shamy, A.M. Review on glucosinolates: Unveiling their potential applications as drug discovery leads in extraction, isolation, biosynthesis, biological activity, and corrosion protection. Food Biosci. 2023, 56, 103071. [Google Scholar] [CrossRef]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef]
- Garcia-Ibañez, P.; Roses, C.; Agudelo, A.; Milagro, F.I.; Barceló, A.M.; Viadel, B.; Nieto, J.A.; Moreno, D.A.; Carvajal, M. The influence of red cabbage extract nanoencapsulated with brassica plasma membrane vesicles on the gut microbiome of obese volunteers. Foods 2021, 10, 1038. [Google Scholar] [CrossRef]
- Yepes-Molina, L.; Carvajal, M. Nanoencapsulation of sulforaphane in broccoli membrane vesicles and their in vitro antiproliferative activity. Pharm. Biol. 2021, 59, 1488–1502. [Google Scholar] [CrossRef]
- Sathayanaran, S.R.; Warke, V.G.; Mahajam, G.B.; Annapure, U.S. Soil free nutrient availability to plants. J. Plant Nutr. 2023, 46, 801–814. [Google Scholar] [CrossRef]
- Baenas, N.; Suárez-Martínez, C.; García-Viguera, C.; Moreno, D.A. Bioavailability and new biomarkers of cruciferous sprouts consumption. Food Res. Int. 2017, 100, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Gutierrez-Docio, A.; Moreno-Fernandez, S.; Alarcon-Cavero, T.; Prodanov, M.; Martinez-Rodriguez, A.J. Procyanidin-rich extract from grape seeds as a putative tool against Helicobacter pylori. Foods 2020, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Silvan, J.M.; Gutierrez-Docio, A.; Guerrero-Hurtado, E.; Domingo-Serrano, L.; Blanco-Suarez, A.; Prodanov, M.; Alarcon-Cavero, T.; Martinez-Rodriguez, A.J. Pre-treatment with grape seed extract reduces inflammatory response and oxidative stress induced by Helicobacter pylori infection in human gastric epithelial cells. Antioxidants 2021, 10, 943. [Google Scholar] [CrossRef]
- Silvan, J.M.; Michalska-Ciechanowska, A.; Martinez-Rodriguez, A.J. Modulation of antibacterial, antioxidant, and anti-inflammatory properties by drying of Prunus domestica L. plum juice extracts. Microorganisms 2020, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Butcher, L.D.; den Hartog, G.; Ernst, P.B.; Crowe, S.E. Oxidative stress resulting from Helicobacter pylori infection contributes to gastric carcinogenesis. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 316–322. [Google Scholar] [CrossRef]
- Kumar, S.; Patel, G.K.; Ghoshal, U.C. Helicobacter pylori-Induced Inflammation: Possible Factors Modulating the Risk of Gastric Cancer. Pathogens 2021, 10, 1099. [Google Scholar] [CrossRef]
- Garcia-Ibañez, P.; Moreno, D.A.; Carvajal, M. Nanoencapsulation of Bimi® extracts increases its bioaccessibility after in vitro digestion and evaluation of its activity in hepatocyte metabolism. Food Chem. 2022, 385, 132680. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef]
- Fahey, J.W.; Stephenson, K.K.; Wallace, A.J. Dietary amelioration of Helicobacter infection. Nutr. Res. 2015, 35, 461–473. [Google Scholar] [CrossRef]
- Fahey, J.W.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435, 1–7. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, C.; Li, Y.; Luo, L.; Xie, F.; Xiong, Q.; Feng, P. Effect of polyphenol compounds on Helicobacter pylori eradication: A systematic review with meta-analysis. BMJ Open 2023, 13, e062932. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.W.; Machado, A.R.T.; De Grandis, R.; Ribeiro, D.L.; Tuttis, K.; Morselli, M.; Aissa, A.F.; Pellegrini, M.; Antunes, L.M.G. Effects of sulforaphane on the oxidative response, apoptosis, and the transcriptional profile of human stomach mucosa cells in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 854–855, 503201. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Suzuki, K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Winter, J.A.; Robinson, K. Differential inflammatory response to Helicobacter pylori infection: Etiology and clinical outcomes. J. Inflamm. Res. 2015, 8, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, E.H.; Hahm, K.B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroenterol. Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef]
- Gobert, A.P.; Wilson, K.T. The immune battle against Helicobacter pylori infection: NO offense. Trends Microbiol. 2016, 24, 366–376. [Google Scholar] [CrossRef]
- Saaed, H.K.; Chiggiato, L.; Webb, D.L.; Rehnberg, A.S.; Rubio, C.A.; Befrits, R.; Hellström, P.M. Elevated gaseous luminal nitric oxide and circulating IL-8 as features of Helicobacter pylori-induced gastric inflammation. Ups. J. Med. Sci. 2021, 126, e8116. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
| Samples | Erucin | Iberin | Indole-3-carbinol (I3C) | Sulforaphane (SFN) | Sulforaphene |
|---|---|---|---|---|---|
| Red cabbage sprout | |||||
| Raw | 0.22 ± 0.01 | 190.00 ± 17.00 a | 4.38 ± 0.67 a | 141.22 ± 12.90 a | - |
| Gastric digested | * | 75.17 ± 0.57 c | * | 11.83 ± 0.11 b | - |
| Gastric digested encapsulated | * | 88.95 ± 2.34 b | 0.17 ± 0.05 b | 14.05 ± 0.10 b | - |
| Red radish sprout | |||||
| Raw | 0.11 ± 0.02 b | 6.74 ± 1.50 a | 4.04 ± 0.70 | 10.60 ± 1.30 a | 9.51 ± 1.01 a |
| Gastric digested | 0.14 ± 0.04 b | * | * | 0.95 ± 0.08 b | 0.37 ± 0.01 c |
| Gastric digested encapsulated | 0.47 ± 0.02 a | 0.73 ± 0.07 b | * | 0.80 ± 0.10 b | 0.81 ± 0.07 b |
| Red radish leaves | |||||
| Raw | - | - | 11.10 ± 1.02 a | 0.56 ± 0.03 a | 2.51 ± 0.07 a |
| Gastric digested | - | - | 0.08 ± 0.02 c | 0.06 ± 0.01 c | 0.10 ± 0.01 b |
| Gastric digested encapsulated | - | - | 0.45 ± 0.03 b | 0.10 ± 0.01 b | 0.13 ± 0.04 b |
| Red radish root | |||||
| Raw | 1.02 ± 0.20 a | - | 0.47 ± 0.10 a | 0.06 ± 0.00 a | 0.10 ± 0.03 a |
| Gastric digested | 0.55 ± 0.05 b | - | * | 0.01 ± 0.00 c | 0.04 ± 0.00 c |
| Gastric digested encapsulated | 0.94 ± 0.04 a | - | 0.27 ± 0.03 b | 0.05 ± 0.01 b | 0.08 ± 0.00 b |
| Samples | Log CFU/mL | Log Reduction | MIC (mg/mL) |
|---|---|---|---|
| Red cabbage sprout | |||
| Raw | <1.5 * | >7.05 | 0.2 |
| Gastric digested | 3.82 ± 0.11 * | 4.74 | 2.0 |
| Gastric digested encapsulated | <1.5 * | >7.05 | 2.0 |
| Red radish sprout | |||
| Raw | <1.5 * | >7.05 | 0.1 |
| Gastric digested | <1.5 * | >7.05 | 2.0 |
| Gastric digested encapsulated | <1.5 * | >7.05 | 2.0 |
| Red radish leaves | |||
| Raw | 8.47 ± 0.04 | 0.08 | - |
| Gastric digested | <1.5 * | >7.05 | 1.0 |
| Gastric digested encapsulated | <1.5 * | >7.05 | 1.0 |
| Red radish root | |||
| Raw | 7.26 ± 0.05 | 1.29 | - |
| Gastric digested | 8.59 ± 0.02 | - | - |
| Gastric digested encapsulated | 8.62 ± 0.03 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Ibañez, P.; Silvan, J.M.; Moreno, D.A.; Carvajal, M.; Martinez-Rodriguez, A.J. Influence of Source Materials, Concentration, Gastric Digestion, and Encapsulation on the Bioactive Response of Brassicaceae-Derived Samples against Helicobacter pylori. Microorganisms 2024, 12, 77. https://doi.org/10.3390/microorganisms12010077
Garcia-Ibañez P, Silvan JM, Moreno DA, Carvajal M, Martinez-Rodriguez AJ. Influence of Source Materials, Concentration, Gastric Digestion, and Encapsulation on the Bioactive Response of Brassicaceae-Derived Samples against Helicobacter pylori. Microorganisms. 2024; 12(1):77. https://doi.org/10.3390/microorganisms12010077
Chicago/Turabian StyleGarcia-Ibañez, Paula, Jose Manuel Silvan, Diego A. Moreno, Micaela Carvajal, and Adolfo J. Martinez-Rodriguez. 2024. "Influence of Source Materials, Concentration, Gastric Digestion, and Encapsulation on the Bioactive Response of Brassicaceae-Derived Samples against Helicobacter pylori" Microorganisms 12, no. 1: 77. https://doi.org/10.3390/microorganisms12010077








