A Comparative Analysis of Molecular Biological Methods for the Detection of SARS-CoV-2 and Testing the In Vitro Infectivity of the Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Loop-Mediated Isothermal Amplification (LAMP)
2.3. RNA Extraction and Nested One-Step RT PCR
2.4. Quantity and Quality Control of Extracted RNA and PCR Products
2.5. Cultivation of the Virus in Cell Culture
2.6. Statistical Analysis
3. Results
3.1. Loop-Mediated Isothermal Amplification (LAMP) and Nested One-Step RT PCR
3.2. Cultivation of the Virus in Cell Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schalk, F.; Hawn, C. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 1931, 78, 418–422. [Google Scholar]
- Doyle, P.; Hutching, M. A transmissible gastroenteritis in pigs. J. Am. Vet. Med. Assoc. 1946, 108, 257–259. [Google Scholar] [PubMed]
- Masters, P.S.; Perlman, S. Coronaviridae. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Cohen, J.I., Griffin, D.E., Lamb, R.A., Martin, M.A., Racaniello, V.R., Roizman, B., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2015; pp. 825–858. [Google Scholar]
- Qian, Z.; Dominguez, S.R.; Holmes, K.V. Role of the spike glycoprotein of human Middle East respiratory syndrome coronavirus (MERS-CoV) in virus entry and syncytia formation. PLoS ONE 2013, 8, e76469. [Google Scholar] [CrossRef]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- de Groot, R.J.; Baker, S.C.; Baric, R.S.; Brown, C.S.; Drosten, C.; Enjuanes, L.; Fouchier, R.A.; Galiano, M.; Gorbalenya, A.E.; Memish, Z.A.; et al. Commentary: Middle east respiratory syndrome coronavirus (mers-cov): Announcement of the coronavirus study group. J. Virol. 2013, 87, 7790–7792. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Shi, Z.; Shu, Y.; Song, J.; Gao, G.F.; Tan, W.; Guo, D. A distinct name is needed for the new coronavirus. Lancet 2020, 395, 949. [Google Scholar] [CrossRef]
- WHO. Severe Acute Respiratory Syndrome (SARS). Available online: www.who.int/ith/diseases/sars/en/ (accessed on 30 September 2020).
- Iqbal, S.; Saki, M.; Sirakov, I.; Akrami, S. Pathogenesis of the SARS Coronavirus-2 and Potential Therapeutic Strategies. J. Commun. Dis. 2022, 202–209. [Google Scholar] [CrossRef]
- Stoyanov, V.; Grigorova, T.; Petkov, D.; Yotov, I. The loss of smell and taste in patients with COVID-19 from Bulgarian population. Det. Infectsiosni Boles. (Pediatr. Infect. Dis.) 2020, 12, 17–23. (In Bulgarian) [Google Scholar]
- Park, M.; Cook, A.R.; Lim, J.T.; Sun, Y.; Dickens, B.L. A systematic review of COVID-19 epidemiology based on current evidence. J. Clin. Med. 2020, 9, 967. [Google Scholar] [CrossRef] [PubMed]
- Cipollaro, L.; Giordano, L.; Padulo, J.; Oliva, F.; Maffulli, N. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J. Orthop. Surg. Res. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Williams, F.M.; Freidin, M.B.; Mangino, M.; Couvreur, S.; Visconti, A.; Bowyer, R.C.; Le Roy, C.I.; Falchi, M.; Mompeó, O.; Sudre, C.; et al. Self-reported symptoms of COVID-19, including symptoms most predictive of SARS-CoV-2 infection, are heritable. Twin Res. Hum. Genet. 2020, 23, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Chaudhary, R.; Khurana, S.K.; Tiwari, R.; Dhama, K.; Gupta, V.K.; Singh, R.K.; Natesan, S. Strengthening of Molecular Diagnosis of SARS-CoV-2/COVID-19 with a Special Focus on India. J. Pure Appl. Microbiol. 2020, 14 (Suppl. S1), 789–798. [Google Scholar] [CrossRef]
- Pal, M.; Parija, S.; Panda, G.; Mishra, S.; Mohapatra, R.K.; Dhama, K. COVID-19 Prognosis from Chest X-ray Images by using Deep Learning Approaches: A Next Generation Diagnostic Tool. J. Pure Appl. Microbiol. 2023, 17, 919–930. [Google Scholar] [CrossRef]
- Sirakov, I.; Stankova, P.; Bakalov, D.; Bardarska, L.; Paraskova, G.; Mitov, I.; Gergova, R. Asymptomatic spread of SARS-CoV-2 during the first wave in Bulgaria: A retrospective study in a region with distinct geography and climate. Was the virus source from the UK? Acta Microbiol. Bulg. 2022, 38, 358–360. [Google Scholar]
- Borges, L.P.; Martins, A.F.; de Melo, M.S.; de Oliveira, M.G.; de Rezende Neto, J.M.; Dósea, M.B.; Cabral, B.C.; Menezes, R.F.; Santos, A.A.; Matos, I.L.; et al. Seroprevalence of SARS-CoV-2 IgM and IgG antibodies in an asymptomatic population in Sergipe, Brazil. Rev. Panam. Salud Pública 2020, 44, e108. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.B.; Grüter, L.; Boltzmann, M.; Rollnik, J.D. Prevalence of serum IgG antibodies against SARS-CoV-2 among clinic staff. PLoS ONE 2020, 15, e0235417. [Google Scholar] [CrossRef] [PubMed]
- Allavarapu, R.S.; Sethumadhavan, K.; Usharani, P.; Tejaswani, B.V.V.V. Comparative and Prospective Study on the Efficacy of RT-PCR and Rapid Antigen Test in Symptomatic COVID-19 Patients at Tertiary Care Hospital. J. Pure Appl. Microbiol. 2023, 17, 1846–1853. [Google Scholar] [CrossRef]
- Saleh, A.H.; Kumar, D.; Sirakov, I.; Shafiee, P.; Arefian, M. Application of nano compounds for the prevention, diagnosis, and treatment of SARS-coronavirus: A review. J. Compos. Compd. 2021, 3, 230–246. [Google Scholar] [CrossRef]
- Iglói, Z.; Abou-Nouar, Z.A.; Weller, B.; Matheeussen, V.; Coppens, J.; Koopmans, M.; Molenkamp, R. Comparison of commercial realtime reverse transcription PCR assays for the detection of SARS-CoV-2. J. Clin. Virol. 2020, 129, 104510. [Google Scholar] [CrossRef]
- Altamimi, A.M.; Obeid, D.A.; Alaifan, T.A.; Taha, M.T.; Alhothali, M.T.; Alzahrani, F.A.; Albarrag, A.M. Assessment of 12 qualitative RT-PCR commercial kits for the detection of SARS-CoV-2. J. Med. Virol. 2021, 93, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Jurek, T.; Rorat, M.; Szleszkowski, Ł.; Tokarski, M.; Pielka, I.; Małodobra-Mazur, M. SARS-CoV-2 viral RNA is detected in the bone marrow in post-mortem samples using RT-LAMP. Diagnostics 2022, 12, 515. [Google Scholar] [CrossRef] [PubMed]
- Seitz, T.; Lickefett, B.; Traugott, M.; Pawelka, E.; Karolyi, M.; Baumgartner, S.; Jansen-Skoupy, S.; Atamaniuk, J.; Fritsche-Polanz, R.; Asenbaum, J.; et al. Evaluation of five commercial SARS-CoV-2 antigen tests in a clinical setting. J. Gen. Intern. Med. 2022, 37, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Meza-Robles, C.; Barajas-Saucedo, C.E.; Tiburcio-Jimenez, D.; Mokay-Ramнrez, K.A.; Melnikov, V.; Rodriguez-Sanchez, I.P.; Martinez-Fierro, M.L.; Garza-Veloz, I.; Zaizar-Fregoso, S.A.; Guzman-Esquivel, J.; et al. One-step nested RT-PCR for COVID-19 detection: A flexible, locally developed test for SARS-CoV2 nucleic acid detection. J. Infect. Dev. Ctries. 2020, 14, 679–684. [Google Scholar] [CrossRef]
- Martin, J.; Klapsa, D.; Wilton, T.; Zambon, M.; Bentley, E.; Bujaki, E.; Fritzsche, M.; Mate, R.; Majumdar, M. Tracking SARS-CoV-2 in sewage: Evidence of changes in virus variant predominance during COVID-19 pandemic. Viruses 2020, 12, 1144. [Google Scholar] [CrossRef]
- Haramoto, E.; Malla, B.; Thakali, O.; Kitajima, M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020, 737, 140405. [Google Scholar] [CrossRef]
- La Rosa, G.; Iaconelli, M.; Mancini, P.; Ferraro, G.B.; Veneri, C.; Bonadonna, L.; Lucentini, L.; Suffredini, E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020, 736, 139652. [Google Scholar] [CrossRef] [PubMed]
- Thommes, L.; Burkert, F.R.; Öttl, K.W.; Goldin, D.; Loacker, L.; Lanser, L.; Griesmacher, A.; Theurl, I.; Weiss, G.; Bellmann-Weiler, R. Comparative evaluation of four SARS-CoV-2 antigen tests in hospitalized patients. Int. J. Infect. Dis. 2021, 105, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Chaimayo, C.; Kaewnaphan, B.; Tanlieng, N.; Athipanyasilp, N.; Sirijatuphat, R.; Chayakulkeeree, M.; Angkasekwinai, N.; Sutthent, R.; Puangpunngam, N.; Tharmviboonsri, T.; et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol. J. 2020, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.S.; Sumana, M.N.; Tejashree, A.; Chitharagi, V.B.; Mahale, R.P.; Rao, M.R.; Sowmya, G.S.; Gowda, R.S.; Deepashree, R.; Sujatha, S.R. Diagnostic Agreement of SARS-CoV-2 Lateral Flow Antigen Assay with the Cycle Threshold Values of RT-PCR. J. Pure Appl. Microbiol. 2023, 17, 1554. [Google Scholar] [CrossRef]
- van Kasteren, P.B.; van Der Veer, B.; van den Brink, S.; Wijsman, L.; de Jonge, J.; van den Brandt, A.; Molenkamp, R.; Reusken, C.B.; Meijer, A. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J. Clin. Virol. 2020, 128, 104412. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Ren, S.; Liu, X.; Zhang, L.; Li, W.; Yu, H. Comparison of the diagnostic efficacy between two PCR test kits for SARS-CoV-2 nucleic acid detection. J. Clin. Lab. Anal. 2020, 34, e23554. [Google Scholar] [CrossRef]
- Artik, Y.; Coşğun, A.B.; Cesur, N.P.; Hızel, N.; Uyar, Y.; Sur, H.; Ayan, A. Comparison of COVID-19 laboratory diagnosis by commercial kits: Effectivity of RT-PCR to the RT-LAMP. J. Med. Virol. 2022, 94, 1998–2007. [Google Scholar] [CrossRef]
- Wang, X.; Tan, L.; Wang, X.; Liu, W.; Lu, Y.; Cheng, L.; Sun, Z. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int. J. Infect. Dis. 2020, 94, 107–109. [Google Scholar] [CrossRef]
- Iwasaki, S.; Fujisawa, S.; Nakakubo, S.; Kamada, K.; Yamashita, Y.; Fukumoto, T.; Sato, K.; Oguri, S.; Taki, K.; Senjo, H.; et al. Comparison of SARS-CoV-2 detection in nasopharyngeal swab and saliva. J. Infect. 2020, 81, e145–e147. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bali, R.K.; Sawhney, H.; Gandhi, D.; Bali, N.K. Coronavirus Disease (COVID-19)—Epidemiology, Detection and Management with Respect to the Indian Subcontinent—Current Updates and Theories. J. Commun. Dis. 2020, 52, 52–62. [Google Scholar]
- Lin, C.; Xiang, J.; Yan, M.; Li, H.; Huang, S.; Shen, C. Comparison of throat swabs and sputum specimens for viral nucleic acid detection in 52 cases of novel coronavirus (SARS-Cov-2)-infected pneumonia (COVID-19). Clin. Chem. Lab. Med. 2020, 58, 1089–1094. [Google Scholar] [CrossRef]
- Clark, J.H.; Pang, S.; Naclerio, R.M.; Kashima, M. Complications of Nasal SARS-CoV-2 Testing: A Review. J. Investig. Med. 2021, 69, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, A.; Tolvi, M.; Jauhiainen, M.; Kekäläinen, E.; Laulajainen-Hongisto, A.; Lamminmäki, S. Complications of COVID-19 nasopharyngeal swab test. JAMA Otolaryngol.-Head Neck Surg. 2021, 147, 672–674. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Maltoni, S. Is naso-pharyngeal swab always safe for SARS-CoV-2 testing? An unusual, accidental foreign body swallowing. Clin. J. Gastroenterol. 2021, 14, 44–47. [Google Scholar] [CrossRef]
- Comaz, A.L.; Buri, P. Nasal mucosa as an absorption barrier. Eur. J. Phann. Biopharm. 1994, 40, 261–270. [Google Scholar]
- Winther, B. Effects on the nasal mucosa of upper respiratory viruses (common cold). Dan. Med. Bull. 1994, 41, 193–204. [Google Scholar]
- Mygind, N.; Pedersen, M.; Nielsen, M.H. Morphology of the upperairway epithelium. In The Nose. Upper Airway Physiology and the Atmospheric Environment; Proctor, D.F., AJdersen, I., Eds.; Elsevier Biomedical Press: Amsterdam, The Netherlands, 1982; pp. 71–97. [Google Scholar]
- Tsang, N.N.; So, H.C.; Ng, K.Y.; Cowling, B.J.; Leung, G.M.; Ip, D.K. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 1233–1245. [Google Scholar] [CrossRef]
- Lee, R.A.; Herigon, J.C.; Benedetti, A.; Pollock, N.R.; Denkinger, C.M. Performance of saliva, oropharyngeal swabs, and nasal swabs for SARS-CoV-2 molecular detection: A systematic review and meta-analysis. J. Clin. Microbiol. 2021, 59, 10–128. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Terada, J.; Kimura, M.; Moriya, A.; Motohashi, A.; Izumi, S.; Kawajiri, K.; Hakkaku, K.; Morishita, M.; Saito, S.; et al. Diagnostic accuracy of nasopharyngeal swab, nasal swab and saliva swab samples for the detection of SARS-CoV-2 using RT-PCR. Infect. Dis. 2021, 53, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://varna-airport.bg/bg/passenger-guide-covid-19-test-center (accessed on 10 March 2022).
- Available online: https://www.genica.bg/test/covid-19-pcr (accessed on 10 March 2022).
- Available online: https://alcotester.bg/produkti/ivd-tests/covid-tests/rapid_antigen_covid-19_test/ (accessed on 10 June 2022).
- Sirakov, I.; Popova-Ilinkina, R.; Ivanova, D.; Rusenova, N.; Mladenov, H.; Mihova, K.; Mitov, I. Development of Nested PCR for SARS-CoV-2 Detection and Its Application for Diagnosis of Active Infection in Cats. Vet. Sci. 2022, 9, 272. [Google Scholar] [CrossRef]
- Sirakov, I.; Rusenova, N.; Rusenov, A.; Gergova, R.; Strateva, T. Human ELISA Detects anti-SARS-CoV-2 Antibodies in Cats: Seroprevalence and Risk Factors for Virus Spread in Domestic and Stray Cats in Bulgaria. Vet. Sci. 2023, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Hinkov, A.; Tsvetkov, V.; Shkondrov, A.; Krasteva, I.; Shishkov, S.; Shishkova, K. Effect of a Total Extract and Saponins from Astragalus glycyphyllos L. on Human Coronavirus Replication In Vitro. Int. J. Mol. Sci 2023, 24, 16525. [Google Scholar] [CrossRef]
- Order No. PД-01-548 Dated 30.06.2021 for the Approval of a Sample of the EU Digital COVID Certificate for Testing for COVID-19. Available online: https://coronavirus.bg/bg/1043 (accessed on 10 July 2021).
- Sirakov, I.; Stankova, P.; Bakalov, D.; Mirani, Y.; Bardarska, L.; Paraskova, G.; Popov, I.; Alexandrova, A.; Dimitrov, G.; Mizgova, G.; et al. Retrospective analysis of the spread of SARS-CoV-2 in the Mediterranean part of Bulgaria, during the first wave of the pandemic. J. Pure Appl. Microbiol. 2024, in press.
- Kalvachev, N.; Sirakov, I. Comparison of different methods for viral RNA isolation and diagnosis of SARS-CoV-2 (COVID-19). Med. Rev. (Med. Pregl.) 2023, 59, 35–42. (In Bulgarian) [Google Scholar]
- Coste, A.T.; Egli, A.; Schrenzel, J.; Nickel, B.; Zbinden, A.; Lienhard, R.; Dumoulin, A.; Risch, M.; Greub, G. on behalf of Coordinated Clinical Commission of Microbiology (CCCM). IVDR: Analysis of the Social, Economic, and Practical Consequences of the Application of an Ordinance of the In Vitro Diagnostic Ordinance in Switzerland. Diagnostics 2023, 13, 2910. [Google Scholar] [CrossRef]
- Available online: https://www.consilium.europa.eu/en/press/press-releases/2021/05/20/eu-supports-start-of-who-process-for-establishment-of-pandemic-treaty-council-decision/ (accessed on 20 October 2021).
- Tapia-Sidas, D.A.; Vargas-Hernández, B.Y.; Ramírez-Pool, J.A.; Núñez-Muñoz, L.A.; Calderón-Pérez, B.; González-González, R.; Brieba, L.G.; Lira-Carmona, R.; Ferat-Osorio, E.; López-Macías, C.; et al. Starting from scratch: Step-by-step development of diagnostic tests for SARS-CoV-2 detection by RT-LAMP. PLoS ONE 2023, 18, e0279681. [Google Scholar] [CrossRef] [PubMed]
- Tavares, E.R.; de Lima, T.F.; Bartolomeu-Gonçalves, G.; de Castro, I.M.; de Lima, D.G.; Borges, P.H.; Nakazato, G.; Kobayashi, R.K.; Venancio, E.J.; Tarley, C.R.; et al. Development of a Melting-Curve-Based Multiplex Real-Time PCR Assay for the Simultaneous Detection of Viruses Causing Respiratory Infection. Microorganisms 2023, 11, 2692. [Google Scholar] [CrossRef] [PubMed]
- Burkardt, H.-J. Standardization and Quality Control of PCR Analyses. Clin. Chem. Lab. Med. 2000, 38, 87–91. [Google Scholar] [CrossRef]
- Bezier, C.; Anthoine, G.; Charki, A. Reliability of RT-PCR tests to detect SARS-CoV-2: Risk analysis. Int. J. Metrol. Qual. Eng. 2020, 11, 15. [Google Scholar] [CrossRef]
- Sirakov, I.N. Nucleic acid isolation and downstream applications. In Nucleic Acids—From Basic Aspects to Laboratory Tools, 1st ed.; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen Limited: London, UK, 2016; Volume 10, pp. 1–26. [Google Scholar] [CrossRef]
- Matzkies, L.M.; Leitner, E.; Stelzl, E.; Assig, K.; Bozic, M.; Siebenhofer, D.; Mustafa, M.E.; Steinmetz, I.; Kessler, H.H. Lack of sensitivity of an IVD/CE-labelled kit targeting the S gene for detection of SARS-CoV-2. Clin. Microbiol. Infect. 2020, 26, 1417-e1. [Google Scholar] [CrossRef]
- Callahan, C.; Lee, R.A.; Lee, G.R.; Zulauf, K.; Kirby, J.E.; Arnaout, R. Nasal swab performance by collection timing, procedure, and method of transport for patients with SARS-CoV-2. J. Clin. Microbiol. 2021, 59, 10–128. [Google Scholar] [CrossRef]
- Liu, L.; Ma, C.; Xu, Q. A rapid nested polymerase chain reaction method to detect circulating cancer cells in breast cancer patients using multiple marker genes. Oncol. Lett. 2014, 7, 2192–2198. [Google Scholar] [CrossRef]
- Higgins, T.S.; Wu, A.W.; Ting, J.Y. SARS-CoV-2 nasopharyngeal swab testing—False-negative results from a pervasive anatomical misconception. JAMA Otolaryngol.-Head Neck Surg. 2020, 146, 993–994. [Google Scholar] [CrossRef]
- Péré, H.; Podglajen, I.; Wack, M.; Flamarion, E.; Mirault, T.; Goudot, G.; Hauw-Berlemont, C.; Le, L.; Caudron, E.; Carrabin, S.; et al. Nasal swab sampling for SARS-CoV-2: A convenient alternative in times of nasopharyngeal swab shortage. J. Clin. Microbiol. 2020, 58, 10–128. [Google Scholar] [CrossRef]
- Konishi, K.; Yamaji, T.; Sakuma, C.; Kasai, F.; Endo, T.; Kohara, A.; Hanada, K.; Osada, N. Whole-Genome Sequencing of Vero E6 (VERO C1008) and Comparative Analysis of Four Vero Cell Sublines. Front. Genet. 2022, 13, 801382. [Google Scholar] [CrossRef] [PubMed]
- Gadenstaetter, A.J.; Mayer, C.D.; Landegger, L.D. Nasopharyngeal versus nasal swabs for detection of SARS-CoV-2: A systematic review. Rhinology 2021, 59, 410–421. [Google Scholar] [CrossRef] [PubMed]
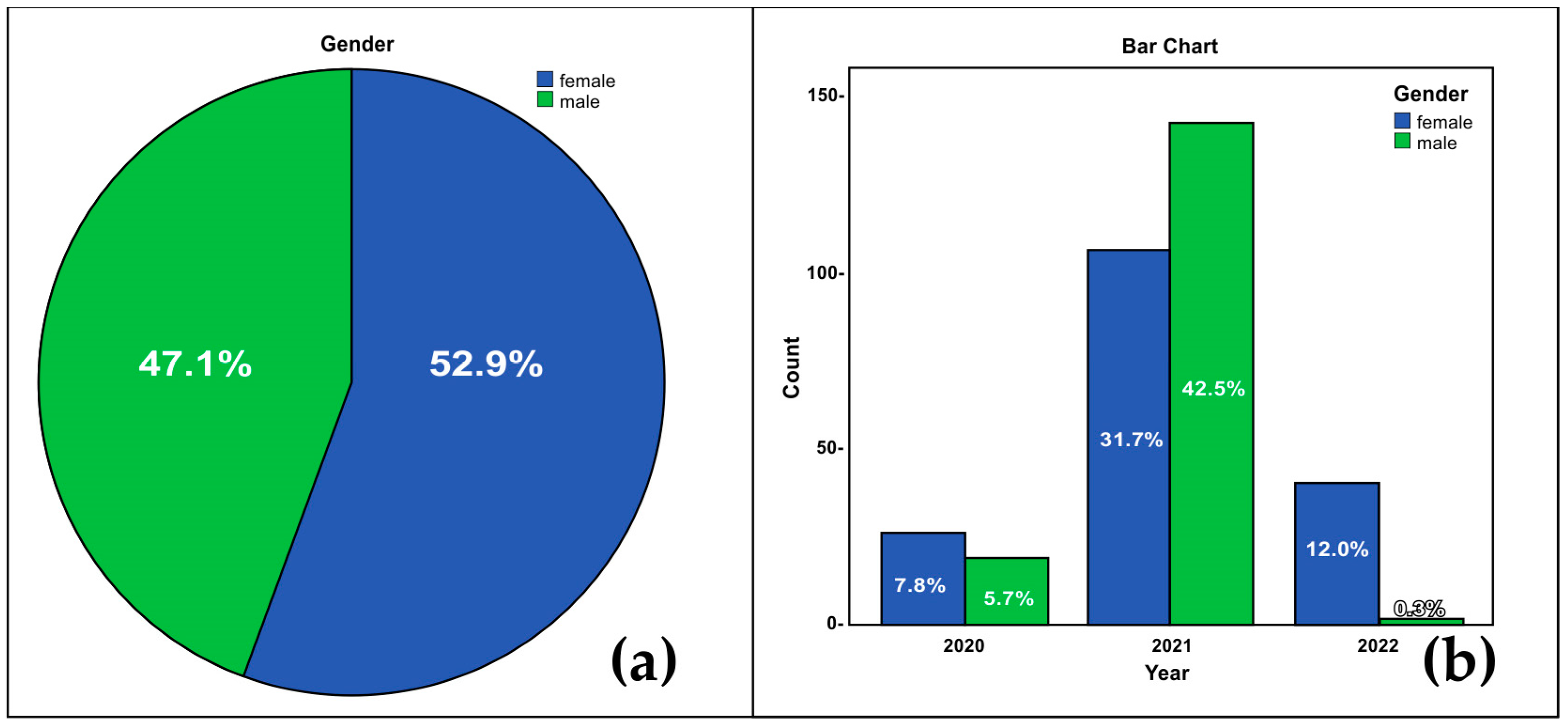

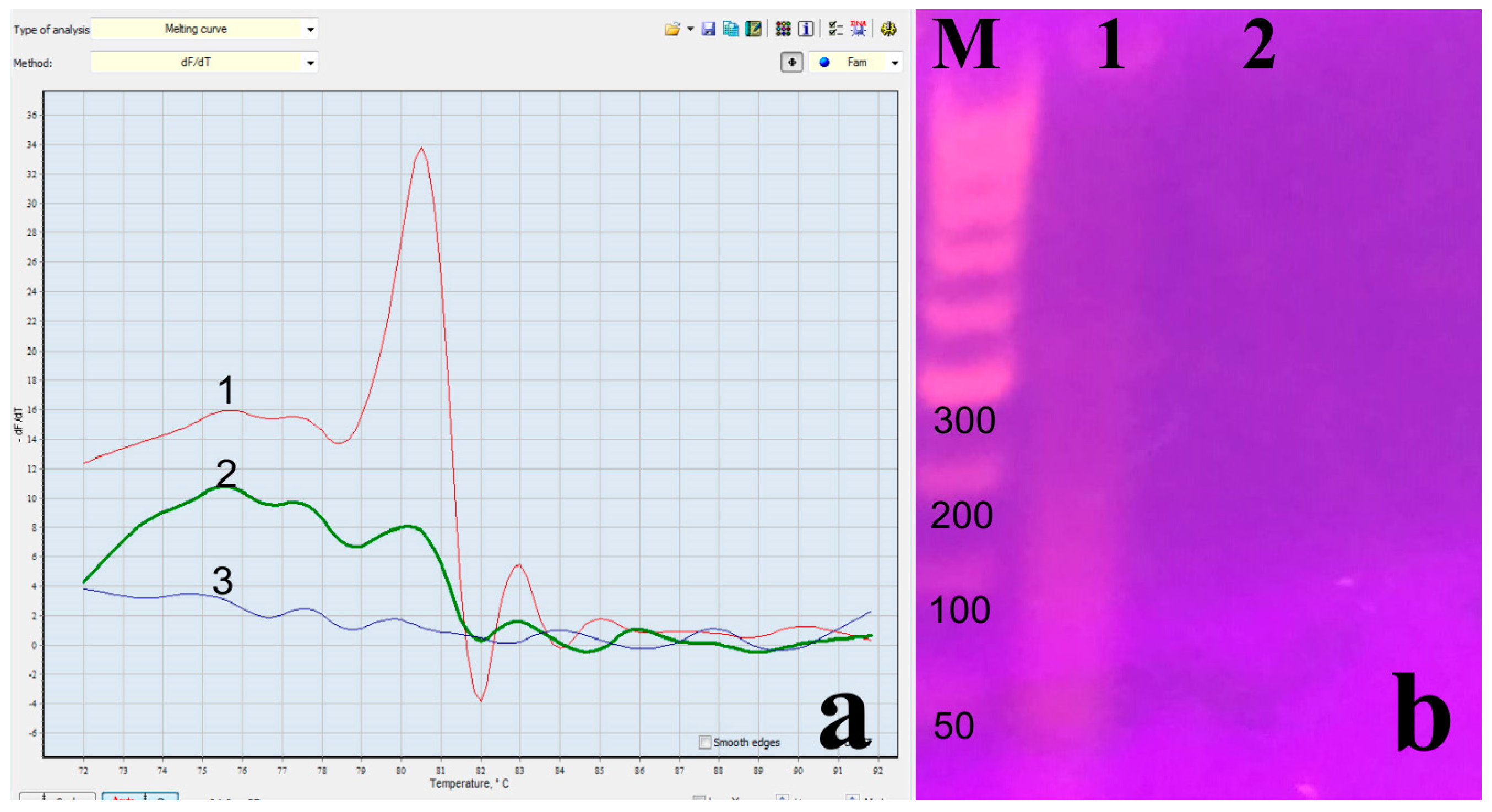
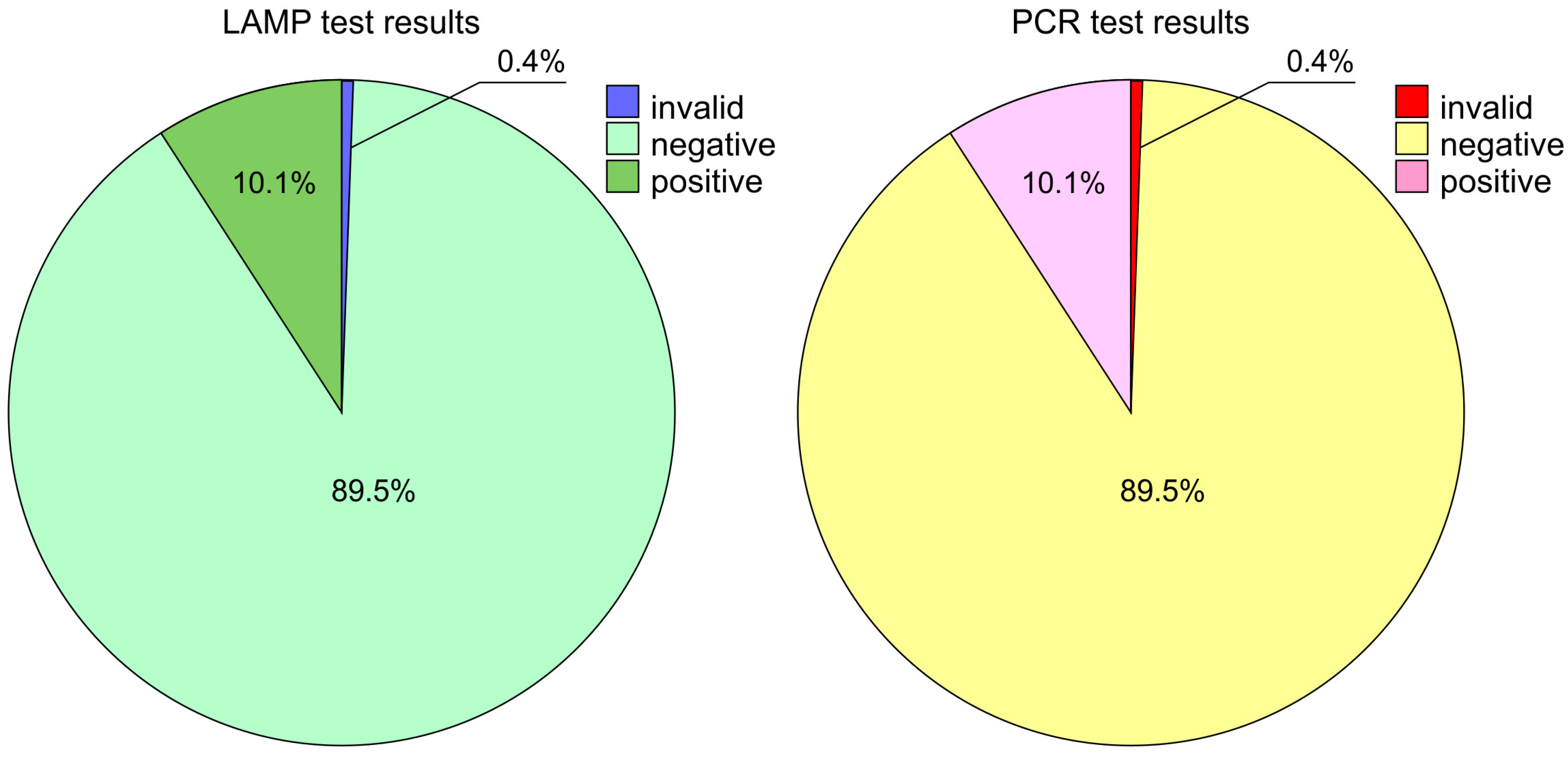

| Oropharyngeal/Nasopharyngeal Result nOne-Step RT PCR Test Result Cross-Tabulation | ||||||
|---|---|---|---|---|---|---|
| nOne-Step RT PCR Test Result | Total | |||||
| Invalid | Neg | Pos | ||||
| Oropharyngeal/ Nasopharyngeal results | 1-Oropharyngeal | Count | 0 | 198 | 18 | 216 |
| % within OP/NP result | 0.0% | 92.1% | 7.9% | 100.0% | ||
| % within PCR test result | 0.0% | 52.0% | 40.5% | 50.6% | ||
| % of total | 0.0% | 46.6% | 4.0% | 50.6% | ||
| 2-Nasopharyngeal | Count | 2 | 182 | 25 | 209 | |
| % within OP/NP result | 1.0% | 87.4% | 11.6% | 100.0% | ||
| % within PCR test result | 100.0% | 47.8% | 57.1% | 48.9% | ||
| % of total | 0.5% | 42.8% | 5.7% | 48.9% | ||
| 1 + 2 | Count | 0 | 1 | 1 | 2 | |
| % within OP/NP result | 0.0% | 50.0% | 50.0% | 100.0% | ||
| % within PCR test result | 0.0% | 0.3% | 2.4% | 0.5% | ||
| % of total | 0.0% | 0.2% | 0.2% | 0.5% | ||
| Total | Count | 2 | 381 | 44 | 427 | |
| % within OP/NP result | 0.5% | 89.6% | 10.1% | 100.0% | ||
| % within PCR test result | 100.0% | 100.0% | 100.0% | 100.0% | ||
| % of total | 0.5% | 89.6% | 10.1% | 100.0% | ||
| Need for Test | |||||
|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
| Valid | By own will | 27 | 6.3 | 79.4 | 79.4 |
| Clinical symptoms—fever | 1 | 0.2 | 2.9 | 82.4 | |
| contact with positive person | 1 | 0.2 | 2.9 | 85.3 | |
| traveling out of the country | 5 | 1.2 | 14.7 | 100 | |
| Total | 34 | 8 | 100 | ||
| Missing | N/A | 393 | 92 | ||
| Total | 427 | 100 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkova, K.; Sirakova, B.; Shishkov, S.; Stoilova, E.; Mladenov, H.; Sirakov, I. A Comparative Analysis of Molecular Biological Methods for the Detection of SARS-CoV-2 and Testing the In Vitro Infectivity of the Virus. Microorganisms 2024, 12, 180. https://doi.org/10.3390/microorganisms12010180
Shishkova K, Sirakova B, Shishkov S, Stoilova E, Mladenov H, Sirakov I. A Comparative Analysis of Molecular Biological Methods for the Detection of SARS-CoV-2 and Testing the In Vitro Infectivity of the Virus. Microorganisms. 2024; 12(1):180. https://doi.org/10.3390/microorganisms12010180
Chicago/Turabian StyleShishkova, Kalina, Bilyana Sirakova, Stoyan Shishkov, Eliya Stoilova, Hristiyan Mladenov, and Ivo Sirakov. 2024. "A Comparative Analysis of Molecular Biological Methods for the Detection of SARS-CoV-2 and Testing the In Vitro Infectivity of the Virus" Microorganisms 12, no. 1: 180. https://doi.org/10.3390/microorganisms12010180






