Elimination of Pathogen Biofilms via Postbiotics from Lactic Acid Bacteria: A Promising Method in Food and Biomedicine
Abstract
:1. Introduction
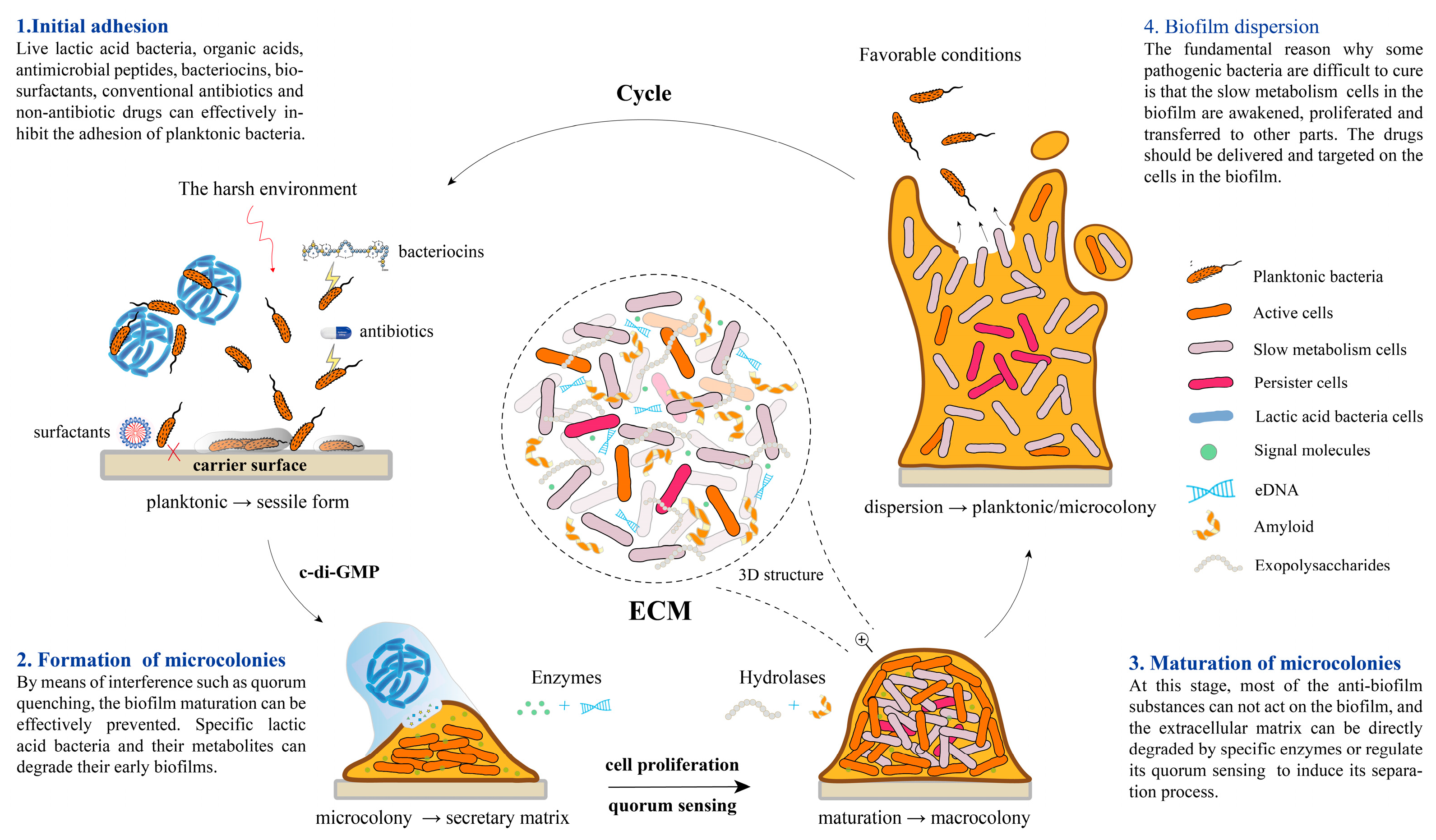
2. The Formation Mechanism of Biofilm
2.1. High Correlation with Quorum Sensing
2.2. Initial Adhesion
2.3. Formation and Maturation of Microcolonies
2.4. Biofilm Dispersion
3. The Summary of Postbiotics from LAB
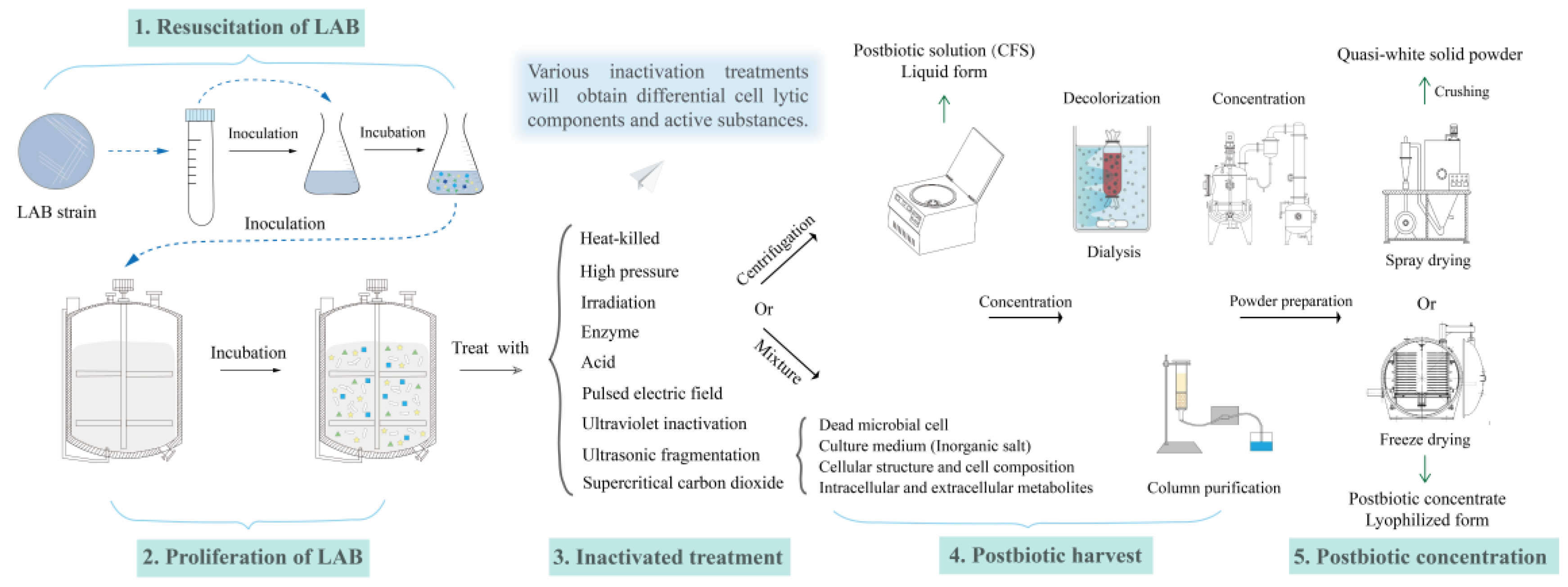
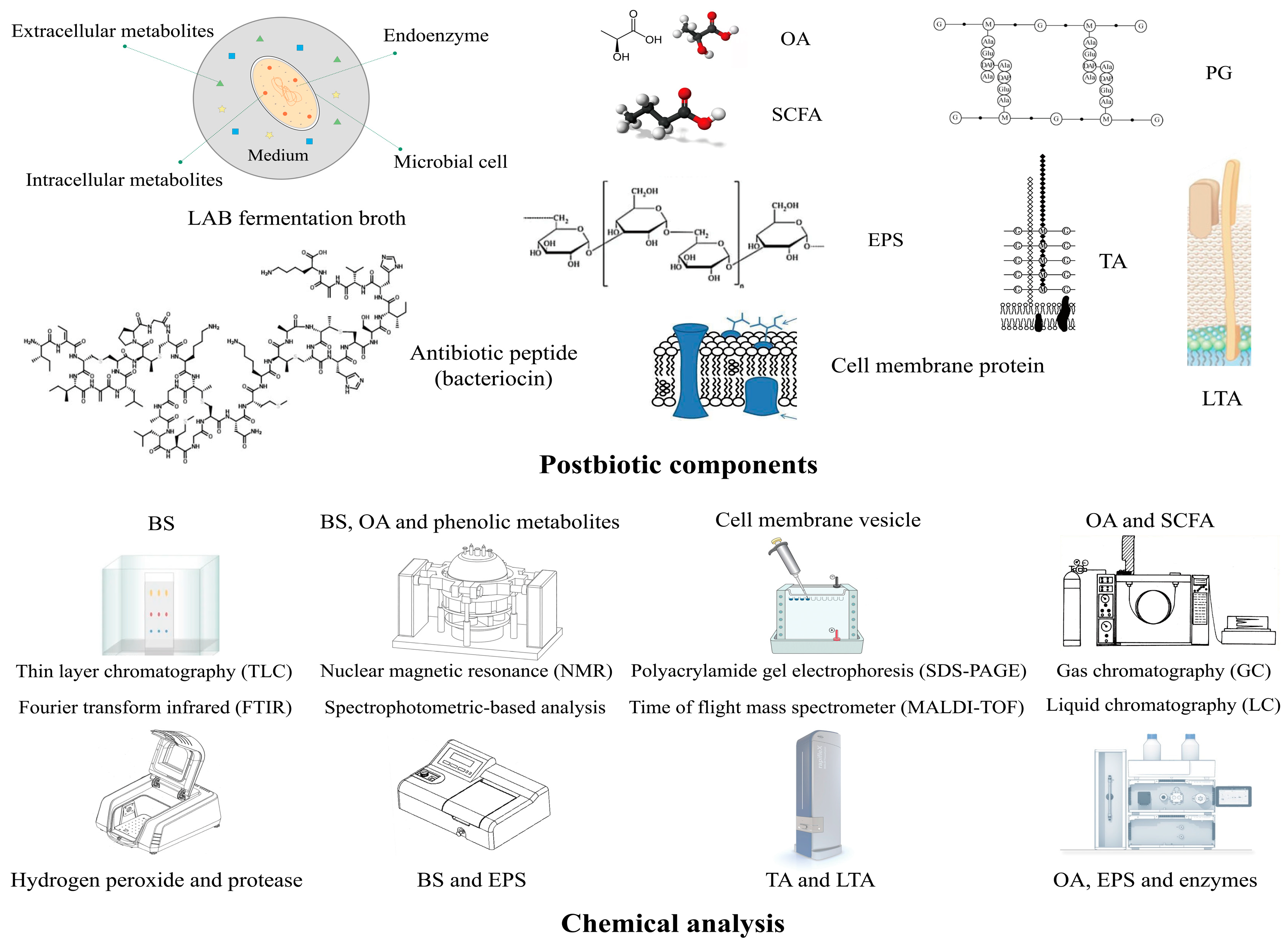
3.1. The Definition and Characteristics of Postbiotics
3.2. The Postbiotic Efficacy Produced by LAB
4. Mechanism of Biofilm Control by LAB Postbiotics
4.1. Inhibition of the QS Pathway
4.2. Reduction in Surface Adhesion
4.3. Antagonistic Effects
4.4. Regulation of Interspecific Interaction
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nahar, S.; Ha, A.J.; Byun, K.H.; Hossain, M.I.; Mizan, M.F.R.; Ha, S.D. Efficacy of flavourzyme against Salmonella typhimurium, Escherichia coli, and Pseudomonas aeruginosa biofilms on food-contact surfaces. Int. J. Food Microbiol. 2021, 336, 108897. [Google Scholar] [CrossRef]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-mediated control of biofilm: A promising new dawn for the future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef] [PubMed]
- Vunduk, J.; Wan-Mohtar, W.A.A.Q.I.; Mohamad, S.A.; Abd Halim, N.H.; Mohd Dzomir, A.Z.; Žižak, Ž.; Klaus, A. Polysaccharides of Pleurotus flabellatus strain Mynuk produced by submerged fermentation as a promising novel tool against adhesion and biofilm formation of foodborne pathogens. Lwt 2019, 112, 108221. [Google Scholar] [CrossRef]
- Funari, R.; Shen, A.Q. Detection and characterization of bacterial biofilms and biofilm-based sensors. Acs Sens. 2022, 7, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella pneumoniae biofilms and their role in disease pathogenesis. Front. Cell Infect. Microbiol. 2022, 12, 877995. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Wang, X.D.; Chang, J.S.; Lee, D.J. Homogeneously and heterogeneously structured biofilm models for wastewater treatment. Bioresour. Technol. 2022, 362, 127763. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, Y.; Lin, S.; Zhang, W.; Shu, G.; Lin, J.; Li, H.; Xu, F.; Tang, H.; Peng, G.; et al. Strategies for interfering with bacterial early stage biofilms. Front. Microbiol. 2021, 12, 675843. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, L.; Fang, Z.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W.; Li, H.; Lu, W. The biofilm-forming ability of six Bifidobacterium strains on grape seed flour. Lwt 2021, 144, 111205. [Google Scholar] [CrossRef]
- Mahto, K.U.; Kumari, S.; Das, S. Unraveling the complex regulatory networks in biofilm formation in bacteria and relevance of biofilms in environmental remediation. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, L.; Miao, J.; Zhang, Z.; Ruan, J.; Xu, L.; Guo, H.; Zhang, M.; Qiao, W. Regulation of the formation and structure of biofilms by quorum sensing signal molecules packaged in outer membrane vesicles. Sci. Total Environ. 2022, 806, 151403. [Google Scholar] [CrossRef]
- Wakade, V.S.; Shende, P. Strategic advancements and multimodal applications of biofilm therapy. Expert. Opin. Biol. Ther. 2021, 21, 395–412. [Google Scholar] [CrossRef]
- Liu, C.; Sun, D.; Zhu, J.; Liu, J.; Liu, W. The regulation of bacterial biofilm formation by cAMP-CRP: A mini-review. Front. Microbiol. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Blumenfeld, R.; Feng, X.Q.; Weitz, D.A. ‘Phase transitions’ in bacteria—From structural transitions in free living bacteria to phenotypic transitions in bacteria within biofilms. Phys. Life Rev. 2022, 43, 98–138. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.B.; Martin, M.; Schafer, D.; Hartmann, R.; Drescher, K.; Brix, S.; Dragos, A.; Kovacs, A.T. Privatization of biofilm matrix in structurally heterogeneous biofilms. Msystems 2020, 5, e00425-20. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Šimunović, K.; Sterniša, M.; Ramić, D.; Smole Možina, S.; Bucar, F. Anti-adhesion activity of phytochemicals to prevent Campylobacter jejuni biofilm formation on abiotic surfaces. Phytochem. Rev. 2020, 20, 55–84. [Google Scholar] [CrossRef]
- Uddin Mahamud, A.; Nahar, S.; Ashrafudoulla, M.; Park, S.H.; Ha, S.D. Insights into antibiofilm mechanisms of phytochemicals: Prospects in the food industry. Crit. Rev. Food Sci. Nutr. 2022, 64, 1736–1763. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Gomes, I.B.; Simoes, M. Antibiofilm activity of glycolic acid and glyoxal and their diffusion-reaction interactions with biofilm components. Food Res. Int. 2022, 152, 110921. [Google Scholar] [CrossRef] [PubMed]
- Domingue, J.C.; Drewes, J.L.; Merlo, C.A.; Housseau, F.; Sears, C.L. Host responses to mucosal biofilms in the lung and gut. Mucosal Immunol. 2020, 13, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Fortes, B.N.; Lincopan, N.; Ishida, K. Caspofungin and polymyxin B reduce the cell viability and total biomass of mixed biofilms of carbapenem-resistant Pseudomonas aeruginosa and Candida spp. Front. Microbiol. 2020, 11, 573263. [Google Scholar] [CrossRef]
- El-Haddad, E.A.; El-Hady, S.A.R.; Hamid, A.E.A.; Fahim, H.A.M. Effect of trans-cinnamaldehyde on preformed biofilm and biofilm formation of Escherichia coli isolated from urine specimens. QJM Int. J. Med. 2021, 114, hcab101. [Google Scholar] [CrossRef]
- Wu, B.C.; Haney, E.F.; Akhoundsadegh, N.; Pletzer, D.; Trimble, M.J.; Adriaans, A.E.; Nibbering, P.H.; Hancock, R.E.W. Human organoid biofilm model for assessing antibiofilm activity of novel agents. NPJ Biofilms Microbiomes 2021, 7, 8. [Google Scholar] [CrossRef]
- Fursov, M.V.; Abdrakhmanova, R.O.; Antonova, N.P.; Vasina, D.V.; Kolchanova, A.D.; Bashkina, O.A.; Rubalsky, O.V.; Samotrueva, M.A.; Potapov, V.D.; Makarov, V.V.; et al. Antibiofilm activity of a broad-range recombinant endolysin LysECD7: In Vitro and in Vivo Study. Viruses 2020, 12, 545. [Google Scholar] [CrossRef]
- Pang, L.; Wang, Y.; Ye, Y.; Zhou, Y.; Zhi, Q.; Lin, H. Metagenomic analysis of dental plaque on pit and fissure sites with and without caries among adolescents. Front. Cell Infect. Microbiol. 2021, 11, 740981. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimaraes, J.T.; Yilmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef]
- Liang, B.; Xing, D. The current and future perspectives of postbiotics. Probiotics Antimicrob. Proteins 2023, 15, 1626–1643. [Google Scholar] [CrossRef] [PubMed]
- Kareb, O.; Aider, M. Quorum sensing circuits in the communicating mechanisms of bacteria and its implication in the biosynthesis of bacteriocins by lactic acid bacteria: A review. Probiotics Antimicrob. Proteins 2020, 12, 5–17. [Google Scholar] [CrossRef]
- Mion, S.; Carriot, N.; Lopez, J.; Plener, L.; Ortalo-Magne, A.; Chabriere, E.; Culioli, G.; Daude, D. Disrupting quorum sensing alters social interactions in Chromobacterium violaceum. NPJ Biofilms Microbiomes 2021, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Coquant, G.; Aguanno, D.; Pham, S.; Grellier, N.; Thenet, S.; Carriere, V.; Grill, J.-P.; Seksik, P. Gossip in the gut: Quorum sensing, a new player in the host-microbiota interactions. World J. Gastroenterol. 2021, 27, 7247–7270. [Google Scholar] [CrossRef]
- Qiu, H.; Pu, F.; Liu, Z.; Deng, Q.; Sun, P.; Ren, J.; Qu, X. Depriving bacterial adhesion-related molecule to inhibit biofilm formation using CeO2-decorated metal-organic frameworks. Small 2019, 15, e1902522. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond risk: Bacterial biofilms and their regulating approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xi, J.; Yang, C.; Cong, W. Quorum sensing regulation methods and their effects on biofilm in biological waste treatment systems: A review. Front. Environ. Sci. Eng. 2021, 16, 87. [Google Scholar] [CrossRef]
- Lu, Y.; Lei, L.; Deng, Y.; Zhang, H.; Xia, M.; Wei, X.; Yang, Y.; Hu, T. RNase III coding genes modulate the cross-kingdom biofilm of Streptococcus mutans and Candida albicans. Front. Microbiol. 2022, 13, 957879. [Google Scholar] [CrossRef] [PubMed]
- Khoury, Z.H.; Vila, T.; Puthran, T.R.; Sultan, A.S.; Montelongo-Jauregui, D.; Melo, M.A.S.; Jabra-Rizk, M.A. The role of Candida albicans secreted polysaccharides in augmenting Streptococcus mutans adherence and mixed biofilm formation: In vitro and in vivo studies. Front. Microbiol. 2020, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Herraez, M.; Costa-Gutierrez, S.B.; Molina-Henares, M.A.; Martinez, M.J.; Espinosa-Urgel, M.; Barriuso, J. The architecture of a mixed fungal-bacterial biofilm is modulated by quorum-sensing signals. Environ. Microbiol. 2021, 23, 2433–2447. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.X.; Tian, J.; Liu, Y.; Dhall, A.; Koo, H.; Hwang, G. Cross-kingdom cell-to-cell interactions in cariogenic biofilm initiation. J. Dent. Res. 2021, 100, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W. Multi-omics reveals the inhibition of Lactiplantibacillus plantarum CCFM8724 in Streptococcus mutans-Candida albicans mixed-species biofilms. Microorganisms 2021, 9, 2368. [Google Scholar] [CrossRef] [PubMed]
- Maan, H.; Povolotsky, T.L.; Porat, Z.; Itkin, M.; Malitsky, S.; Kolodkin-Gal, I. Imaging flow cytometry reveals a dual role for exopolysaccharides in biofilms: To promote self-adhesion while repelling non-self-community members. Comput. Struct. Biotechnol. J. 2022, 20, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Buzzo, J.R.; Devaraj, A.; Gloag, E.S.; Jurcisek, J.A.; Robledo-Avila, F.; Kesler, T.; Wilbanks, K.; Mashburn-Warren, L.; Balu, S.; Wickham, J.; et al. Z-form extracellular DNA is a structural component of the bacterial biofilm matrix. Cell 2021, 184, 5740–5758.e5717. [Google Scholar] [CrossRef]
- Palencia, S.L.; Garcia, A.; Palencia, M. Multiple surface interaction mechanisms direct the anchoring, co-aggregation and formation of dual-species biofilm between Candida albicans and Helicobacter pylori. J. Adv. Res. 2022, 35, 169–185. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, M.; Lu, Z. The LuxS/AI-2 system regulates the probiotic activities of lactic acid bacteria. Trends Food Sci. Technol. 2022, 127, 272–279. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Recent advances and future challenges in the use of nanoparticles for the dispersal of infectious biofilms. J. Mater. Sci. Technol. 2021, 84, 208–218. [Google Scholar] [CrossRef]
- Bridges, A.A.; Fei, C.; Bassler, B.L. Identification of signaling pathways, matrix-digestion enzymes, and motility components controlling Vibrio cholerae biofilm dispersal. Proc. Natl. Acad. Sci. USA 2020, 117, 32639–32647. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.P.; Pires, R.P.S.; Guimaraes, J.T.; Abud, Y.K.D.; Almada, C.N.; Pimentel, T.C.; Sant’Anna, C.; De-Melo, L.D.B.; Duarte, M.C.K.H.; Silva, M.C.; et al. Ohmic heating as a method of obtaining paraprobiotics: Impacts on cell structure and viability by flow cytometry. Food Res. Int. 2021, 140, 110061. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jeon, B.; Kim, W.J.; Chung, D.-K. Effect of paraprobiotic prepared from Kimchi-derived Lactobacillus plantarum K8 on skin moisturizing activity in human keratinocyte. J. Funct. Foods 2020, 75, 104244. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, T.; Luo, R.; Liu, J.; Jin, R.; Peng, X. Recent advances and potentiality of postbiotics in the food industry: Composition, inactivation methods, current applications in metabolic syndrome, and future trends. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, S.; Di, H.; Deng, Z.; Liu, J.; Wang, H. Gut health benefit and application of postbiotics in animal production. J. Anim. Sci. Biotechnol. 2022, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Ghasempour, Z.; Sabahi, S.; Kafil, H.S.; Hasannezhad, P.; Saadat, Y.R.; Shahbazi, N. The biological activities of postbiotics in gastrointestinal disorders. Crit. Rev. Food Sci. Nutr. 2022, 62, 5983–6004. [Google Scholar] [CrossRef]
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, S.; Lv, H.; Peng, L.; Yang, W.; Chen, J.; Wu, Z.; Wan, C. Effect of Bifidobacterium animalis subsp. lactis SF on enhancing the tumor suppression of irinotecan by regulating the intestinal flora. Pharmacol. Res. 2022, 184, 106406. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Q.; Ruan, G.; Chen, Y.; Zhao, M.; Shi, D.; Pan, B.; Xu, Z.; Zhang, T.; Wang, F.; et al. Efficacy of probiotics against dental caries in children: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 63, 9977–9994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qin, S.; Xu, X.; Zhao, J.; Zhang, H.; Liu, Z.; Chen, W. Inhibitory effect of Lactobacillus plantarum CCFM8724 towards Streptococcus mutans- and Candida albicans-induced caries in rats. Oxid. Med. Cell Longev. 2020, 2020, 4345804. [Google Scholar] [CrossRef] [PubMed]
- Masebe, R.D.; Thantsha, M.S. Anti-biofilm activity of cell free supernatants of selected lactic acid bacteria against Listeria monocytogenes isolated from avocado and cucumber fruits, and from an avocado processing plant. Foods 2022, 11, 2872. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Fundamental study on the improvement of the antifungal activity of Lactobacillus reuteri R29 through increased production of phenyllactic acid and reuterin. Food Control 2018, 88, 139–148. [Google Scholar] [CrossRef]
- Liu, J.; Huang, R.; Song, Q.; Xiong, H.; Ma, J.; Xia, R.; Qiao, J. Combinational antibacterial activity of nisin and 3-phenyllactic acid and their co-production by engineered Lactococcus lactis. Front. Bioeng. Biotechnol. 2021, 9, 612105. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Han, Y.; Xiao, H.; Zhou, Z. Pediococcus acidilactici inhibit biofilm formation of food-borne pathogens on abiotic surfaces. Trans. Tianjin Univ. 2016, 23, 70–77. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Bandara, H.M.; Mayer, M.P.; Samaranayake, L.P. Probiotics as antifungals in mucosal candidiasis. Clin. Infect. Dis. 2016, 62, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Benmouna, Z.; Dalache, F.; Zadi-Karam, H.; Karam, N.E.; Vuotto, C. Ability of three lactic acid bacteria to grow in sessile mode and to inhibit biofilm formation of pathogenic bacteria. Adv. Exp. Med. Biol. 2020, 1282, 105–114. [Google Scholar] [CrossRef]
- Mohd-Zubri, N.S.; Ramasamy, K.; Abdul-Rahman, N.Z. Characterization and potential oral probiotic properties of Lactobacillus plantarum FT 12 and Lactobacillus brevis FT 6 isolated from Malaysian fermented food. Arch. Oral. Biol. 2022, 143, 105515. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef] [PubMed]
- Toushik, S.H.; Park, J.-H.; Kim, K.; Ashrafudoulla, M.; Senakpon Isaie Ulrich, M.; Mizan, M.F.R.; Roy, P.K.; Shim, W.-B.; Kim, Y.-M.; Park, S.H.; et al. Antibiofilm efficacy of Leuconostoc mesenteroides J.27-derived postbiotic and food-grade essential oils against Vibrio parahaemolyticus, Pseudomonas aeruginosa, and Escherichia coli alone and in combination, and their application as a green preservative in the seafood industry. Food Res. Int. 2022, 156, 111163. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; De Paepe, K.; Van de Wiele, T. Postbiotics and their health modulatory biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Kim, J.-H.; Oh, S.-W. Review of multi-species biofilm formation from foodborne pathogens: Multi-species biofilms and removal methodology. Crit. Rev. Food Sci. Nutr. 2021, 62, 5783–5793. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Ye, T.; Li, Q.; Bhatt, P.; Zhang, L.; Chen, S. Potential of a quorum quenching bacteria isolate Ochrobactrum intermedium D-2 against soft rot pathogen Pectobacterium carotovorum subsp. carotovorum. Front. Microbiol. 2020, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Rasamiravaka, T.; El Jaziri, M. Quorum-sensing mechanisms and bacterial response to antibiotics in P. aeruginosa. Curr. Microbiol. 2016, 73, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Wang, C.; Han, M.F.; Tong, Z.; Hu, X.R.; Lin, Y.T.; Zhao, X. Reduction of biofilm adhesion strength by adjusting the characteristics of biofilms through enzymatic quorum quenching. Chemosphere 2022, 288, 132465. [Google Scholar] [CrossRef] [PubMed]
- El-Mokhtar, M.A.; Hassanein, K.M.; Ahmed, A.S.; Gad, G.F.M.; Amin, M.M.; Hassanein, O.F.E. Antagonistic activities of cell-free supernatants of Lactobacilli against extended-spectrum beta-lactamase producing Klebsiella pneumoniae and Pseudomonas aeruginosa. Infect. Drug Resist. 2020, 13, 543–552. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, C.; Gulhane, R.D.; Rokana, N.; Singh, B.P.; Puniya, A.K.; Attri, S.; Goel, G.; Panwar, H. Inhibitory effects of lactobacilli of goat’s milk origin against growth and biofilm formation by pathogens: An in vitro study. Food Biosci. 2018, 22, 129–138. [Google Scholar] [CrossRef]
- Ciandrini, E.; Campana, R.; Baffone, W. Live and heat-killed Lactobacillus spp. interfere with Streptococcus mutans and Streptococcus oralis during biofilm development on titanium surface. Arch. Oral. Biol. 2017, 78, 48–57. [Google Scholar] [CrossRef]
- Koohestani, M.; Moradi, M.; Tajik, H.; Badali, A. Effects of cell-free supernatant of Lactobacillus acidophilus LA5 and Lactobacillus casei 431 against planktonic form and biofilm of Staphylococcus aureus. Vet. Res. Forum 2018, 9, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Dube, Y.; Khan, A.; Marimani, M.; Ahmad, A. Lactobacillus rhamnosus cell-free extract targets virulence and antifungal drug resistance in Candida albicans. Can. J. Microbiol. 2020, 66, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gu, S.; Cui, X.; Shi, Y.; Wen, S.; Chen, H.; Ge, J. Antimicrobial, anti-adhesive and anti-biofilm potential of biosurfactants isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microb. Pathog. 2019, 127, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Siddiqui, A.J.; Hamadou, W.S.; Surti, M.; Awadelkareem, A.M.; Ashraf, S.A.; Alreshidi, M.; Snoussi, M.; Rizvi, S.M.D.; Bardakci, F.; et al. Inhibition of bacterial adhesion and antibiofilm activities of a glycolipid biosurfactant from Lactobacillus rhamnosus with its physicochemical and functional properties. Antibiotics 2021, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, A.; Ramalhete, R.; Blunn, G.; Gibbs, H.; Pumilia, C.A.; Meckmongkol, T.; Lovejoy, J.; Coathup, M.J. Lactobacillus cell-free supernatant as a novel bioagent and biosurfactant against Pseudomonas aeruginosa in the prevention and treatment of orthopedic implant infection. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Harjai, K.; Shukla, G. Effect of bacteriocin and exopolysaccharides isolated from probiotic on P. aeruginosa PAO1 biofilm. Folia Microbiol. 2018, 63, 181–190. [Google Scholar] [CrossRef]
- MacAlpine, J.; Daniel-Ivad, M.; Liu, Z.; Yano, J.; Revie, N.M.; Todd, R.T.; Stogios, P.J.; Sanchez, H.; O’Meara, T.R.; Tompkins, T.A.; et al. A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase. Nat. Commun. 2021, 12, 6151. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Wang, Y.; Bandara, H.; Mayer, M.P.A.; Samaranayake, L.P. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl. Microbiol. Biotechnol. 2016, 100, 6415–6426. [Google Scholar] [CrossRef]
- Zhong, S.; He, S. Quorum sensing inhibition or quenching in Acinetobacter baumannii: The novel therapeutic strategies for new drug development. Front. Microbiol. 2021, 12, 558003. [Google Scholar] [CrossRef]
- Dong, W.; Cai, Y.; Xu, Z.; Fu, B.; Chen, Q.; Cui, Y.; Ruan, Z.; Liang, Y.; Peng, N.; Zhao, S. Heterologous expression of AHL lactonase AiiK by Lactobacillus casei MCJDelta1 with great quorum quenching ability against Aeromonas hydrophila AH-1 and AH-4. Microb. Cell Fact. 2020, 19, 191. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, X.; Li, J.; Ye, T.; Mishra, S.; Zhang, L.; Chen, S. Exploration of the quorum-quenching mechanism in Pseudomonas nitroreducens W-7 and its potential to attenuate the virulence of Dickeya zeae EC1. Front. Microbiol. 2021, 12, 694161. [Google Scholar] [CrossRef] [PubMed]
- Kusada, H.; Tamaki, H.; Kamagata, Y.; Hanada, S.; Kimura, N. A novel quorum-quenching N-acylhomoserine lactone acylase from Acidovorax sp. strain MR-S7 mediates antibiotic resistance. Appl. Environ. Microbiol. 2017, 83, e00080-17. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hill, P.; Liu, J.; Qian, J.; Ma, Y.; Zhou, S. Marine-source quorum quenching enzyme YtnP to improve hygiene quality in dental units. Mar. Drugs 2021, 19, 225. [Google Scholar] [CrossRef]
- Yu, H.; Du, C.; Qu, F.; He, J.; Rong, H. Efficient biostimulants for bacterial quorum quenching to control fouling in MBR. Chemosphere 2022, 286, 131689. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; D’Morris, S.; Paul, V.; Warrier, S.; Vasudevan, A.K.; Vanuopadath, M.; Nair, S.S.; Paul-Prasanth, B.; Mohan, C.G.; Biswas, R. Mechanistic understanding of phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2017, 101, 8223–8236. [Google Scholar] [CrossRef] [PubMed]
- Aman, M.; Aneeqha, N.; Bristi, K.; Deeksha, J.; Afza, N.; Sindhuja, V.; Shastry, R.P. Lactic acid bacteria inhibits quorum sensing and biofilm formation of Pseudomonas aeruginosa strain JUPG01 isolated from rancid butter. Biocatal. Agric. Biotechnol. 2021, 36, 102115. [Google Scholar] [CrossRef]
- Chappell, T.C.; Nair, N.U. Engineered lactobacilli display anti-biofilm and growth suppressing activities against Pseudomonas aeruginosa. NPJ Biofilms Microbiomes 2020, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ramesh, C.; Mallappa, R.H. Functional group characterization of lactic bacterial biosurfactants and evaluation of antagonistic actions against clinical isolates of methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2021, 73, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Dimitrijevic, M.; Aleksic, A.; Neffe-Skocinska, K.; Zielinska, D.; Kolozyn-Krajewska, D.; Sharifi-Rad, J.; Stojanovic-Radic, Z.; Prabu, S.M.; Rodrigues, C.F.; et al. Human microbiome and homeostasis: Insights into the key role of prebiotics, probiotics, and symbiotics. Crit. Rev. Food Sci. Nutr. 2021, 61, 1415–1428. [Google Scholar] [CrossRef]
- Shazadi, K.; Arshad, N. Evaluation of inhibitory and probiotic properties of lactic acid bacteria isolated from vaginal microflora. Folia Microbiol. 2022, 67, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Ray Mohapatra, A.; Jeevaratnam, K. Inhibiting bacterial colonization on catheters: Antibacterial and antibiofilm activities of bacteriocins from Lactobacillus plantarum SJ33. J. Glob. Antimicrob. Resist. 2019, 19, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Chen, J.; Zhou, S.; Zhao, Y.; Xu, M.; Xu, H. Dual mode of anti-biofilm action of G3 against Streptococcus mutans. ACS Appl. Mater. Interfaces 2020, 12, 27866–27875. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Song, X.; Chen, M.; Tian, S.; Lu, Z.; Sun, J.; Li, X.; Lu, Y.; Yuk, H.G. Combating biofilms of foodborne pathogens with bacteriocins by lactic acid bacteria in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1657–1676. [Google Scholar] [CrossRef] [PubMed]
- Chandla, S.; Harjai, K.; Shukla, G. Synergistic effect of biogenics derived from potential probiotics together with zingerone against biofilm formation by Pseudomonas aeruginosa PAO1. Probiotics Antimicrob. Proteins 2021, 13, 1481–1497. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, J.; Liu, Z.; Pei, J.; Brennan, C.; Abd El-Aty, A.M. Isolation and characterization of bacteriocin-producing Lacticaseibacillus rhamnosus XN2 from Yak yoghurt and its bacteriocin. Molecules 2022, 27, 2066. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hao, X.; Yang, F.; Wang, Y.; Fan, X.; Wang, Y. Antifungal activity of Lactobacillus plantarum ZZUA493 and its application to extend the shelf life of Chinese steamed buns. Foods 2022, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, A.L.; Correia, A.F.; Pereira, L.C.; de Alencar, B.M.; Silva, F.B.A.; Almeida, R.M.; de Medeiros Nobrega, Y.K. Inhibitory effects of Lactobacillus casei Shirota against both Candida auris and Candida spp. isolates that cause vulvovaginal candidiasis and are resistant to antifungals. BMC Complement. Med. Ther. 2021, 21, 237. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Sun, Z.; Wang, F.; Liu, Y.; Zhu, Y.; Du, L.; Wang, D.; Xu, W. Inhibition of biofilm formation and exopolysaccharide synthesis of Enterococcus faecalis by phenyllactic acid. Food Microbiol. 2020, 86, 103344. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Han, J.-H.; Rather, I.A.; Park, C.; Lim, J.; Paek, W.K.; Lee, J.S.; Yoon, J.-I.; Park, Y.-H. Characterization and antibacterial potential of lactic acid bacterium Pediococcus pentosaceus 4I1 isolated from freshwater fish Zacco koreanus. Front. Microbiol. 2016, 7, 226725. [Google Scholar] [CrossRef]
- Shokri, D.; Khorasgani, M.R.; Mohkam, M.; Fatemi, S.M.; Ghasemi, Y.; Taheri-Kafrani, A. The inhibition effect of Lactobacilli against growth and biofilm formation of Pseudomonas aeruginosa. Probiotics Antimicrob. Proteins 2018, 10, 34–42. [Google Scholar] [CrossRef]
- Divyashree, S.; Anjali, P.G.; Deepthi, B.V.; Somashekaraiah, R.; Mottawea, W.; Hammami, R.; Sreenivasa, M.Y. Black cherry fruit as a source of probiotic candidates with antimicrobial and antibiofilm activities against Salmonella. S. Afr. J. Bot. 2022, 150, 861–872. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Radziwill-Bienkowska, J.M.; Robert, V.; Drabot, K.; Chain, F.; Cherbuy, C.; Langella, P.; Thomas, M.; Bardowski, J.K.; Mercier-Bonin, M.; Kowalczyk, M. Contribution of plasmid-encoded peptidase S8 (PrtP) to adhesion and transit in the gut of Lactococcus lactis IBB477 strain. Appl. Microbiol. Biotechnol. 2017, 101, 5709–5721. [Google Scholar] [CrossRef] [PubMed]
- Merino, L.; Trejo, F.M.; De Antoni, G.; Golowczyc, M.A. Lactobacillus strains inhibit biofilm formation of Salmonella sp. isolates from poultry. Food Res. Int. 2019, 123, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Hao, L.; Zhou, R.; Jin, Y.; Huang, J.; Wu, C. Formation of biofilm by Tetragenococcus halophilus benefited stress tolerance and anti-biofilm activity against S. aureus and S. Typhimurium. Front. Microbiol. 2022, 13, 819302. [Google Scholar] [CrossRef] [PubMed]
- Jara, J.; Perez-Ramos, A.; Del Solar, G.; Rodriguez, J.M.; Fernandez, L.; Orgaz, B. Role of Lactobacillus biofilms in Listeria monocytogenes adhesion to glass surfaces. Int. J. Food Microbiol. 2020, 334, 108804. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-T.; Matsuo, M.; Niemann, S.; Herrmann, M.; Goetz, F. Lipoproteins in gram-positive bacteria: Abundance, function, fitness. Front. Microbiol. 2020, 11, 582582. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Siddiqui, A.J.; Hamadou, W.S.; Ashraf, S.A.; Hassan, M.I.; Snoussi, M.; Badraoui, R.; Jamal, A.; Bardakci, F.; Awadelkareem, A.M.; et al. Functional and structural characterization of Pediococcus pentosaceus-derived biosurfactant and its biomedical potential against bacterial adhesion, quorum sensing, and biofilm formation. Antibiotics 2021, 10, 1371. [Google Scholar] [CrossRef]
- Wang, L.; Si, W.; Xue, H.; Zhao, X. A fibronectin-binding protein (FbpA) of Weissella cibaria inhibits colonization and infection of Staphylococcus aureus in mammary glands. Cell Microbiol. 2017, 19, e12731. [Google Scholar] [CrossRef]
- Martorell, P.; Alvarez, B.; Llopis, S.; Navarro, V.; Ortiz, P.; Gonzalez, N.; Balaguer, F.; Rojas, A.; Chenoll, E.; Ramón, D.; et al. Heat-Treated Bifidobacterium longum CECT-7347: A Whole-Cell Postbiotic with Antioxidant, Anti-Inflammatory, and Gut-Barrier Protection Properties. Antioxidants 2021, 10, 536. [Google Scholar] [CrossRef]
- Miyazawa, K.; He, F.; Kawase, M.; Kubota, A.; Yoda, K.; Hiramatsu, M. Enhancement of immunoregulatory effects of Lactobacillus gasseri TMC0356 by heat treatment and culture medium. Lett. Appl. Microbiol. 2011, 53, 210–216. [Google Scholar] [CrossRef]
- Ishikawa, H.; Kutsukake, E.; Fukui, T.; Sato, I.; Shirai, T.; Kurihara, T.; Okada, N.; Danbara, H.; Toba, M.; Kohda, N.; et al. Oral administration of heat-killed Lactobacillus plantarum strain b240 protected mice against Salmonella enterica Serovar Typhimurium. Biosci. Biotechnol. Biochem. 2010, 74, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Iwabuchi, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Hachimura, S. Orally administered heat-killed Lactobacillus paracasei MCC1849 enhances antigen-specific IgA secretion and induces follicular helper T cells in mice. PLoS ONE 2018, 13, e0199018. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Sim, J.R.; Yun, C.H.; Han, S.H. Lipoteichoic acids as a major virulence factor causing inflammatory responses via Toll-like receptor 2. Arch. Pharmacal Res. 2016, 39, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.J.; Underhill, D.M. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 2018, 18, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Umemoto, E.; Fujita, S.; Hayashi, A.; Kikuta, J.; Kimura, I.; Haneda, T.; Imai, T.; Inoue, A.; Mimuro, H.; et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1+ cells by bacterial metabolites. Nature 2019, 566, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Beebout, C.J.; Sominsky, L.A.; Eberly, A.R.; Van Horn, G.T.; Hadjifrangiskou, M. Cytochrome bd promotes Escherichia coli biofilm antibiotic tolerance by regulating accumulation of noxious chemicals. NPJ Biofilms Microbiomes 2021, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Kollef, M. The epidemiology and pathogenesis and treatment of Pseudomonas aeruginosa infections: An update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Wang, L.; Chen, L. Iron metabolism in Pseudomonas aeruginosa biofilm and the involved iron-targeted anti-biofilm strategies. J. Drug Target. 2021, 29, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Holban, A.M.; Curutiu, C.; Chifiriuc, M.C. Modulation of quorum sensing and biofilms in less investigated gram-negative ESKAPE pathogens. Front. Microbiol. 2021, 12, 676510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, S.; Li, J.; Bu, X.; Dong, X.; Chen, N.; Li, F.; Zhu, J.; Sang, L.; Zeng, Y.; et al. Dual-sensitive antibacterial peptide nanoparticles prevent dental caries. Theranostics 2022, 12, 4818–4833. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.H.N.; Casey, E. A review of nanomaterials and technologies for enhancing the antibiofilm activity of natural products and phytochemicals. ACS Appl. Nano Mater. 2020, 3, 8537–8556. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhao, I.S.; Mei, M.L.; Li, Q.; Yu, O.Y.; Chu, C.H. Use of silver nanomaterials for caries prevention: A concise review. Int. J. Nanomed. 2020, 15, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xin, X.; Qiu, W.; Li, D.; Liu, Z.; Ma, J. Role of nano-Fe3O4 particle on improving membrane bioreactor (MBR) performance: Alleviating membrane fouling and microbial mechanism. Water Res. 2021, 209, 117897. [Google Scholar] [CrossRef] [PubMed]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Rubini, D.; Banu, S.F.; Subramani, P.; Hari, B.N.V.; Gowrishankar, S.; Pandian, S.K.; Wilson, A.; Nithyanand, P. Extracted chitosan disrupts quorum sensing mediated virulence factors in urinary tract infection causing pathogens. Pathog. Dis. 2019, 77, ftz009. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.S.E.M.; Riad, O.K.M.; Taher, F.A.; Zaki, S.A. Chitosan and chitosan-zinc oxide nanocomposite inhibit expression of LasI and RhlI genes and quorum sensing dependent virulence factors of Pseudomonas aeruginosa. Int. J. Biol. Macromol. 2020, 149, 1109–1117. [Google Scholar] [CrossRef]
- Gu, Z.; Meng, S.; Wang, Y.; Lyu, B.; Li, P.; Shang, N. A novel bioactive postbiotics: From microbiota-derived extracellular nanoparticles to health promoting. Crit. Rev. Food Sci. Nutr. 2022, 63, 6885–6899. [Google Scholar] [CrossRef]
- Incili, G.K.; Karatepe, P.; Akgol, M.; Kaya, B.; Kanmaz, H.; Hayaloglu, A.A. Characterization of Pediococcus acidilactici postbiotic and impact of postbiotic-fortified chitosan coating on the microbial and chemical quality of chicken breast fillets. Int. J. Biol. Macromol. 2021, 184, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Li, Y.H.; Luo, Y.B.; Zhou, Y.; Wen, J.; Chen, L.; Liang, X.P.; Wu, T.; Tan, C.Y.; Liu, Y. Gut microbiome and metabolites: The potential key roles in pulmonary fibrosis. Front. Microbiol. 2022, 13, 943791. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, H.; Tada, A.; Vizoso-Pinto, M.G.; Salva, S.; Kanmani, P.; Agüero, G.; Alvarez, S.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic Lactobacillus rhamnosus modulates inflammation-coagulation interactions and reduces influenza virus-associated pulmonary damage. Inflamm. Res. 2015, 64, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Takahashi, T.; Oishi, K.; Tanaka, H.; Masuda, M.; Takahashi, S.; Takano, M.; Kawakami, T.; Fukushima, K.; Kanazawa, H.; et al. Consecutive oral administration of Bifidobacterium longum MM-2 improves the defense system against influenza virus infection by enhancing natural killer cell activity in a murine model. Microbiol. Immunol. 2015, 59, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef]
- Mjösberg, J.; Rao, A. Lung inflammation originating in the gut. Science 2018, 359, 36–37. [Google Scholar] [CrossRef]

| Name of LAB | Postbiotic form | Functional Components | Mechanism of Antibiofilm | Application Potential | Reference |
|---|---|---|---|---|---|
| Lb. acidophilus | CFS | Exopolysaccharides and biosurfactants | Inhibition of initial adhesion to the biological surface | Controlling or preventing ESBL colonization or infection | [69] |
| Lactobacillus spp. | CFS in neutralized and heat-treated form | Organic acids (lactic, formic, and acetic acids) and bacteriocins | Inhibition of initial adhesion to the biological surface/promotion of the dispersion of biofilm | Bio-control agents used to prevent infections | [70] |
| Lactobacillus spp. | Heat-killed cells/CFS | hydrogen peroxide, bacteriocins and biosurfactants | Competition for the specific salivary receptors/displacement of biofilm via high co-aggregation ability | Products for oral hygiene | [71] |
| Lb. acidophilus LA5 Lb. casei 431 | CFS | Exopolysaccharide and biosurfactants | Inhibition of adhesion to the biological surface | Biofilm removal compounds to control the foodborne pathogens | [72] |
| Lb. acidophilus La14 150B Lb. plantarum B411 Lb. rhamnosus ATCC 53103 | CFS | Organic acids (lactic and acetic acids) and bacteriocins | downregulation of prfA gene involved in biofilm formation | Food-grade sanitizers | [55] |
| Lb. rhamnosus | cell-free extracts (solution) | oleic and myristic acid | Downregulation of virulence gene/inhibition of yeast-to-hyphae transition | Therapeutic agents to treat Candida infections | [73] |
| Enterococcus sp. CM9 and CM18 | CFS | bacteriocins | Competition for adhesion site | Control of food contamination | [60] |
| P. acidilactici 27167 Lb. plantarum 27172 | cell-free extracts (solution) | biosurfactants | Reduction in expression levels of biofilm-related genes/interference with the release of AI-2 | Therapeutic agents to treat S. aureus infection | [74] |
| L. mesenteroides J.27 | CFS (lyophilized) | Organic acids (lactic, acetic, and citric acid) | downregulation of the mRNA expression of virulence-related genes | Green preservative in seafood processing | [63] |
| Lb. rhamnosus | CFS (lyophilized) | Glycolipid biosurfactants | Inhibition of initial adhesion to surfaces/altering the integrity and viability of biofilm cells | Green antibiofilm agents | [75] |
| Lactobacillus spp. | CFS | biosurfactants, lactic acid, and exopolysaccharides | Inhibition of initial adhesion to surfaces/induction of pore formation on the bacterial cell surface/suppression in short-chain AHL production | The prevention and treatment of orthopedic infection | [76] |
| Lb. fermentum KT998657 | CFS in neutralized/cell-free extracts (solution) | Exopolysaccharides and bacteriocins | Reduction in quorum sensing signals needed for biofilm formation/matrix modification/restriction on cell assembly and attachment | Prophylactic agents for medical devices | [77] |
| Lb. plantarum FT 12 Lb. brevis FT 6 | CFS | Organic acids and bacteriocins | Interfere with quorum sensing/high co-aggregation ability with pathogens | Supportive oral health treatment | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, J.; Shi, J.; Fang, C.; Zeng, X.; Wu, Z.; Du, Q.; Tu, M.; Pan, D. Elimination of Pathogen Biofilms via Postbiotics from Lactic Acid Bacteria: A Promising Method in Food and Biomedicine. Microorganisms 2024, 12, 704. https://doi.org/10.3390/microorganisms12040704
Che J, Shi J, Fang C, Zeng X, Wu Z, Du Q, Tu M, Pan D. Elimination of Pathogen Biofilms via Postbiotics from Lactic Acid Bacteria: A Promising Method in Food and Biomedicine. Microorganisms. 2024; 12(4):704. https://doi.org/10.3390/microorganisms12040704
Chicago/Turabian StyleChe, Jiahao, Jingjing Shi, Chenguang Fang, Xiaoqun Zeng, Zhen Wu, Qiwei Du, Maolin Tu, and Daodong Pan. 2024. "Elimination of Pathogen Biofilms via Postbiotics from Lactic Acid Bacteria: A Promising Method in Food and Biomedicine" Microorganisms 12, no. 4: 704. https://doi.org/10.3390/microorganisms12040704





