Rare Plasmid-Mediated AmpC Beta-Lactamase DHA-1 Located on Easy Mobilized IS26-Related Genetic Element Detected in Escherichia coli from Livestock and Food in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation of E. coli
2.2. Antimicrobial Susceptibility Testing
2.3. Molecular Analysis
2.4. Sequencing Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority. Scientific Opinion on the public health risks of bacterial strains producing extended-spectrum β-lactamases and/or AmpC β-lactamases in food and food-producing animals. EFSA J. 2011, 9, 2322. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Systematic Review of the Effectiveness of Infection Control Measures to Prevent the Transmission of Carbapenemase-Producing Enterobacteriaceae through Cross-Border Transfer of Patients; ECDC: Stockholm, Sweden, 2014. Available online: https://data.europa.eu/doi/10.2900/418192 (accessed on 30 January 2024).
- Livermore, D. β -Lactamases- the threat renews. Curr. Protein Pept. Sci. 2009, 10, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Peter-Getzlaff, S.; Polsfuss, S.; Poledica, M.; Hombach, M.; Giger, J.; Böttger, E.C.; Zbinden, R.; Bloemberg, G.V. Detection of AmpC beta-lactamase in Escherichia coli: Comparison of three phenotypic confirmation assays and genetic analysis. J. Clin. Microbiol. 2011, 49, 2924–2932. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A. AmpC beta-lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Jacoby, G.A. Updated Functional Classification of β-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Reisbig, M.D.; Hanson, N.D. The ACT-1 plasmid-encoded AmpC beta-lactamase is inducible: Detection in a complex beta-lactamase background. J. Antimicrob. Chemother. 2002, 49, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, N.; Jung, B.Y.; Cha, S.B.; Shin, M.K.; Kim, A.; Kang, M.S.; Lee, K.M.; Yoo, H.S. Antibiotic resistance patterns and detection of blaDHA-1 in Salmonella species isolates from chicken farms in South Korea. Appl. Environ. Microbiol. 2010, 76, 4760–4764. [Google Scholar] [CrossRef] [PubMed]
- Pai, H.; Kang, C.-I.; Byeon, J.-H.; Lee, K.-D.; Park, W.B.; Kim, H.-B.; Kim, E.-C.; Oh, M.; Choe, K.-W. Epidemiology and clinical features of bloodstream infections caused by AmpC-type-β-lactamase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 3720–3728. [Google Scholar] [CrossRef] [PubMed]
- Barnaud, G.; Arlet, G.; Verdet, C.; Gaillot, O.; Lagrange, P.H.; Philippon, A. Salmonella enteritidis: AmpC plasmid-mediated inducible β-Lactamase (DHA-1) with an ampR gene from Morganella morganii. Antimicrob. Agents Chemother. 1998, 42, 2352–2358. [Google Scholar] [CrossRef]
- Gaillot, O.; Clément, C.; Simonet, M.; Philippon, A. Novel transferable beta-lactam resistance with cephalosporinase characteristics in Salmonella enteritidis. J. Antimicrob. Chemother. 1997, 39, 85–87. [Google Scholar] [CrossRef]
- Rayamajhi, N.; Kang, S.; Lee, D.; Kang, M.; Lee, S.; Park, K.; Lee, H.; Yoo, H. Characterization of TEM-, SHV- and AmpC-type β-lactamases from cephalosporin-resistant Enterobacteriaceae isolated from swine. Int. J. Food Microbiol. 2008, 124, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, M.; Shin, J.H.; Lee, M.H.; Kang, S.H.; Park, A.J.; Yong, D.; Chong, Y. Prevalence of plasmid-mediated AmpC β -lactamases in Escherichia coli and Klebsiella pneumoniae in Korea. Microb. Drug Resist. 2006, 12, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.G.; Novais, Â.; Rodrigues, C.; Nascimento, R.; Freitas, F.; Machado, E.; Peixe, L. Dynamics of clonal and plasmid backgrounds of Enterobacteriaceae producing acquired AmpC in Portuguese clinical settings over time. Int. J. Antimicrob. Agents 2019, 53, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Kis, Z.; Tóth, Á.; Jánvári, L.; Damjanova, I. Countrywide dissemination of a DHA-1-type plasmid-mediated AmpC β-lactamase-producing Klebsiella pneumoniae ST11 international high-risk clone in Hungary, 2009–2013. J. Med. Microbiol. 2016, 65, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.; Machado, E.; Ribeiro, T.G.; Novais, Â.; Peixe, L. Long-term dissemination of acquired AmpC β-lactamases among Klebsiella spp. and Escherichia coli in Portuguese clinical settings. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 551–558. [Google Scholar] [CrossRef]
- Empel, J.; Hrabák, J.; Kozińska, A.; Bergerová, T.; Urbášková, P.; Kern-Zdanowicz, I.; Gniadkowski, M. DHA-1-producing Klebsiella pneumoniae in a teaching hospital in the Czech Republic. Microb. Drug Resist. 2010, 16, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Diestra, K.; Miró, E.; Martí, C.; Navarro, D.; Cuquet, J.; Coll, P.; Navarro, F. Multiclonal epidemic of Klebsiella pneumoniae isolates producing DHA-1 in a Spanish hospital. Clin. Microbiol. Infect. 2011, 17, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Estaleva, C.E.L.; Zimba, T.F.; Sekyere, J.O.; Govinden, U.; Chenia, H.Y.; Simonsen, G.S.; Haldorsen, B.; Essack, S.Y.; Sundsfjord, A. High prevalence of multidrug resistant ESBL- and plasmid mediated AmpC-producing clinical isolates of Escherichia coli at Maputo Central Hospital, Mozambique. BMC Infect. Dis. 2021, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Liebana, E.; Batchelor, M.; Clifton-Hadley, F.A.; Davies, R.H.; Hopkins, K.L.; Threlfall, E.J. First report of Salmonella isolates with the DHA-1 AmpC beta-lactamase in the United Kingdom. Antimicrob. Agents Chemother. 2004, 48, 4492. [Google Scholar] [CrossRef]
- Moland, E.S.; Black, J.A.; Ourada, J.; Reisbig, M.D.; Hanson, N.D.; Thomson, K.S. Occurrence of newer β-Lactamases in Klebsiella pneumoniae isolates from 24 U.S. hospitals. Antimicrob. Agents Chemother. 2002, 46, 3837–3842. [Google Scholar] [CrossRef]
- Alvarez, M.; Tran, J.H.; Chow, N.; Jacoby, G.A. Epidemiology of conjugative plasmid-mediated AmpC β-Lactamases in the United States. Antimicrob. Agents Chemother. 2004, 48, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Lynne, A.M.; Kaldhone, P.; David, D.; White, D.G.; Foley, S.L. Characterization of antimicrobial resistance in Salmonella enterica serotype Heidelberg isolated from food animals. Foodborne Pathog. Dis. 2009, 6, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.M.; Elkenany, R.M.; Zakaria, A.I.; Badawy, B.M. Epidemiological study on Listeria monocytogenes in Egyptian dairy cattle farms’ insights into genetic diversity of multi-antibiotic-resistant strains by ERIC-PCR. Environ. Sci. Pollut. Res. 2022, 29, 54359–54377. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-S.; Pan, Y.; Agustie, H.; Loh, H.-S.; Rayamajhi, N.; Fang, C.-M. Characterisation of ESBL/AmpC-Producing Enterobacteriaceae isolated from poultry farms in Peninsular Malaysia. Lett. Appl. Microbiol. 2023, 76, ovac044. [Google Scholar] [CrossRef]
- Faife, S.L.; Zimba, T.; Sekyere, J.O.; Govinden, U.; Chenia, H.Y.; Simonsen, G.S.; Sundsfjord, A.; Essack, S.Y. β-lactam and fluoroquinolone resistance in Enterobacteriaceae from imported and locally-produced chicken in Mozambique. J. Infect. Dev. Ctries. 2020, 14, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Chinnam, B.K.; Nelapati, S.; Tumati, S.R.; Bobbadi, S.; Chaitanya Peddada, V.; Bodempudi, B. Detection of β-lactamase–producing Proteus mirabilis strains of animal origin in Andhra Pradesh, India and their genetic diversity. J. Food Prot. 2021, 84, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Rezaloo, M.; Motalebi, A.; Mashak, Z.; Anvar, A. Prevalence, antimicrobial resistance, and molecular description of Pseudomonas aeruginosa isolated from meat and meat products. J. Food Qual. 2022, 2022, 9899338. [Google Scholar] [CrossRef]
- Poursina, S.; Ahmadi, M.; Fazeli, F.; Ariaii, P. Assessment of virulence factors and antimicrobial resistance among the Pseudomonas aeruginosa strains isolated from animal meat and carcass samples. Vet. Med. Sci. 2023, 9, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, S.M.H.; Anvar, S.A.; Rahimi, E.; Ahari, H.; Ataee, M. Prevalence, phenotypic and genotypic diversity, antibiotic resistance, and frequency of virulence genes in Pseudomonas aeruginosa isolated from shrimps. Aquacult Int. 2022, 30, 131–156. [Google Scholar] [CrossRef]
- Dehkordi, S.M.H.; Anvar, S.A.; Rahimi, E.; Ahari, H.; Ataee, M. Molecular investigation of prevalence, phenotypic and genotypic diversity, antibiotic resistance, frequency of virulence genes and genome sequencing in Pseudomonas aeruginosa strains isolated from lobster. Int. J. Food Microbiol. 2022, 382, 109901. [Google Scholar] [CrossRef]
- Matloko, K.; Fri, J.; Ateba, T.P.; Molale-Tom, L.G.; Ateba, C.N. Evidence of potentially unrelated AmpC beta-lactamase producing Enterobacteriaceae from cattle, cattle products and hospital environments commonly harboring the blaACC resistance determinant. PLoS ONE 2021, 16, e0253647. [Google Scholar] [CrossRef] [PubMed]
- Yaici, L.; Haenni, M.; Métayer, V.; Saras, E.; Mesbah Zekar, F.; Ayad, M.; Touati, A.; Madec, J.-Y. Spread of ESBL/AmpC-producing Escherichia coli and Klebsiella pneumoniae in the community through ready-to-eat sandwiches in Algeria. Int. J. Food Microbiol. 2017, 245, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.T.; Alter, T.; Roesler, U.; Roschanski, N.; Huehn, S. Investigation of extended-spectrum and AmpC β-lactamase–producing Enterobacteriaceae from retail seafood in Berlin, Germany. J. Food Prot. 2018, 81, 1079–1086. [Google Scholar] [CrossRef]
- 2013/652/EU: Commission Implementing Decision of 12 November 2013 on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria. Available online: http://data.europa.eu/eli/dec_impl/2013/652/o (accessed on 29 January 2024).
- 2020/1729/EU: Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria and Repealing Implementing Decision 2013/652/EU. Available online: http://data.europa.eu/eli/dec_impl/2020/1729/o (accessed on 29 January 2024).
- Roschanski, N.; Fischer, J.; Guerra, B.; Roesler, U. Development of a multiplex Real-Time PCR for the rapid detection of the predominant beta-lactamase genes CTX-M, SHV, TEM and CIT-Type AmpCs in Enterobacteriaceae. PLoS ONE 2014, 9, e100956. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, F.J.; Hanson, N.D. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 2002, 40, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Barownick, W.; Helmuth, R.; Mendoza, M.C.; Rodicio, M.R.; Schroeter, A.; Guerra, B. Extended-spectrum ß-lactamases and AmpC ß-lactamases in ceftiofur-resistant Salmonella enterica isolates from food and livestock obtained in Germany during 2003–2007. J. Antimicrob. Chemother. 2009, 64, 301–309. [Google Scholar] [CrossRef]
- Hammerl, J.A.; Klein, I.; Lanka, E.; Appel, B.; Hertwig, S. Genetic and functional properties of the self-transmissible Yersinia enterocolitica plasmid pYE854, which mobilizes the virulence plasmid pYV. J. Bacteriol. 2008, 190, 991–1010. [Google Scholar] [CrossRef]
- Deneke, C.; Brendebach, H.; Uelze, L.; Borowiak, M.; Malorny, B.; Tausch, S.H. Species-specific quality control, assembly and contamination detection in microbial isolate sequences with AQUAMIS. Genes 2021, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, M.B.; Overballe-Petersen, S.; Lund, O.; Hasman, H.; Clausen, P.T.L.C. MINTyper: An outbreak-detection method for accurate and rapid SNP typing of clonal clusters with noisy long reads. Biol. Methods Protoc. 2021, 6, bpab008. [Google Scholar] [CrossRef]
- Zhou, Z.; Alikhan, N.-F.; Mohamed, K.; Fan, Y.; Achtman, M. The EnteroBase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Res. 2020, 30, 138–152. [Google Scholar] [CrossRef]
- Clausen, P.T.L.C.; Aarestrup, F.M.; Lund, O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform. 2018, 19, 307. [Google Scholar] [CrossRef] [PubMed]
- Deneke, C.; Uelze, L.; Brendebach, H.; Tausch, S.H.; Malorny, B. Decentralized investigation of bacterial outbreaks based on hashed cgMLST. Front. Microbiol. 2021, 12, 649517. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021. EFSA J. 2023, 21, e07867. [Google Scholar] [CrossRef]
- Mattioni Marchetti, V.; Bitar, I.; Mercato, A.; Nucleo, E.; Marchesini, F.; Mancinelli, M.; Prati, P.; Scarsi, G.S.; Hrabak, J.; Pagani, L.; et al. Deadly puppy infection caused by an MDR Escherichia coli O39 blaCTX–M–15, blaCMY–2, blaDHA–1, and aac(6)-Ib-cr positive in a breeding kennel in Central Italy. Front. Microbiol. 2020, 11, e00584. [Google Scholar] [CrossRef] [PubMed]
- Clément, M.; Keller, P.M.; Bernasconi, O.J.; Stirnimann, G.; Frey, P.M.; Bloemberg, G.V.; Sendi, P.; Endimiani, A. First clinical case of in vivo acquisition of DHA-1 plasmid-mediated AmpC in a Salmonella enterica subsp. enterica isolate. Antimicrob. Agents Chemother. 2019, 63, e00992-19. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, K.; Borowiak, M.; Tausch, S.H.; Malorny, B.; Käsbohrer, A.; Otani, S.; Schwarz, S.; Meemken, D.; Deneke, C.; Hammerl, J.A. Outcome of different sequencing and assembly approaches on the detection of plasmids and localization of antimicrobial resistance genes in commensal Escherichia coli. Microorganisms 2021, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Ravet, V.; Robin, F. Plasmids carrying DHA-1 β-lactamases. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Mahrouki, S.; Chihi, H.; Bourouis, A.; Ayari, K.; Ferjani, M.; Moussa, M.B.; Belhadj, O. Nosocomial dissemination of plasmids carrying blaTEM-24, blaDHA-1, aac(6’)-Ib-cr, and qnrA6 in Providencia spp. strains isolated from a Tunisian hospital. Diagn. Microbiol. Infect. Dis. 2015, 81, 50–52. [Google Scholar] [CrossRef]
- Harmer, C.J.; Hall, R.M. IS 26 -mediated formation of transposons carrying antibiotic resistance genes. mSphere 2016, 1, e00038-16. [Google Scholar] [CrossRef]
- Harmer, C.J.; Moran, R.A.; Hall, R.M. Movement of IS 26-associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. mBio 2014, 5, e01801-14. [Google Scholar] [CrossRef]
- Harmer, C.J.; Hall, R.M. IS26-mediated precise excision of the IS26-aphA1a translocatable unit. mBio 2015, 6, e01866-15. [Google Scholar] [CrossRef]
- Cain, A.K.; Liu, X.; Djordjevic, S.P.; Hall, R.M. Transposons related to Tn 1696 in IncHI2 plasmids in multiply antibiotic resistant Salmonella enterica serovar Typhimurium from Australian animals. Microb. Drug Resist. 2010, 16, 197–202. [Google Scholar] [CrossRef]
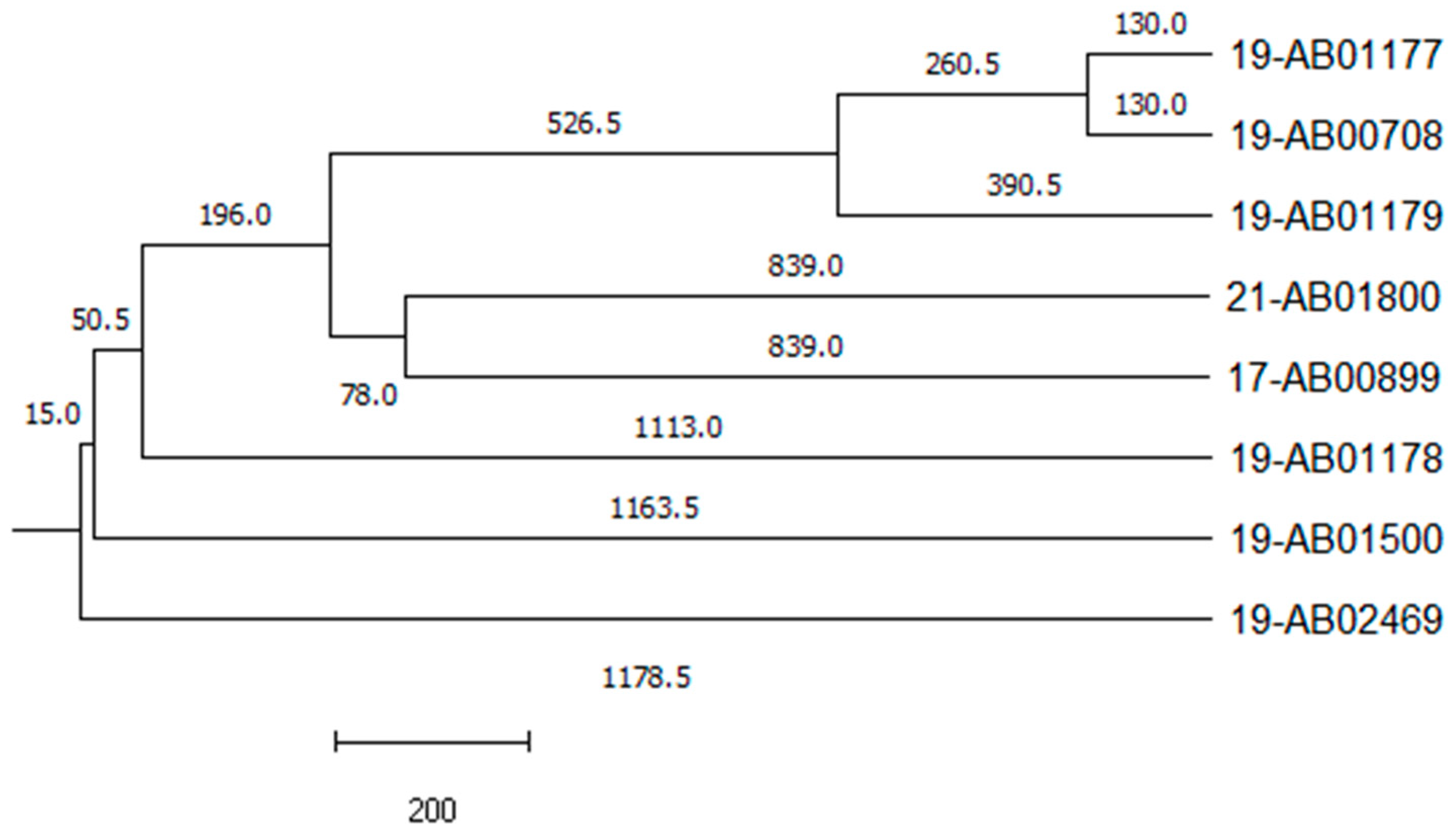
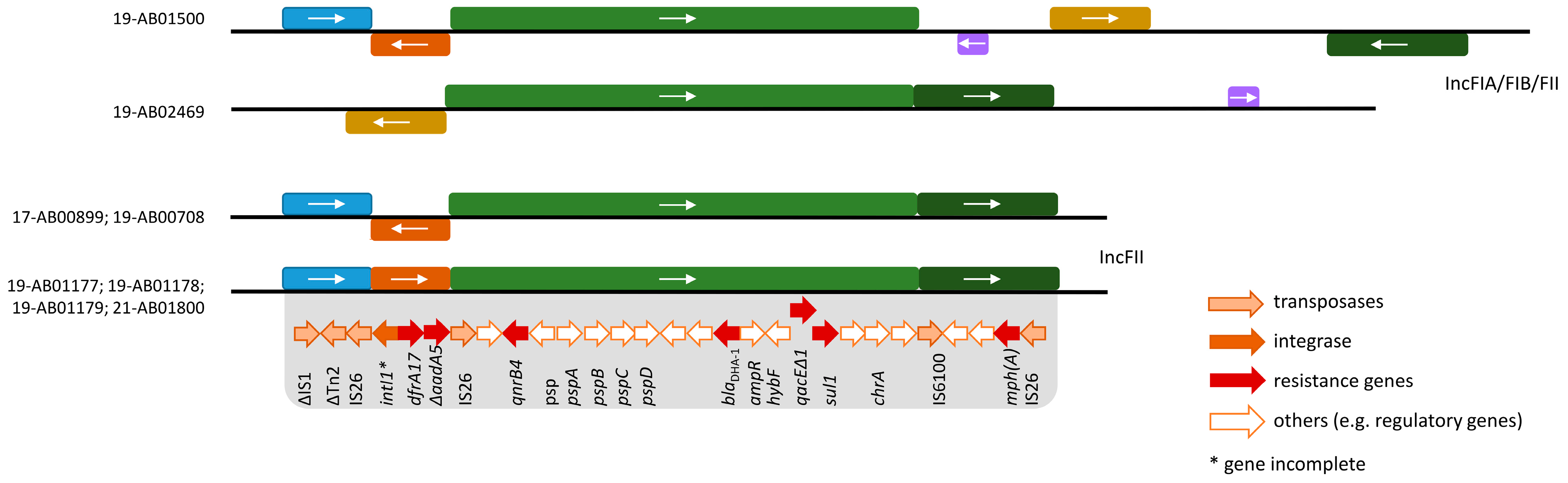
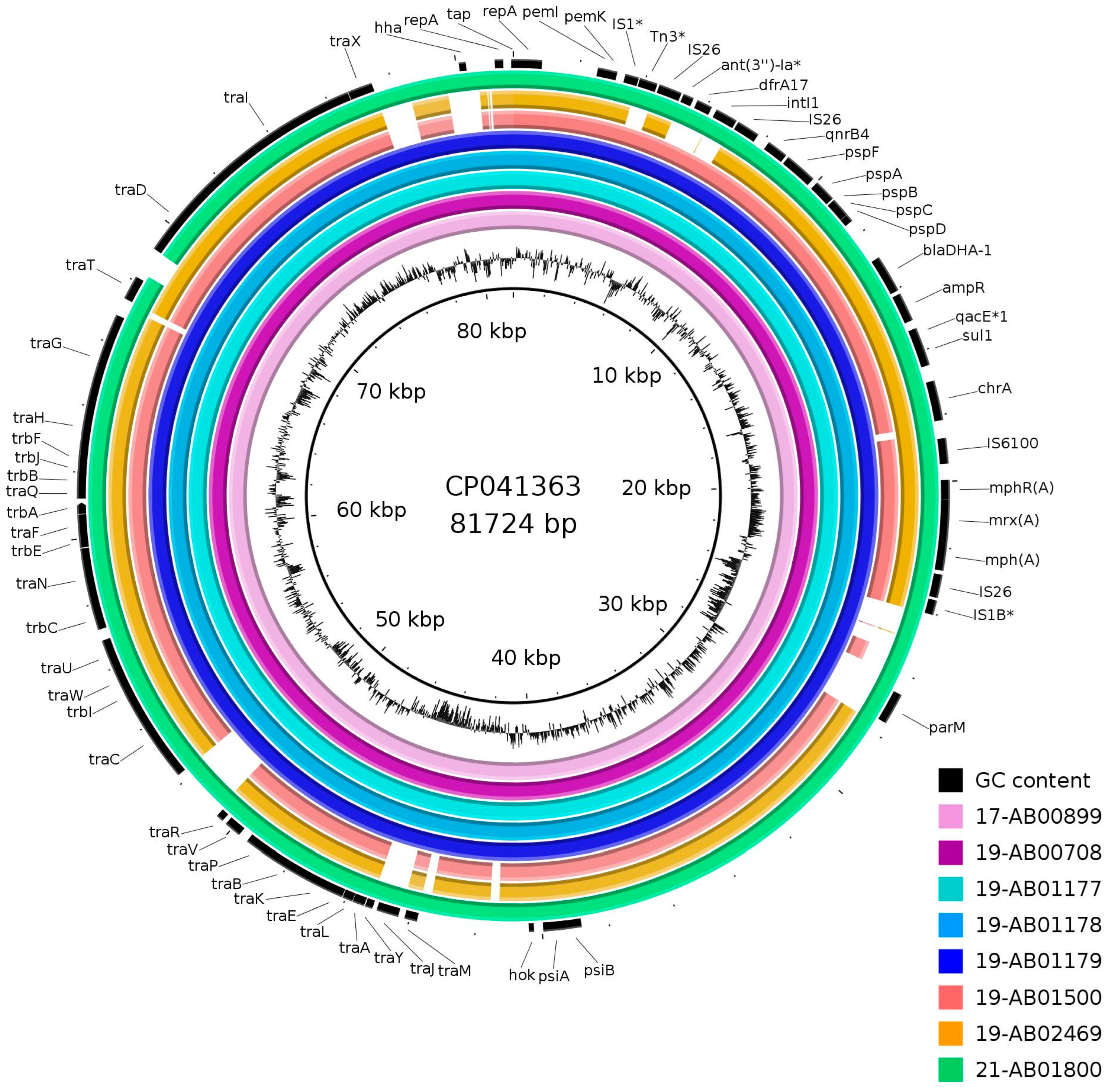
| Isolate ID | Matrix | Resistance Profile 1 [Beta-Lactam Phenotype 2] | Phylogenetic Group | MLST | Resistance Genes 3 |
|---|---|---|---|---|---|
| 17-AB00899 | Calves, feces | AMP, AZI, CIP, FOT, NAL, TAZ, TET, TMP [AmpC] | B1 | 446 | blaDHA-1; dfrA17; mph(A); qnrB4; sul1; tet(A); tet(B) |
| 19-AB00708 | Fattening pig, feces | AMP, AZI, CIP, FOT, NAL, TAZ, TET, TMP [AmpC] | C | 23 | blaDHA-1; dfrA17; mph(A); qnrB4; sul1; tet(A) |
| 19-AB01177 | Fattening pig, feces | AMP, AZI, CIP, FOT, NAL, SMX, TAZ, TET, TMP [AmpC] | C | 369 | blaDHA-1; blaTEM-1; dfrA1; dfrA17; mph(A); qnrB4; sul1; sul2; tet(A) |
| 19-AB01178 | Fattening pig, feces | AMP, AZI, CIP, FOT, NAL, SMX, TAZ, TET, TMP [AmpC] | E | 118 | aadA1; aph(3’)-Ia; blaDHA-1; blaTEM-34; dfrA1; dfrA17; emrD; mph(A); qnrB4; sat2; sul1; sul2; tet(B) |
| 19-AB01179 | Fattening pig, feces | AMP, AZI, CIP, FOT, NAL, SMX, TAZ, TET, TMP [AmpC] | C | 5271 | aadA1; aph(3’’)-Ib; aph(6)-Id; blaDHA-1, blaTEM-1; dfrA1; dfrA17; mph(A); qnrB4; sul1; sul2; tet(A) |
| 19-AB01500 | Freshwater fish | AMP, AZI, CIP, FOT, NAL, SMX, TAZ, TET, TMP [ESBL/AmpC] | D | 69 | aph(3’’)-Ib; aph(6)-Id; blaCTX-M-27; blaDHA-1; blaTEM-1; cyaA_S352T; dfrA14; dfrA17; erm(B) *; gyrA_S83L; mdtM; mph(A); parE_D476N; qnrB4; sul1; sul2; tet(B) * |
| 19-AB02469 | Wild goose, feces | AMP, AZI, CIP, FOT, NAL, SMX, TAZ, TET [ESBL/AmpC] | B2 | 131 | aph(3’’)-Ib; aph(6)-Id; blaCTX-M-15; blaDHA-1; gyrA_D87N; gyrA_S83L; mph(A); parC_E84V; parC_S80I; parE_I529L; ptsI_V25I; qnrB4; sul1; sul2, tet(A); uhpT_E350Q |
| 21-AB01800 | Freshwater fish | AMP, AZI, CIP, FOT, SMX, TAZ, TMP [AmpC] | B1 | 3570 | blaDHA-1; blaTEM-1; dfrA17; mph(A); qnrB4; sul1 |
| Isolate | Plasmid Size (in kb) | Incompatibility Group | Conjugative | |
|---|---|---|---|---|
| PFGE, Transformed Plasmid | WGS 1 | |||
| 17-AB00899 | 92/74 | 83.5 | FII | yes |
| 19-AB00708 | 210/193 | 82 | FII | yes |
| 19-AB01177 | 87 | 77 | FII | yes |
| 19-AB01178 | 75 | 82 | FII | yes |
| 19-AB01179 | 141/178/88/148 | 78.5 | FII | yes |
| 19-AB01500 | 141 | 149 | FII, FIA, FIB | yes |
| 19-AB02469 | 152 | 160 | FII, FIA, FIB | yes |
| 21-AB01800 | 58/110 | 63.5 | FII | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfreda, C.; Kaesbohrer, A.; Schmoger, S.; Skladnikiewicz-Ziemer, T.; Grobbel, M.; Irrgang, A. Rare Plasmid-Mediated AmpC Beta-Lactamase DHA-1 Located on Easy Mobilized IS26-Related Genetic Element Detected in Escherichia coli from Livestock and Food in Germany. Microorganisms 2024, 12, 632. https://doi.org/10.3390/microorganisms12030632
Manfreda C, Kaesbohrer A, Schmoger S, Skladnikiewicz-Ziemer T, Grobbel M, Irrgang A. Rare Plasmid-Mediated AmpC Beta-Lactamase DHA-1 Located on Easy Mobilized IS26-Related Genetic Element Detected in Escherichia coli from Livestock and Food in Germany. Microorganisms. 2024; 12(3):632. https://doi.org/10.3390/microorganisms12030632
Chicago/Turabian StyleManfreda, Chiara, Annemarie Kaesbohrer, Silvia Schmoger, Tanja Skladnikiewicz-Ziemer, Mirjam Grobbel, and Alexandra Irrgang. 2024. "Rare Plasmid-Mediated AmpC Beta-Lactamase DHA-1 Located on Easy Mobilized IS26-Related Genetic Element Detected in Escherichia coli from Livestock and Food in Germany" Microorganisms 12, no. 3: 632. https://doi.org/10.3390/microorganisms12030632






