Metagenomic Insight into the Effect of Probiotics on Nitrogen Cycle in the Coilia nasus Aquaculture Pond Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design, Sampling, and Water Quality Determination
2.2. DNA Extraction and Metagenomic Sequencing
2.3. Reads-Based Phylogenetic Annotation
2.4. Metagenomic De Novo Assembly, Gene Prediction, Gene Abundance
2.5. Gene Function Annotation Based on Unique Gene
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Nitrogen Element Transformation in Different Forms
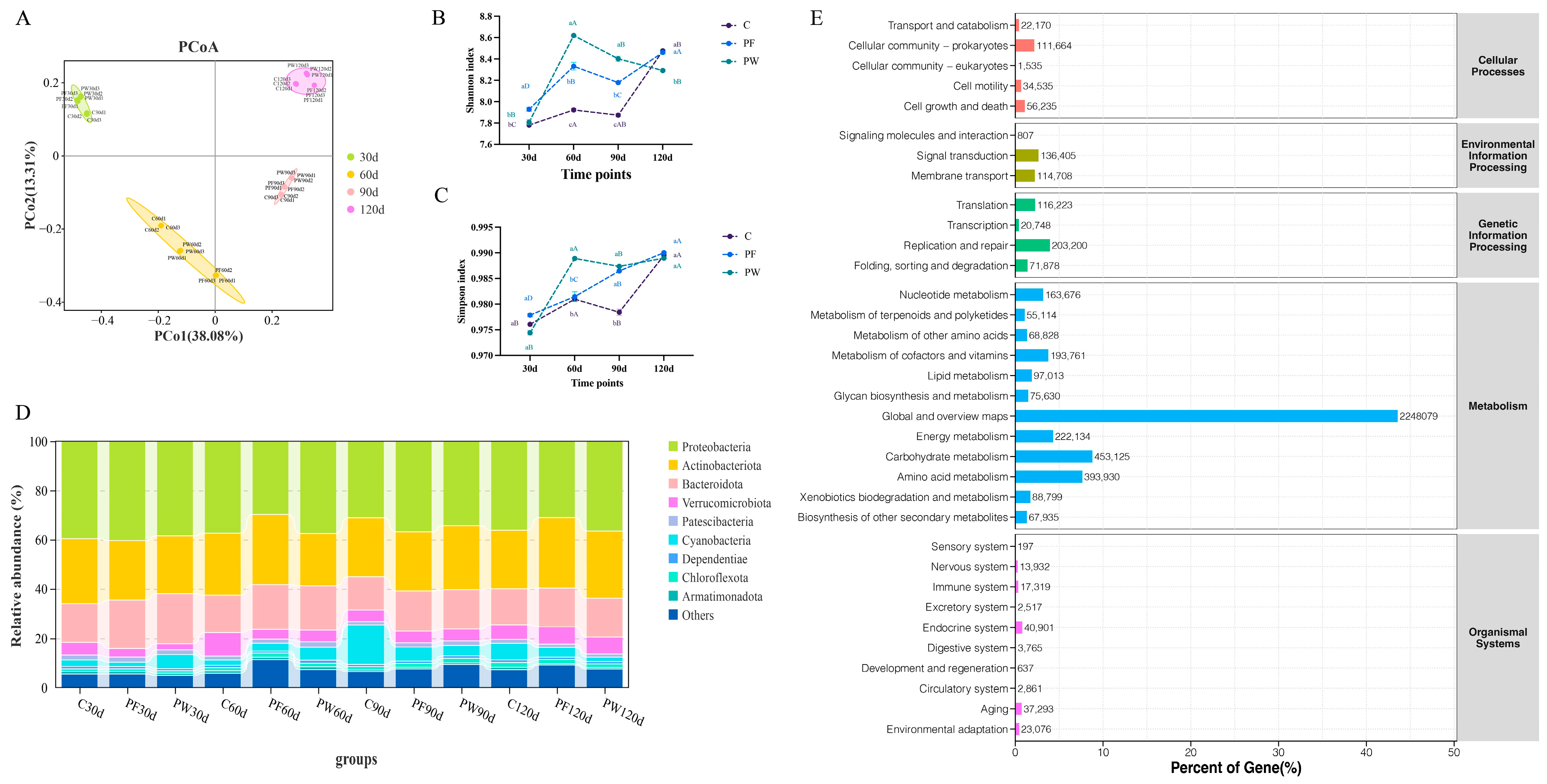
3.2. Characteristics of Microbial Diversity and Community Structure Differences
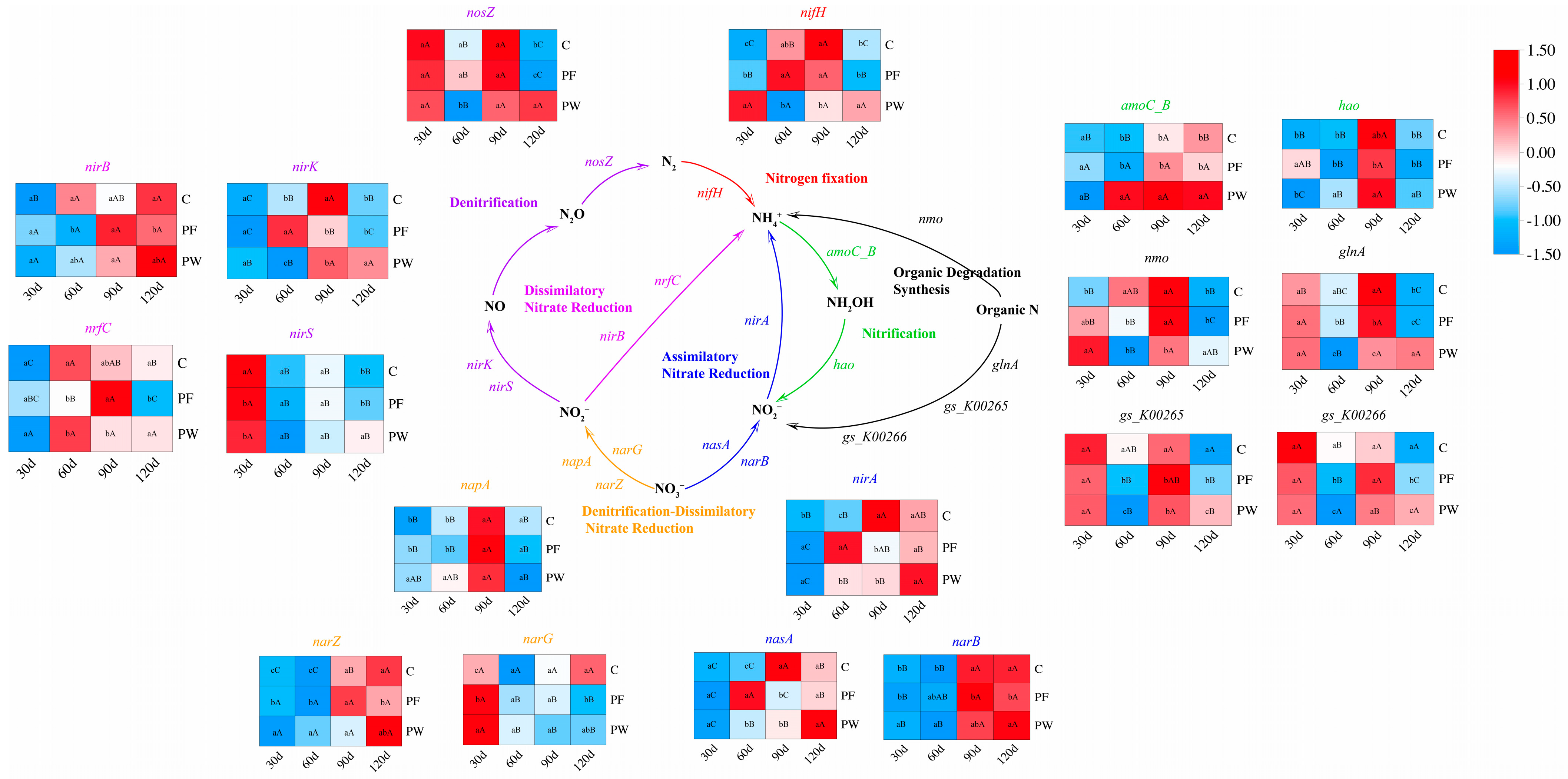
3.3. Nitrogen Cycling Pathways and Their Key Functional Genes
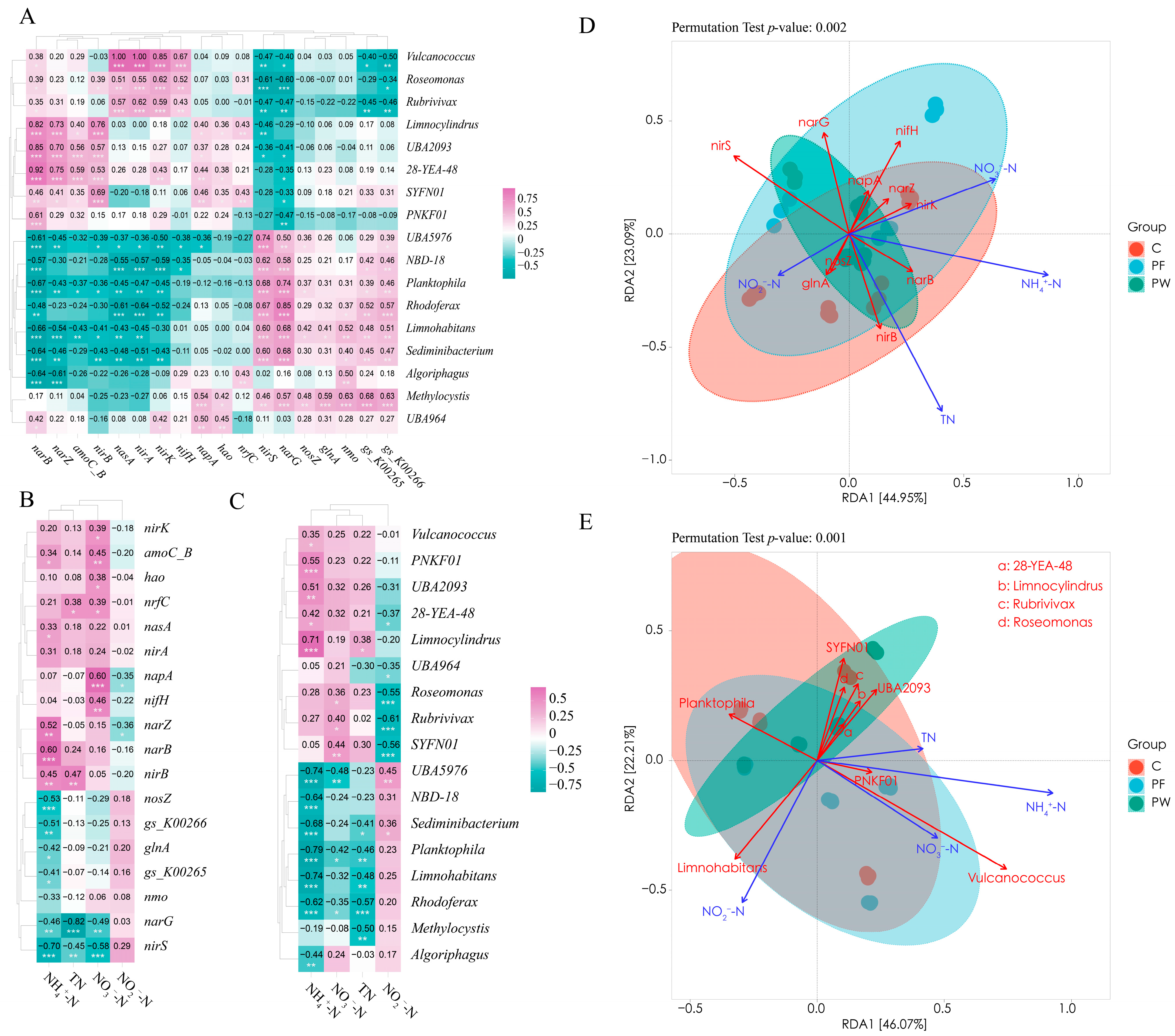
3.4. Correlation Analysis of Key Functional Genes, Microorganisms, and Water Quality in Nitrogen Cycling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, L.; Liu, C.; Peng, L.; Song, C.; Li, X.; Tao, L.; Li, G. Different Distribution Patterns of Microorganisms between Aquaculture Pond Sediment and Water. J. Microbiol. 2021, 59, 376–388. [Google Scholar] [CrossRef]
- Herbeck, L.S.; Krumme, U.; Nordhaus, I.; Jennerjahn, T.C. Pond Aquaculture Effluents Feed an Anthropogenic Nitrogen Loop in a SE Asian Estuary. Sci. Total Environ. 2021, 756, 144083. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, Z.; Ma, Z.; Chen, J.; Song, Z. Immobilization of Rhodopseudomonas palustris P1 on Glass Pumice to Improve the Removal of NH4+-N and NO2−-N from Aquaculture Pond Water. Biotechnol. Appl. Biochem. 2020, 67, 323–329. [Google Scholar] [CrossRef]
- Luo, S.; Wu, B.; Xiong, X.; Wang, J. Short-Term Toxicity of Ammonia, Nitrite, and Nitrate to Early Life Stages of the Rare Minnow (Gobiocypris rarus). Environ. Toxicol. Chem. 2016, 35, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, Y.J.; Kim, K.I.; Kim, S.K.; Kim, J.H. Toxic Effects of Nitrogenous Compounds (Ammonia, Nitrite, and Nitrate) on Acute Toxicity and Antioxidant Responses of Juvenile Olive Flounder, Paralichthys olivaceus. Environ. Toxicol. Pharmacol. 2019, 67, 73–78. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, M.J.; da Costa, F.F.B.; Leme, F.P.; Takata, R.; Costa, D.C.; Mattioli, C.C.; Luz, R.K.; Miranda-Filho, K.C. Biological Responses of Neotropical Freshwater Fish Lophiosilurus alexandri Exposed to Ammonia and Nitrite. Sci. Total Environ. 2018, 616–617, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, H.M.; Ferreira, T.O.; Taniguchi, C.A.K.; Barcellos, D.; do Nascimento, J.C.; Nóbrega, G.N.; Otero, X.L.; Artur, A.G. Nitrogen Mineralization and Eutrophication Risks in Mangroves Receiving Shrimp Farming Effluents. Environ. Sci. Pollut. Res. 2020, 27, 34941–34950. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, C.; Wang, C.; Zheng, J.; Hu, Y.; Li, D. Microbial Succession and Nitrogen Cycling in Cultured Biofilms as Affected by the Inorganic Nitrogen Availability. Microb. Ecol. 2017, 73, 1–15. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Wooller, M.J.; Pohlman, J.W.; Tiedje, J.M.; Leigh, M.B. Methane-Derived Carbon Flow through Microbial Communities in Arctic Lake Sediments. Environ. Microbiol. 2015, 17, 3233–3250. [Google Scholar] [CrossRef] [PubMed]
- Mosley, O.E.; Gios, E.; Close, M.; Weaver, L.; Daughney, C.; Handley, K.M. Nitrogen Cycling and Microbial Cooperation in the Terrestrial Subsurface. ISME J. 2022, 16, 2561–2573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ruan, X.; Li, R.; Zhang, Y. Microbial Interaction Patterns and Nitrogen Cycling Regularities in Lake Sediments under Different Trophic Conditions. Sci. Total Environ. 2024, 907, 167926. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, H.; Yang, Y.; Liu, F.; Li, M.; Huang, J.; Yu, Y.; Wang, C.; Jiang, F.; He, Z.; et al. Ecological Interactions and the Underlying Mechanism of Anammox and Denitrification across the Anammox Enrichment with Eutrophic Lake Sediments. Microbiome 2023, 11, 82. [Google Scholar] [CrossRef]
- Wen, X.; Zhou, Y.; Liang, X.; Li, J.; Huang, Y.; Li, Q. A Novel Carbon-Nitrogen Coupled Metabolic Pathway Promotes the Recyclability of Nitrogen in Composting Habitats. Bioresour. Technol. 2023, 381, 129134. [Google Scholar] [CrossRef]
- Niu, S.; Song, L.; Wang, J.; Luo, Y.; Yu, G. Dynamic Carbon-Nitrogen Coupling under Global Change. Sci. China Life Sci. 2023, 66, 771–782. [Google Scholar] [CrossRef]
- Ma, B.; Stirling, E.; Liu, Y.; Zhao, K.; Zhou, J.; Singh, B.K.; Tang, C.; Dahlgren, R.A.; Xu, J. Soil Biogeochemical Cycle Couplings Inferred from a Function-Taxon Network. Research 2023, 2021, 7102769. [Google Scholar] [CrossRef]
- Ansari, K.; Khandeshwar, S.; Waghmare, C.; Mehboob, H.; Gupta, T.; Shrikhande, A.N.; Abbas, M. Experimental Evaluation of Industrial Mushroom Waste Substrate Using Hybrid Mechanism of Vermicomposting and Effective Microorganisms. Materials 2022, 15, 2963. [Google Scholar] [CrossRef]
- Reszka, P.; Cygan-Szczegielniak, D.; Jankowiak, H.; Cebulska, A.; Mikołajczak, B.; Bogucka, J. Effects of Effective Microorganisms on Meat Quality, Microstructure of the Longissimus Lumborum Muscle, and Electrophoretic Protein Separation in Pigs Fed on Different Diets. Animals 2020, 10, 1755. [Google Scholar] [CrossRef]
- Yesuf, Y.K.; Lejamo, S.B.; Abduljebar, T.H. Effect of Effective Microorganisms (EM) Treated Taro (Colocasia esculenta) Root on the Growth Performance of Broiler Chickens. Anim. Biotechnol. 2023, 34, 593–601. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.-Y.; Zhang, J.; Liu, Y.-H.; Tang, D.-Y.; Zhao, J.; Dai, C.-C. By Reconstructing a Multifunctional Intensive Microbiome, Effective Microorganisms (EM) Improve the Ecological Environment of Rice–Crayfish Cocropping. Agric. Ecosyst. Environ. 2023, 357, 108698. [Google Scholar] [CrossRef]
- Du, G.; Shi, J.; Zhang, J.; Ma, Z.; Liu, X.; Yuan, C.; Zhang, B.; Zhang, Z.; Harrison, M.D. Exogenous Probiotics Improve Fermentation Quality, Microflora Phenotypes, and Trophic Modes of Fermented Vegetable Waste for Animal Feed. Microorganisms 2021, 9, 644. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Li, G.; Shen, J.; Tao, Z.; Tian, Y.; Chen, L.; Li, C.; Lu, L. Dynamic Comparison on the Usage of Probiotics in Organic Wastewater Treatment under Aerobic Conditions in a Diurnal Environment. J. Air Waste Manage Assoc. 2016, 66, 1183–1190. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Fu, B.; Mu, X. Improvement of Aquaculture Water Quality by Mixed Bacillus and Its Effects on Microbial Community Structure. Environ. Sci. Pollut. Res. 2022, 29, 69731–69742. [Google Scholar] [CrossRef]
- Gamoori, R.; Rashidian, G.; Ahangarzadeh, M.; Najafabadi, M.; Dashtebozorg, M.; Mohammadi, Y.; Morshedi, V. Improvement of Water Quality with Probiotics Inclusion during Simulated Transport of Yellowfin Seabream (Acanthopagrus latus) Larvae. J. Aquat. Anim. Health 2023, 35, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, Y.; Guo, Z.; Xu, G.; Xu, P. Transcriptomic Analysis Reveals Different Responses to Ammonia Stress and Subsequent Recovery between Coilia nasus Larvae and Juveniles. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 230, 108710. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mawolo, P.Y.; Gao, J.; Chu, L.; Wang, Y.; Nie, Z.; Song, L.; Shao, N.; Gao, J.; Xu, P.; et al. Effects of Supplemental Effective Microorganisms in Feed on the Growth, Immunity, and Appetite Regulation in Juvenile GIFT Tilapia. Aquac. Rep. 2021, 19, 100577. [Google Scholar] [CrossRef]
- Ridha, M.T.; Azad, I.S. Preliminary Evaluation of Growth Performance and Immune Response of Nile Tilapia Oreochromis niloticus Supplemented with Two Putative Probiotic Bacteria. Aquac. Res. 2012, 43, 843–852. [Google Scholar] [CrossRef]
- Suphoronski, S.A.; de Souza, F.P.; Chideroli, R.T.; Mantovani Favero, L.; Ferrari, N.A.; Ziemniczak, H.M.; Gonçalves, D.D.; Lopera Barrero, N.M.; Pereira, U.d.P. Effect of Enterococcus faecium as a Water and/or Feed Additive on the Gut Microbiota, Hematologic and Immunological Parameters, and Resistance against Francisellosis and Streptococcosis in Nile Tilapia (Oreochromis niloticus). Front. Microbiol. 2021, 12, 743957. [Google Scholar] [CrossRef]
- Gao, J.; Shen, L.; Nie, Z.; Zhu, H.; Cao, L.; Du, J.; Dai, F.; Xu, G. Microbial and Planktonic Community Characteristics of Eriocheir sinensis Culture Ponds Experiencing Harmful Algal Blooms. Fishes 2022, 7, 180. [Google Scholar] [CrossRef]
- Tu, Q.; Lin, L.; Cheng, L.; Deng, Y.; He, Z. NCycDB: A Curated Integrative Database for Fast and Accurate Metagenomic Profiling of Nitrogen Cycling Genes. Bioinformatics 2019, 35, 1040–1048. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lai, J.; Li, Z.; Wang, Y.; Xi, H.; Luo, X. Tritium and Carbon-14 Contamination Reshaping the Microbial Community Structure, Metabolic Network, and Element Cycle in the Seawater Environment. Environ. Sci. Technol. 2023, 57, 5305–5316. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jing, W.; Wang, T.; Hu, Z.; Lu, H. Functional Metabolomics Revealed the Dual-Activation of CAMP-AMP Axis Is a Novel Therapeutic Target of Pancreatic Cancer. Pharmacol. Res. 2023, 187, 106554. [Google Scholar] [CrossRef] [PubMed]
- Turcios, A.E.; Papenbrock, J. Sustainable Treatment of Aquaculture Effluents-What Can We Learn from the Past for the Future? Sustainability 2014, 6, 836–856. [Google Scholar] [CrossRef]
- Robles-Porchas, G.R.; Gollas-Galván, T.; Martínez-Porchas, M.; Martínez-Cordova, L.R.; Miranda-Baeza, A.; Vargas-Albores, F. The Nitrification Process for Nitrogen Removal in Biofloc System Aquaculture. Rev. Aquac. 2020, 12, 2228–2249. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Zheng, J.-A.; Huang, S.-C.; Hung, G.-Y.; Horng, J.-L. Ammonia Exposure Impairs Lateral-Line Hair Cells and Mechanotransduction in Zebrafish Embryos. Chemosphere 2020, 257, 127170. [Google Scholar] [CrossRef]
- Esam, F.; Khalafalla, M.M.; Gewaily, M.S.; Abdo, S.; Hassan, A.M.; Dawood, M.A.O. Acute Ammonia Exposure Combined with Heat Stress Impaired the Histological Features of Gills and Liver Tissues and the Expression Responses of Immune and Antioxidative Related Genes in Nile Tilapia. Ecotoxicol. Environ. Saf. 2022, 231, 113187. [Google Scholar] [CrossRef]
- Kajimura, M.; Takimoto, K.; Takimoto, A. Acute Toxicity of Ammonia and Nitrite to Siamese Fighting Fish (Betta splendens). BMC Zool. 2023, 8, 25. [Google Scholar] [CrossRef]
- Kellock, K.A.; Moore, A.P.; Bringolf, R.B. Chronic Nitrate Exposure Alters Reproductive Physiology in Fathead Minnows. Environ. Pollut. 2018, 232, 322–328. [Google Scholar] [CrossRef]
- Banerjee, G.; Ray, A.K. The Advancement of Probiotics Research and Its Application in Fish Farming Industries. Res. Vet. Sci. 2017, 115, 66–77. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y. A Review on the Application of Bacillus as Probiotics in Aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Hassan, M.A.; Fathallah, M.A.; Elzoghby, M.A.; Salem, M.G.; Helmy, M.S. Influence of Probiotics on Water Quality in Intensified Litopenaeus vannamei Ponds under Minimum-Water Exchange. AMB Express 2022, 12, 22. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, T.; Jiang, Y.; Zheng, C.; Yang, H.; Liu, Q. Long-Term Effects of Three Compound Probiotics on Water Quality, Growth Performances, Microbiota Distributions and Resistance to Aeromonas veronii in Crucian Carp Carassius auratus Gibelio. Fish Shellfish Immunol. 2022, 120, 233–241. [Google Scholar] [CrossRef]
- Nimrat, S.; Suksawat, S.; Boonthai, T.; Vuthiphandchai, V. Potential Bacillus Probiotics Enhance Bacterial Numbers, Water Quality and Growth during Early Development of White Shrimp (Litopenaeus vannamei). Vet. Microbiol. 2012, 159, 443–450. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, L.; Deng, B.; Liang, Q.; Zheng, J.; Sun, J.; Zhu, H.; Peng, L.; Wang, Y.; Wenying, S.; et al. Bacillus subtilis SC02 Supplementation Causes Alterations of the Microbial Diversity in Grass Carp Water. World J. Microbiol. Biotechnol. 2013, 29, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Koba, K.; Makabe, A.; Li, X.-D.; Yoh, M.; Liu, C.-Q. Ammonium First: Natural Mosses Prefer Atmospheric Ammonium but Vary Utilization of Dissolved Organic Nitrogen Depending on Habitat and Nitrogen Deposition. New Phytol. 2013, 199, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Orellana, L.H.; Ben Francis, T.; Ferraro, M.; Hehemann, J.-H.; Fuchs, B.M.; Amann, R.I. Verrucomicrobiota Are Specialist Consumers of Sulfated Methyl Pentoses during Diatom Blooms. ISME J. 2022, 16, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Herlemann, D.P.; Lundin, D.; Labrenz, M.; Jürgens, K.; Zheng, Z.; Aspeborg, H.; Andersson, A.F. Metagenomic De Novo Assembly of an Aquatic Representative of the Verrucomicrobial Class Spartobacteria. mBio 2013, 4, e00569-12. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The Evolution and Future of Earth’s Nitrogen Cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, Y.; Lan, S.; Hu, C. Metagenomic Insight into Patterns and Mechanism of Nitrogen Cycle during Biocrust Succession. Front. Microbiol. 2021, 12, 633428. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I. Nitric Oxide: Interaction with the Ammonia Monooxygenase and Regulation of Metabolic Activities in Ammonia Oxidizers. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2008; Volume 440, pp. 121–135. ISBN 0076-6879. [Google Scholar]
- Yu, Y.; Han, P.; Zhou, L.-J.; Li, Z.; Wagner, M.; Men, Y. Ammonia Monooxygenase-Mediated Cometabolic Biotransformation and Hydroxylamine-Mediated Abiotic Transformation of Micropollutants in an AOB/NOB Coculture. Environ. Sci. Technol. 2018, 52, 9196–9205. [Google Scholar] [CrossRef] [PubMed]
- Schatteman, A.; Wright, C.L.; Crombie, A.T.; Murrell, J.C.; Lehtovirta-Morley, L.E. Hydrazines as Substrates and Inhibitors of the Archaeal Ammonia Oxidation Pathway. Appl. Environ. Microbiol. 2022, 88, e02470-21. [Google Scholar] [CrossRef]
- Zeng, Y.; Kasalický, V.; Šimek, K.; Koblízek, M. Genome Sequences of Two Freshwater Betaproteobacterial Isolates, Limnohabitans Species Strains Rim28 and Rim47, Indicate Their Capabilities as Both Photoautotrophs and Ammonia Oxidizers. J. Bacteriol. 2012, 194, 6302–6303. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, P.; Li, P.; He, Y. Co-Degradation of Ofloxacin and Its Impact on Solid Phase Denitrification with Polycaprolactone as Carbon Source. Bioresour. Technol. 2022, 350, 126938. [Google Scholar] [CrossRef]
- Hu, E.; Hu, L.; Zheng, Y.; Wu, Y.; Wang, X.; Sun, C.; Su, Y. Bacterial Abundance and Community Structure in Response to Nutrients and Photodegraded Terrestrial Humic Acids in a Eutrophic Lake. Environ. Sci. Pollut. Res. 2022, 29, 8218–8231. [Google Scholar] [CrossRef]
- Lu, H.; Chandran, K.; Stensel, D. Microbial Ecology of Denitrification in Biological Wastewater Treatment. Water Res. 2014, 64, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Koebke, K.J.; Tebo, A.G.; Manickas, E.C.; Deb, A.; Penner-Hahn, J.E.; Pecoraro, V.L. Nitrite Reductase Activity within an Antiparallel De Novo Scaffold. JBIC J. Biol. Inorg. Chem. 2021, 26, 855–862. [Google Scholar] [CrossRef]
- Torres, M.J.; Simon, J.; Rowley, G.; Bedmar, E.J.; Richardson, D.J.; Gates, A.J.; Delgado, M.J. Chapter Seven—Nitrous Oxide Metabolism in Nitrate-Reducing Bacteria: Physiology and Regulatory Mechanisms. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 68, pp. 353–432. ISBN 0065-2911. [Google Scholar]
- Li, E.; Lu, S. Denitrification Processes and Microbial Communities in a Sequencing Batch Reactor Treating Nanofiltration (NF) Concentrate from Coking Wastewater. Water Sci. Technol. 2017, 76, 3289–3298. [Google Scholar] [CrossRef] [PubMed]
- Reyna, L.; Wunderlin, D.A.; Genti-Raimondi, S. Identification and Quantification of a Novel Nitrate-Reducing Community in Sediments of Suquía River Basin along a Nitrate Gradient. Environ. Pollut. 2010, 158, 1608–1614. [Google Scholar] [CrossRef]
- Feng, W.-W.; Liu, J.-F.; Gu, J.-D.; Mu, B.-Z. Nitrate-Reducing Community in Production Water of Three Oil Reservoirs and Their Responses to Different Carbon Sources Revealed by Nitrate-Reductase Encoding Gene (NapA). Int. Biodeterior. Biodegrad. 2011, 65, 1081–1086. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, K.; Li, Z.; Wang, G.; Li, H.; Xia, Y.; Tian, J.; Yu, E.; Gong, W.; Xie, J. Nitrification and Denitrification Processes in a Zero-Water Exchange Aquaculture System: Characteristics of the Microbial Community and Potential Rates. Front. Mar. Sci. 2023, 10, 1072911. [Google Scholar] [CrossRef]
- Deng, M.; Hou, J.; Song, K.; Chen, J.; Gou, J.; Li, D.; He, X. Community Metagenomic Assembly Reveals Microbes That Contribute to the Vertical Stratification of Nitrogen Cycling in an Aquaculture Pond. Aquaculture 2020, 520, 734911. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mang, Q.; Gao, J.; Li, Q.; Sun, Y.; Xu, G.; Xu, P. Metagenomic Insight into the Effect of Probiotics on Nitrogen Cycle in the Coilia nasus Aquaculture Pond Water. Microorganisms 2024, 12, 627. https://doi.org/10.3390/microorganisms12030627
Mang Q, Gao J, Li Q, Sun Y, Xu G, Xu P. Metagenomic Insight into the Effect of Probiotics on Nitrogen Cycle in the Coilia nasus Aquaculture Pond Water. Microorganisms. 2024; 12(3):627. https://doi.org/10.3390/microorganisms12030627
Chicago/Turabian StyleMang, Qi, Jun Gao, Quanjie Li, Yi Sun, Gangchun Xu, and Pao Xu. 2024. "Metagenomic Insight into the Effect of Probiotics on Nitrogen Cycle in the Coilia nasus Aquaculture Pond Water" Microorganisms 12, no. 3: 627. https://doi.org/10.3390/microorganisms12030627



