Biosynthesis Progress of High-Energy-Density Liquid Fuels Derived from Terpenes
Abstract
:1. Introduction
2. Properties of HED Fuels Derived from Terpenes
2.1. Monoterpene-Derived Fuels
2.2. Sesquiterpene-Derived Fuels
| Fuel | Structure | NHOC/(MJ/L) | Density/(g/mL) | Viscosity/mm2/s | Freezing Point/°C | Ref. |
|---|---|---|---|---|---|---|
| β-Pinene | 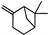 | 36.89 | 0.86 (20 °C) | — | — | [16] |
| α-Pinene |  | 36.89 | 0.86 (20 °C) | — | — | [16] |
| α-Pinane | 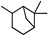 | 37.08 | 0.86 (15 °C) | 11.23 (−40 °C) | <−75 | [21] |
| Limonene | 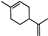 | 39.15 | ~0.87 (20 °C) | 2.35 (−20 °C) | −74 | [19] |
| Sabinane | 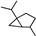 | 35.05 | 0.81 (15 °C) | 4.809 (−40 °C) | — | [41] |
| Myrcene |  | 34.32 | 0.79 (20 °C) | — | — | [27] |
| p-Menthane | 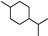 | 34.72 | 0.804 (15 °C) | 5.19 (−40 °C) | <−70 | [22,42] |
| Hydrogenated β-pinene dimer | 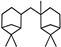 | 39.50 | 0.938 (20 °C) | 35.05 (40 °C) | −30 | [16] |
| α-Pinene dimer | - | 39.31 | 0.935 (20 °C) | 34.68 (40 °C) | −52 | [16] |
| Camphene dimer | - | 39.58 | 0.941 (20 °C) | 34.96 (40 °C) | −54 | [16] |
| Limonene dimer | - | 38.36 | 0.914 (20 °C) | 25.86 (40 °C) | −78 | [16] |
| Farnesane |  | 33.93 | 0.77 (20 °C) | 2.35 (40 °C) | −52 | [32] |
| Pentalenene | 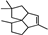 | 40.73 | 0.91 (20 °C) | — | −63.5 | [32] |
| Pentalenane |  | 40.55 | 0.89 (20 °C) | — | <−67 | [32] |
| Pentalenene cyclopropanation |  | 38.43 | 0.90 (20 °C) | — | <−53 | [32] |
| Presilphiperfol-1-ane |  | 39.24 | 0.92 (20 °C) | — | −47 | [32] |
| Hydrogenated cedarwood oil | - | 39.25 | 0.92 (20 °C) | 6.2 (40 °C) | <−80 | [37] |
| Valencane | 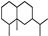 | 37.73 | 0.88 (20 °C) | 50.2 (−20 °C) | — | [34] |
| Premnaspirodiane | 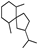 | 37.78 | 0.88 (20 °C) | 42.9 (−20 °C) | — | [34] |
| Caryophyllane | 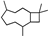 | 37.01 | 0.85 (20 °C) | 60.5 (−20 °C) | — | [34] |
| Cedrane | 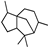 | 39.47 | 0.923 (20 °C) | 33 (−20 °C) | — | [37] |
3. Microbial Synthesis of Monoterpene and Sesquiterpene Fuels
3.1. Microbial Synthesis of Monoterpenes
3.2. Microbial Synthesis of Sesquiterpenes
| Product | Chassis | Synthetase | Conditions | Carbon Source | Production | Ref. |
|---|---|---|---|---|---|---|
| Monoterpene | ||||||
| Limonene | E. coli BL21 (DE3) | Limonene synthase from Mentha spicata | Fed-batch fermentation/3.1 L | Glycerol | 24 h, 3.63 g/L | [45] |
| α-Pinene | E. coli BW25113 | PS from Pinus taeda | Whole-cell biocatalysis/50 mL | Glucose | 28 h, 166.50 mg/L | [47] |
| E. coli MG1655 | PS from Abies grandis | Shake-flask fermentation/10 mL | Glucose | 48 h, 165.10 mg/L | [48] | |
| Sabinene | E. coli BL21(DE3) | SabS1 from Salvia pomifera | Fed-batch fermentation/5 L | Glycerol | 24 h, 2.65 g/L | [49] |
| Myrcene | E. coli MG1655 | CsMS from Cannabis sativa | Shake-flask fermentation/10 mL | Glucose | 72 h, 510.38 mg/L | [70] |
| γ-Terpinene | E. coli BL21 (DE3) | γ-Terpinene synthase Thymus vulgaris | Fed-batch fermentation/2 L | Glycerol | 72 h, 275.41 mg/L | [71] |
| 1,8-Cineole | R. toruloides IFO0880 | HYP3 from Hypoxylon sp. E7406B | Fed-batch fermentation/2 L | Corn stover hydrolysate | 168 h, 1.4 g/L | [50] |
| Geraniol | E. coli DH5α | GES from Valeriana officinalis | Shake-flask fermentation | Glycerol | 48 h, 2.12 g/L | [72] |
| S. cerevisiae CEN.PK102-5B | geraniol synthase from Valeriana officinalis | Fed-batch fermentation/1 L | Glucose | 120 h, 1.69 g/L | [73] | |
| (S)-Linalool | P. ananatis | AaLINS from Actinidia arguta | Test tube/5 mL | Glucose | 24 h, 5.6 g/L | [51] |
| (R)-Linalool | P. ananatis | ScLINS from Streptomyces clavuligerus | Test tube/5 mL | Glucose | 24 h, 3.71 g/L | [51] |
| Nerolidol | S. cerevisiae CEN.PK2-1C | NES from Actinidia chinensis | Shake-flask fermentation/50 mL | Glucose | 72 h, 4.2 g/L | [74] |
| Sesquiterpene | ||||||
| β-Farnesene | S. cerevisiae CEN.PK2-1C | FS from Artemisia annua | Industrial bioreactors/200,000 L | Glucose and sucrose | 2.24 g/L/h, 130 g/L | [52] |
| Y. lipolytica Po1f | FS from A. annua | Fed-batch fermentation/2 L | Glucose | 240 h, 28.9 g/L | [53] | |
| Y. lipolytica Po1f | FS from A. annua | Fed-batch fermentation/5 L | Waste cooking oil | 216 h, 35.2 g/L | [54] | |
| Y. lipolytica MYA-2613 | FS from A. annua | Fed-batch fermentation/2 L | Glucose | 175 h, 22.8 g/L | [75] | |
| α-Farnesene | S. cerevisiae CEN.PK2-1D | AFS from Malus domestica | Fed-batch fermentation/15 L | Glucose and sucrose | 120 h, 38.8 g/L | [32] |
| S. cerevisiae CEN.PK113-5D | CsAFS from Camellia sinensis | Fed-batch fermentation/5 L | Glucose and sucrose | 145 h, 28.3 g/L | [76] | |
| Y. lipolytica Po1f | FS from apple seeds | Fed-batch fermentation/1 L | Glucose | 288 h, 25.5 g/L | [77] | |
| Pentalenene | S. cerevisiae CEN.PK2-1D | PentS from Streptomyces exfoliatus | Fed-batch fermentation/15 L | Glucose and sucrose | 120 h, 10.8 g/L | [32] |
| Presilphiperfol-1-ene | S. cerevisiae CEN.PK2-1D | Cgl06493-COP from Colletotrichum gloeosporioides | Fed-batch fermentation/15 L | Glucose and sucrose | 120 h, 22.7 g/L | [32] |
| β-Copaene | S. cerevisiae CEN.PK2-1D | Copu2 from Coniophora puteana | Fed-batch fermentation/15 L | Glucose and sucrose | 120 h, 6.8 g/L | [32] |
| Epi-isozizaene | S. cerevisiae CEN.PK2-1D | SCO5222 from Streptomyces | Fed-batch fermentation/15 L | Glucose and sucrose | 120 h, 4.7 g/L | [32] |
| Thujopsene | S. cerevisiae CEN.PK2-1D | BarS from Arabidopsis thaliana | Fed-batch fermentation/15 L | Glucose and sucrose | 120 h, 1.2 g/L | [32] |
| α-Barbatene | S. cerevisiae CEN.PK2-1D | BarS from Arabidopsis thaliana | Fed-batch fermentation/15 L | Glucose and sucrose | 120 h, 1.6 g/L | [32] |
| Protoilludene | S. cerevisiae CEN.PK2-1D | OMP7 from Omphalotus olearius | Fed-batch fermentation/15 L | Glucose and sucrose | 120 h, 12.1 g/L | [32] |
| α-Bisabolene | Y. lipolytica Po1g | BIS from Abies grandis | Fed-batch fermentation/5 L | Waste cooking oil | 144 h, 15.5 g/L | [55] |
| β-Bisabolene | Y. lipolytica Po1g | BS from Zingiber officinale | Shake-flask fermentation/25 mL | Glucose | 120 h, 68.2 mg/L | [78] |
| γ-Bisabolene | S. cerevisiae CEN.PK2-1D | AcTPS5 from Acremonium chrysogenum | Fed-batch fermentation/5 L | Glucose and ethanol | 34.7 h, 2.69 g/L | [56] |
| Germacrene A | Y. lipolytica W29 | dlGAS from Daldinia loculata | Fed-batch fermentation/5 L | Glucose | 240 h, 39 g/L | [57] |
| (-)-Germacrene D | S. cerevisiae CEN.PK2-1D | AcTPS1 from A. chrysogenum | Fed-batch fermentation/5 L | Glucose and ethanol | 94 h, 7.9 g/L | [58] |
| (+)-Bicyclogermacrene | E. coli BL21(DE3) | PeTS4 from Penicillium expansum | Shake-flask fermentation/50 mL | Glucose | 72 h, 188 mg/L | [59] |
| Valencene | S. cerevisiae CEN.PK2-1D | EgVS from Eryngium glaciale | Fed-batch fermentation/15 L | Glucose and sucrose | 120 h, 16.6 g/L | [79] |
| Premnaspirodiene | S. cerevisiae BY4741 | HPS from Hyoscyamus muticus | Shake-flask fermentation/30 mL | Glucose | 144 h, 170.23 mg/L | [80] |
| Caryophyllene | E. coli BL21(DE3) | TPS7 from Nicotiana tabacum | Fed-batch fermentation/5 L | Glucose | 72 h, 5.14 g/L | [81] |
| α-Humulene | Y. lipolytica Po1f | ACHS2 from Aquilaria crassna | Fed-batch fermentation/5 L | Glucose | 40 h, 21.7 g/L | [82] |
| δ-Cadinene | S. cerevisiae CEN.PK2-1C | LsSqTPS2 from Leonurus sibiricus L. | Shake-flask fermentation/50 mL | Glucose | 120 h, 76.23 mg/L | [83] |
| Longifolene | S. cerevisiae CEN.PK113-5D | psTPS from Pinus sylvestris | Fed-batch fermentation | Glucose | 180 h, 1.24 g/L | [84] |
| α-Santalene | K. phaffii | SAS | Fed-batch fermentation | Glucose | 90 h, 21.5 | [85] |
| α-Isocomene | E. coli DH1 | MrTPS2 from Matricaria recutita | Shake-flask fermentation | Glucose | 72 h, 77.5 mg/L | [38] |
| (-)-Eremophilene | S. cerevisiae CEN.PK2-1D | OsaTPS07 from Ocimum sanctum | Fed-batch fermentation/5 L | Glucose and ethanol | 90 h, 34.6 g/L | [60] |
| Zizaene | E. coli BL21(DE3) | ZS from Chrysopogon zizanioides | Fed-batch fermentation/2 L | Glucose | 72 h, 211.13 mg/L | [86] |
| Viridiflorol | E. coli MG1655 | VS from Agrocybe aegerita | Fed-batch fermentation/250 ml | Glucose | 60 h, 25.7 g/L | [61] |
| Patchoulol | Y. lipolytica Po1f | Patchoulol synthase from Pogostemon cablin | Fed-batch fermentation/5 L | Glucose | 180 h, 2.86 g/L | [87] |
| τ-Cadinol | E. coli BL21(DE3) | τ-Cadinol synthase from Lavandula angustifolia | Fed-batch fermentation/5 L | Glucose | 168 h, 15.2 g/L | [88] |
| Nerolidol | S. cerevisiae CEN.PK2-1C | NES from Actinidia chinensis | Shake-flask fermentation/50 mL | Glucose | 72 h, 4.2 g/L | [74] |
4. Strategies for Constructing Efficient Microbial Cell Factories for Terpenes
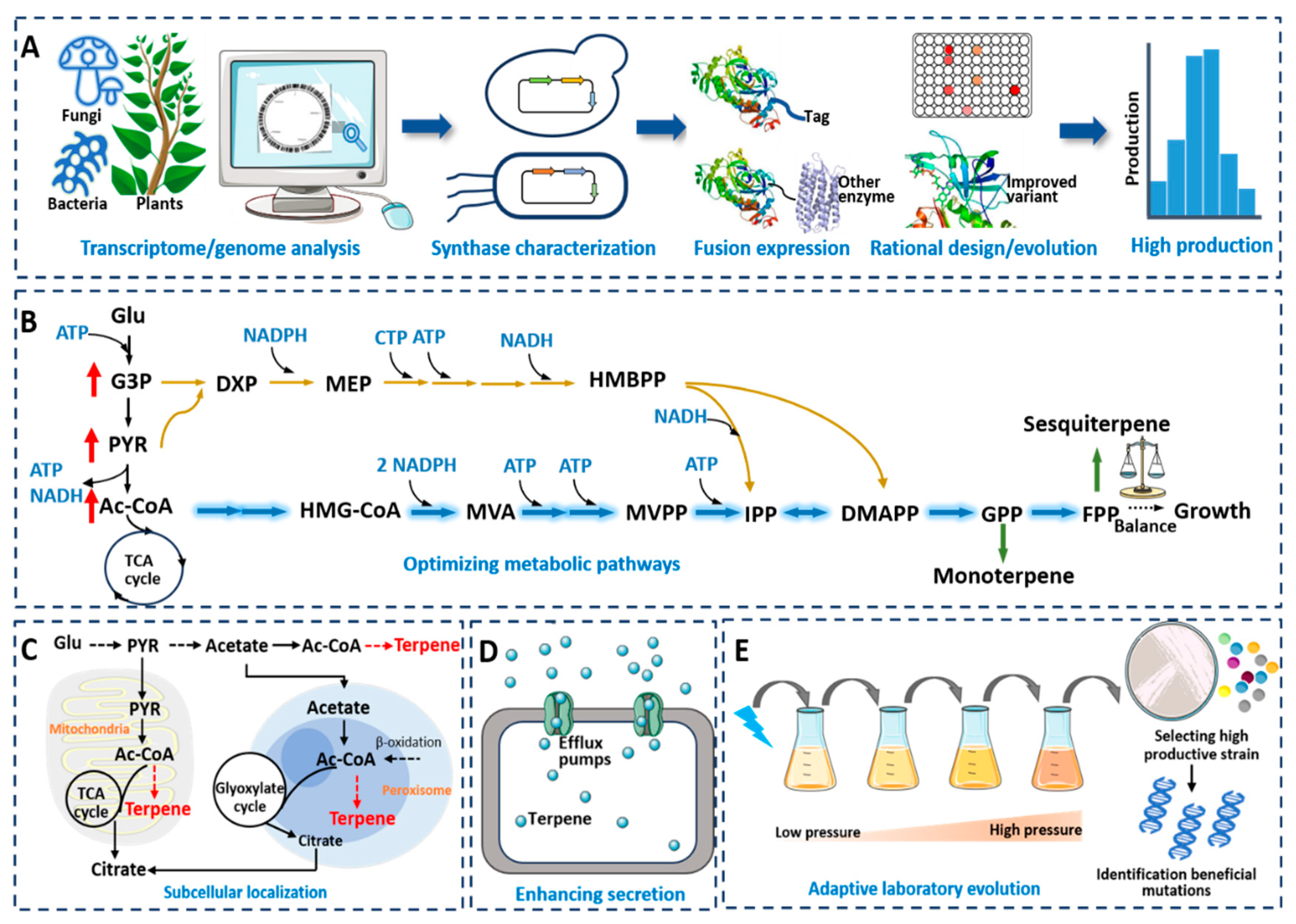
4.1. Terpene Synthase Mining and Engineering
4.1.1. Mining of Terpene Synthases
4.1.2. Engineering of Terpene Synthases
4.2. Optimizing Metabolic Pathways
4.2.1. Optimization of MEP and MVA Pathways
4.2.2. Optimization of the Upstream and Downstream Pathways
4.3. Cell-Level Optimization
4.3.1. Subcellular Localization of Terpene Synthesis
4.3.2. Enhancing Product Efflux Efficiency
4.3.3. Cell Mutagenesis and Adaptive Evolution
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chung, H.S.; Chen, C.S.H.; Kremer, R.A.; Boulton, J.R. Recent developments in high-energy density liquid hydrocarbon fuels. Energy Fuels 1999, 13, 641–649. [Google Scholar] [CrossRef]
- Pan, L.; Deng, Q.; Xiu, T.F.; Nie, G.K.; Zhang, X.W.; Zou, J.J. Synthesis chemistry of high-density fuels for aviation and aerospace propulsion. Prog. Chem. 2015, 27, 1531–1541. [Google Scholar]
- Zhang, X.; Pan, L.; Wang, L.; Zou, J.J. Review on synthesis and properties of high-energy-density liquid fuels: Hydrocarbons, nanofluids and energetic ionic liquids. Chem. Eng. Sci. 2018, 180, 95–125. [Google Scholar] [CrossRef]
- John, J.E.; Isaac, S.A.; Elwood, W.R. Process for Isomerisation of Tetraidromdimetildiciclopentadiene. U.S. Patent 4288644A, 8 September 1981. [Google Scholar]
- Xiong, Z.Q.; Mi, Z.T.; Zhang, X.W.; Xing, E.H. Development of synthesized high-density hydrocarbon fuels. Prog. Chem. Beijing 2005, 17, 359–367. [Google Scholar]
- Bruno, T.J.; Huber, M.L.; Laesecke, A.R.; Lemmon, E.W.; Perkins, R.A. Thermochemical and Thermophysical Properties of JP-10; NIST Interagency/Internal Report (NISTIR)-6640; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2006. [Google Scholar]
- Zhou, J.S.; Feng, J.C.; Zhang, Z.Y. Synthesis of high density hydrocarbon fuel from cyclopentadiene for cruise missile. Chem. Propellants Polym. Mater. 2003, 1, 25. [Google Scholar]
- Du, Z.G.; Shi, X.M.; Fu, Q.J. Development status and prospect of higher energy liquid propellant. J. Rocket. Propuls. 2005, 31, 30–34. [Google Scholar]
- Alexander, D.D.; Vladimir, A.B.; Chernoivanov, V.A. Norbornadiene-quadricyclane as an abiotic system for the storage of solar energy. Russ. Chem. Rev. 2002, 71, 917–927. [Google Scholar]
- Feng, X.T.; Pan, L.; Wang, F.; Wang, L.; Zhang, X.; Zou, J.J. Al-nanoparticle-containing nanofluid fuel: Synthesis, stability, properties, and propulsion performance. Ind. Eng. Chem. Res. 2016, 55, 2738–2745. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Wang, C.L.; Pfleger, B.F.; Kim, S.W. Reassessing Escherichia coli as a cell factory for biofuel production. Curr. Opin. Biotechnol. 2017, 45, 92–103. [Google Scholar] [CrossRef]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd, A.A.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar]
- Mewalal, R.; Rai, D.K.; Kainer, D.; Chen, F.; Külheim, C.; Peter, G.F.; Tuskan, G.A. Plant-derived terpenes: A feedstock for specialty biofuels. Trends Biotechnol. 2017, 35, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, T.; Tang, K.; Zhou, X.; Lu, M.; Ounkham, W.L.; Spain, S.M.; Frost, B.J.; Lin, H. Highly efficient conversion of terpenoid biomass to Jet-fuel range cycloalkanes in a biphasic tandem catalytic process. Green Chem. 2017, 19, 3566–3573. [Google Scholar] [CrossRef]
- Meylemans, H.A.; Quintana, R.L.; Harvey, B.G. Efficient conversion of pure and mixed terpene feedstocks to high density fuels. Fuel 2012, 97, 560–568. [Google Scholar] [CrossRef]
- Zou, J.J.; Zhang, X.W.; Wang, L.; Wang, Q.F. The Invention Relates to a Mixed Jet Fuel Containing bio-Fuel and a Preparation Method. Chinese Patent 103013589A, 3 April 2013. [Google Scholar]
- Tracy, N.I.; Chen, D.; Crunkleton, D.W.; Price, G.L. Hydrogenated monoterpenes as diesel fuel additives. Fuel 2009, 88, 2238–2240. [Google Scholar] [CrossRef]
- Chuck, C.J.; Donnelly, J. The compatibility of potential bioderived fuels with Jet A-1 aviation kerosene. Appl. Energy 2014, 118, 83–91. [Google Scholar] [CrossRef]
- Nie, G.; Zou, J.J.; Feng, R.; Zhang, X.; Wang, L. HPW/MCM-41 catalyzed isomerization and dimerization of pure pinene and crude turpentine. Catal. Today 2014, 234, 271–277. [Google Scholar] [CrossRef]
- Meylemans, H.A.; Baldwin, L.C.; Harvey, B.G. Low-temperature properties of renewable high-density fuel blends. Energy Fuels 2023, 27, 883–888. [Google Scholar] [CrossRef]
- Sutton, G.P. History of liquid propellant rocket engines in the United States. J. Propuls. Power 2003, 19, 978. [Google Scholar] [CrossRef]
- Cruz-Morales, P.; Yin, K.; Landera, A.; Cort, J.R.; Young, R.P.; Kyle, J.E.; Bertrand, R.; Iavarone, A.T.; Acharya, S.; Cowan, A.; et al. Biosynthesis of polycyclopropanated high energy biofuels. Joule 2022, 6, 1590–1605. [Google Scholar] [CrossRef]
- Langlois, A.; Lebel, O. To cyclopropanate or not to cyclopropanate? A look at the effect of cyclopropanation on the performance of biofuels. Energy Fuels 2010, 24, 5257–5263. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Shi, C.; Pan, L.; Zou, J.J. Synthesis of strained high-energy rocket bio-kerosene via cyclopropanation of myrcene. Fuel Process. Technol. 2020, 201, 106339. [Google Scholar] [CrossRef]
- Woodroffe, J.D.; Harvey, B.G. Chemoselective hydrogenation of ring-strained monoterpenes: A route to high-performance sustainable aviation fuels. Energy Technol. 2021, 9, 2100221. [Google Scholar] [CrossRef]
- Woodroffe, J.D.; Lupton, D.V.; Garrison, M.D.; Nagel, E.M.; Harvey, B.G. Synthesis and fuel properties of high-energy density cyclopropanated monoterpenes. Fuel Process. Technol. 2021, 222, 106952. [Google Scholar] [CrossRef]
- Brennan, T.C.; Williams, T.C.; Schulz, B.L.; Palfreyman, R.W.; Krömer, J.O.; Nielsen, L.K. Evolutionary engineering improves tolerance for replacement Jet fuels in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2015, 81, 3316–3325. [Google Scholar] [CrossRef] [PubMed]
- Meylemans, H.A.; Quintana, R.L.; Rex, M.L.; Harvey, B.G. Low-temperature, solvent-free dehydration of cineoles with heterogeneous acid catalysts for the production of high-density biofuels. J. Chem. Technol. Biotechnol. 2014, 89, 957–962. [Google Scholar] [CrossRef]
- Meylemans, H.A.; Quintana, R.L.; Goldsmith, B.R.; Harvey, B.G. Solvent-free conversion of linalool to methylcyclopentadiene dimers: A route to renewable high-density fuels. Chemsuschem 2011, 4, 465–469. [Google Scholar] [CrossRef]
- Keller, C.L.; Doppalapudi, K.R.; Woodroffe, J.D.; Harvey, B.G. Solvent-free dehydration, cyclization, and hydrogenation of linalool with a dual heterogeneous catalyst system to generate a high-performance sustainable aviation fuel. Commun. Chem. 2022, 5, 113. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, Z.; Wan, X.; Yao, G.; Duan, J.; Liu, J.; Yao, M.; Sun, X.; Deng, Z.; Shen, K.; et al. Systematic mining and evaluation of the sesquiterpene skeletons as high energy aviation fuel molecules. Adv. Sci. 2023, 10, e2300889. [Google Scholar] [CrossRef]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef]
- Harvey, B.G.; Meylemans, H.A.; Gough, R.V.; Quintana, R.L.; Garrison, M.D.; Bruno, T.J. High-density biosynthetic fuels: The intersection of heterogeneous catalysis and metabolic engineering. Phys. Chem. Chem. Phys. 2014, 16, 9448–9457. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.G.; Merriman, W.W.; Koontz, T.A. High-density renewable diesel and jet fuels prepared from multicyclic sesquiterpanes and a 1-hexene-derived synthetic paraffinic kerosene. Energy Fuels 2015, 29, 150220083739002. [Google Scholar] [CrossRef]
- Harvey, B.G.; Harrison, K.W. High Density Turbine and Diesel Fuels from Tricyclic Sesquiterpenes. U.S. Patent 10106475, 23 October 2018. [Google Scholar]
- Harrisona, K.W.; Harvey, B.G. Renewable high density fuels containing tricyclic sesquiterpanes and alkyl diamondoids. Sustain. Energy Fuels 2017, 1, 467–473. [Google Scholar] [CrossRef]
- Liu, C.L.; Tian, T.; Gutierrez, J.A.; Garabedian, B.; Wang, S.; Baidoo, E.K.; Benites, V.; Chen, Y.; Petzold, C.J.; Adams, P.D.; et al. Renewable production of high density jet fuel precursor sesquiterpenes from Escherichia coli. Biotechnol. Biofuels 2018, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Geiselman, G.M.; Kirby, J.; Landera, A.; Otoupal, P.; Papa, G.; Barcelos, C.; Sundstrom, E.R.; Das, L.; Magurudeniya, H.D.; Wehrs, M.; et al. Conversion of poplar biomass into high-energy density tricyclic sesquiterpene jet fuel blendstocks. Microb. Cell Fact. 2020, 19, 208. [Google Scholar] [CrossRef] [PubMed]
- Butcher, M.G.; Meyer, P.A.; Hallen, R.T.; Albrecht, K.O.; Clayton, C.K.; Polikarpov, E.; Rappe, K.G.; Jones, S.B.; Magnuson, J.K. Fungal metabolites as precursors to renewable transportation fuels. Fuel 2018, 215, 123–141. [Google Scholar] [CrossRef]
- Woodroffe, J.D.; Harvey, B.G. High-performance, biobased, jet fuel blends containing hydrogenated monoterpenes and synthetic paraffinic kerosenes. Energy Fuels 2020, 34, 5929–5937. [Google Scholar] [CrossRef]
- Ryder, J.A. Jet Fuel Compositions. U.S. Patent US7935156B2, 3 May 2011. [Google Scholar]
- Maurya, R.; Gohil, N.; Nixon, S.; Kumar, N.; Noronha, S.B.; Dhali, D.; Trabelsi, H.; Alzahrani, K.J.; Reshamwala, S.S.; Awasthi, M.K.; et al. Rewiring of metabolic pathways in yeasts for sustainable production of biofuels. Bioresour. Technol. 2023, 372, 128668. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X. Biosynthesis of monoterpenoid and sesquiterpenoid as natural flavors and fragrances. Biotechnol. Adv. 2023, 65, 108151. [Google Scholar] [CrossRef]
- Rolf, J.; Julsing, M.K.; Rosenthal, K.; Lütz, S. A gram-scale limonene production process with engineered Escherichia coli. Molecules 2020, 25, 1881. [Google Scholar] [CrossRef]
- Tashiro, M.; Kiyota, H.; Noma, S.K.; Saito, K.; Ikeuchi, M.; Iijima, Y.; Umeno, D. Bacterial production of pinene by a laboratory-evolved pinene-synthase. ACS Synth. Biol. 2016, 5, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.X.; He, X.; Wu, Y.Q.; Liu, J.Z. Enhancing production of pinene in Escherichia coli by using a combination of tolerance, evolution, and modular co-culture engineering. Front. Microbiol. 2018, 9, 1623. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Jiang, H.; Zhu, L.; Yao, G.; Han, P.; Wan, X.; Wang, K.; Song, T.; Liu, C.; Wang, S.; et al. A dynamic and multilocus metabolic regulation strategy using quorum-sensing-controlled bacterial small RNA. Cell Rep. 2021, 36, 109413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Q.; Cao, Y.; Feng, X.; Zheng, Y.; Zou, H.; Liu, H.; Yang, J.; Xian, M. Microbial production of sabinene—A new terpene-based precursor of advanced biofuel. Microb. Cell. Fact. 2014, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.; Geiselman, G.M.; Yaegashi, J.; Kim, J.; Zhuang, X.; Tran-Gyamfi, M.B.; Prahl, J.P.; Sundstrom, E.R.; Gao, Y.; Munoz, N.; et al. Further engineering of R. toruloides for the production of terpenes from lignocellulosic biomass. Biotechnol. Biofuels 2021, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.; Moriya, M.; Matsudaira, A.; Katashkina, J.; Nitta, N.; Nishio, Y.; Usuda, Y. Stereospecific linalool production utilizing two-phase cultivation system in Pantoea ananatis. J. Biotechnol. 2020, 324, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Meadows, T.M.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Xu, C.; Bao, Y.; Zhang, C.; Wang, K.; Zhang, Y.; Wang, M.; Chen, B.; Fang, Y.; Tan, T. Enhancing precursor supply and modulating metabolism to achieve high-level production of β-farnesene in Yarrowia lipolytica. Bioresour. Technol. 2023, 382, 129171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Li, Q.; Wang, Z.; Cui, Z.; Su, T.; Lu, X.; Qi, Q.; Hou, J. Engineering Yarrowia lipolytica for the sustainable production of β-farnesene from waste oil feedstock. Biotechnol. Biofuels Bioprod. 2022, 15, 101. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Wang, Y.; Lu, Z.; Miao, L.; Wang, S.; Li, Z.; Sun, X.; Han, Y.; He, S. Biosynthesis of α-bisabolene from low-cost renewable feedstocks by peroxisome engineering and systems metabolic engineering of the yeast Yarrowia lipolytica. Green Chem. 2023, 25, 8145–8159. [Google Scholar] [CrossRef]
- Liu, J.; Yao, G.; Wan, X.; Wang, F.; Han, P.; Bao, S.; Wang, K.; Song, T.; Jiang, H. Highly efficient biosynthesis of γ-bisabolene with a new sesquiterpene synthase AcTPS5 by dual cytoplasmic-peroxisomal engineering in Saccharomyces cerevisiae. Fermentation 2023, 9, 779. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, G.; Su, L.; Liu, P.; Jia, S.; Wang, Q.; Dai, Z. Reprogramming the metabolism of oleaginous yeast for sustainably biosynthesizing the anticarcinogen precursor germacrene A. Green Chem. 2023, 25, 7988–7997. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Wan, X.; Yao, G.; Bao, S.; Wang, F.; Wang, K.; Song, T.; Han, P.; Jiang, H. Identification of the sesquiterpene synthase AcTPS1 and high production of (-)-germacrene D in metabolically engineered Saccharomyces cerevisiae. Microb. Cell Fact. 2022, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, Q.; Li, C.; Yu, H.; Xu, J. Facile production of (+)-aristolochene and (+)-bicyclogermacrene in Escherichia coli using newly discovered sesquiterpene synthases from Penicillium expansum. J. Agric. Food Chem. 2022, 70, 5860–5868. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shi, B.; Ye, Z.; Huang, M.; Chen, R.; Cai, Y.; Kuang, Z.; Sun, X.; Bian, G.; Deng, Z. Systematic identification of Ocimum sanctum sesquiterpenoid synthases and (−)-eremophilene overproduction in engineered yeast. Metab. Eng. 2022, 69, 122–133. [Google Scholar] [CrossRef]
- Shukal, S.; Chen, X.; Zhang, C. Systematic engineering for high-yield production of viridiflorol and amorphadiene in auxotrophic Escherichia coli. Metab. Eng. 2019, 55, 170–178. [Google Scholar] [CrossRef]
- Stoppacher, N.; Kluger, B.; Zeilinger, S.; Krska, R.; Schuhmacher, R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods 2010, 81, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Strobel, G.; Yan, D. The production of 1,8-cineole, a potential biofuel, from an endophytic strain of Annulohypoxylon sp. FPYF3050 when grown on agricultural residues. J. Sustain. Bioenergy Syst. 2017, 7, 65–84. [Google Scholar] [CrossRef]
- Dutta, K.; Daverey, A.; Lin, J.G. Evolution retrospective for alternative fuels: First to fourth generation. Renew. Energy 2014, 69, 114–122. [Google Scholar] [CrossRef]
- Hernández-García, A.; Velásquez-Orta, S.B.; Novelo, E.; Yáñez-Noguez, I.; Monje-Ramírez, I.; Orta Ledesma, M.T. Wastewater-leachate treatment by microalgae: Biomass, carbohydrate and lipid production. Ecotoxicol. Environ. Saf. 2019, 174, 435–444. [Google Scholar] [CrossRef]
- Abedi, S.; Astaraei, F.R.; Ghobadian, B.; Tavakoli, O.; Jalili, H.; Greenwell, H.C.; Cummins, I.; Chivasa, S. Decoupling a novel Trichormus variabilis-Synechocystis sp. interaction to boost phycoremediation. Sci. Rep. 2019, 9, 2511. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Zhang, F.; Pakrasi, H.B. Enhanced limonene production in a fast-growing cyanobacterium through combinatorial metabolic engineering. Metab. Eng. Commun. 2021, 12, e00164. [Google Scholar] [CrossRef] [PubMed]
- Blanc-Garin, V.; Chenebault, C.; Diaz-Santos, E.; Vincent, M.; Sassi Jean-François Cassier-Chauvat, C.; Chauvat, F. Exploring the potential of the model cyanobacterium Synechocystis PCC 6803 for the photosynthetic production of various high-value terpenes. Biotechnol. Biofuels Bioprod. 2022, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, S.B.; Sun, T.; Chen, L.; Zhang, W.W. Light and carbon dioxide-driven synthesis of high-density fuel in Synechococcus elongates UTEX 2973. Chin. J. Biotechnol. 2020, 36, 2126–2138. [Google Scholar]
- Chen, C.; Liu, J.; Yao, G.; Bao, S.; Wan, X.; Wang, F.; Wang, K.; Song, T.; Han, P.; Liu, T.; et al. A novel, genetically encoded whole-cell biosensor for directed evolution of myrcene synthase in Escherichia coli. Biosens. Bioelectron. 2023, 228, 115176. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhao, H.; Li, W.; Li, X.; Xiang, H.; Zhang, G.; Liu, H.; Wang, Q.; Wang, Y.; Xian, M. Production of γ-terpinene by metabolically engineered Escherichia coli using glycerol as feedstock. RSC Adv. 2018, 8, 30851–30859. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Zhang, J.; Zhou, Y.; Zhang, Y.; Wang, F.; Li, X. Engineering Escherichia coli for production of geraniol by systematic synthetic biology approaches and laboratory-evolved fusion tags. Metab. Eng. 2021, 66, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, C.; Zhang, Y.; Shen, Y.; Hou, J.; Bao, X. Dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism contributes to the improvement of geraniol production in Saccharomyces cerevisiae. Microb. Cell. Fact. 2017, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Cheah, L.C.; Liu, L.; Stark, T.; Plan, M.R.; Peng, B.; Lu, Z.; Schenk, G.; Sainsbury, F.; Vickers, C.E. Metabolic flux enhancement from the translational fusion of terpene synthases is linked to terpene synthase accumulation. Metab. Eng. 2023, 77, 143–151. [Google Scholar] [CrossRef]
- Shi, T.; Li, Y.; Zhu, L.; Tong, Y.; Yang, J.; Fang, Y.; Wang, M.; Zhang, J.; Jiang, Y.; Yang, S. Engineering the oleaginous yeast Yarrowia lipolytica for β-farnesene overproduction. Biotechnol. J. 2021, 16, e2100097. [Google Scholar] [CrossRef]
- Wang, S.; Zhan, C.; Nie, S.; Tian, D.; Lu, J.; Wen, M.; Qiao, J.; Zhu, H.; Caiyin, Q. Enzyme and metabolic engineering strategies for biosynthesis of α-farnesene in Saccharomyces cerevisiae. J. Agric. Food Chem. 2023, 71, 12452–12461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, X.; Cui, Z.; Wang, Z.; Qi, Q.; Hou, J. Engineering the oleaginous yeast Yarrowia lipolytica for production of α-farnesene. Biotechnol. Biofuels 2019, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, K.; Li, J.; Zhao, Y.; Li, S.; Zhang, C.; Xiao, D.; Yu, A. High-efficiency production of bisabolene from waste cooking oil by metabolically engineered Yarrowia lipolytica. Microb. Biotechnol. 2021, 14, 2497–2513. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Huang, Y.; Shi, B.; Xiang, Z.; Tian, Z.; Huang, M.; Wu, L.; Deng, Z.; Shen, K.; Liu, T. Coupling cell growth and biochemical pathway induction in Saccharomyces cerevisiae for production of (+)-valencene and its chemical conversion to (+)-nootkatone. Metab. Eng. 2022, 72, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Chappell, J. Building terpene production platforms in yeast. Biotechnol. Bioeng. 2015, 112, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, K.; Guo, J.; Yang, Q.; Li, Y.; Xian, M.; Zhang, R. Highly efficient biosynthesis of β-caryophyllene with a new sesquiterpene synthase from tobacco. Biotechnol. Biofuels Bioprod. 2022, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Milker, S.; Sydow, A.; Torres-Monroy, I.; Jach, G.; Faust, F.; Kranz, L.; Tkatschuk, L.; Holtmann, D. Gram-scale production of the sesquiterpene α-humulene with Cupriavidus necator. Biotechnol. Bioeng. 2021, 118, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, W.; Liang, D.; Zhao, G.; Caiyin, Q.; Qiao, J. Comparative transcriptome analysis of sesquiterpene biosynthesis and functional characterization of sesquiterpene synthases in Leonurus sibiricus L. Planta 2021, 253, 71. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Du, J.; Wang, K.; Liu, L.; Ba, L.; Liu, H.; Liu, Y. Application of multiple strategies to debottleneck the biosynthesis of longifolene by engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2022, 70, 11336–11343. [Google Scholar] [CrossRef]
- Zuo, Y.; Xiao, F.; Gao, J.; Ye, C.; Jiang, L.; Dong, C.; Lian, J. Establishing Komagataella phaffii as a cell factory for efficient production of sesquiterpenoid α-Santalene. J. Agric. Food Chem. 2022, 70, 8024–8031. [Google Scholar] [CrossRef]
- Aguilar, F.; Scheper, T.; Beutel, S. Improved production and in situ recovery of sesquiterpene (+)-zizaene from metabolically-engineered E. coli. Molecules 2019, 24, 3356. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Guo, Q.; Chen, C.; Song, P.; Wang, Y.; Ji, X.; Ye, C.; Shi, T.Q. High-level production of patchoulol in Yarrowia lipolytica via systematic engineering strategies. J. Agric. Food Chem. 2023, 71, 4638–4645. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Q.; Shen, J.; Li, Y.; Zhang, H.; Zhang, X.; Yang, S.; Jiang, Z.; Wang, M.; Li, J.; et al. Metabolic engineering of glycolysis in Escherichia coli for efficient production of patchoulol and τ-cadinol. Bioresour. Technol. 2024, 391, 130004. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, B.O.; Van Lanen, S.G.; Baltz, R.H. Microbial genome mining for accelerated natural products discovery: Is a renaissance in the making? J. Ind. Microbiol. Biotechnol. 2014, 41, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.; Leferink, N.G.H.; Umemura, M.; Ahmed, S.T.; Breitling, R.; Scrutton, N.S.; Takano, E. Exploring novel bacterial terpene synthases. PLoS ONE 2020, 15, e0232220. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yao, G.; Wang, F.; Bao, S.; Wan, X.; Han, P.; Wang, K.; Song, T.; Jiang, H. Identification of a (+)-cubenene synthase from filamentous fungi Acremonium chrysogenum. Biochem. Biophys. Res. Commun. 2023, 677, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bao, X.; Li, C.; Shen, Y.; Hou, J. Improving monoterpene geraniol production through geranyl diphosphate synthesis regulation in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2016, 100, 4561–4571. [Google Scholar] [CrossRef]
- Hassan, J.; Kaleem, I.; Rasool, A.; Xu, K.; Tahir, A.; Lv, B.; Li, C. Engineered Saccharomyces cerevisiae for the de novo synthesis of the aroma compound longifolene. Chem. Eng. Sci. 2020, 226, 115799. [Google Scholar] [CrossRef]
- Chica, R.A.; Doucet, N.; Pelletier, J.N. Semi-rational approaches to engineering enzyme activity: Combining the benefits of directed evolution and rational design. Curr. Opin. Biotechnol. 2005, 16, 378–384. [Google Scholar] [CrossRef]
- Denby, C.M.; Li, R.A.; Vu, V.T.; Costello, Z.; Lin, W.; Chan, L.J.G.; Williams, J.; Donaldson, B.; Bamforth, C.W.; Petzold, C.J.; et al. Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer. Nat. Commun. 2018, 9, 965. [Google Scholar] [CrossRef]
- Jiang, G.; Yao, M.; Wang, Y.; Xiao, W.; Yuan, Y. A “push-pull-restrain” strategy to improve citronellol production in Saccharomyces cerevisiae. Metab. Eng. 2021, 66, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, J.; Chen, J.; Xiao, L.; Zhang, Y.; Wang, F.; Li, X. Efficient biosynthesis of R-(-)-linalool through adjusting expression strategy and increasing GPP Supply in Escherichia coli. J. Agric. Food Chem. 2020, 68, 8381–8390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Zhang, X.; Zhang, Y.; Wang, F.; Li, X. Sesquiterpene synthase engineering and targeted engineering of α-santalene overproduction in Escherichia coli. J. Agric. Food Chem. 2022, 70, 5377–5385. [Google Scholar] [CrossRef] [PubMed]
- Ignea, C.; Pontini, M.; Maffei, M.E.; Makris, A.M.; Kampranis, S.C. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase. ACS Synth. Biol. 2014, 3, 298. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, L.; Chen, Y.; Bach, L.S.; Rattleff, S.; Maury, J.; Brix, S.; Nielsen, J.; Mortensen, U.H. Diversion of flux toward sesquiterpene production in Saccharomyces cerevisiae by fusion of host and heterologous enzymes. Appl. Environ. Microbiol. 2011, 77, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Sarria, S.; Wong, B.; García Martín, H.; Keasling, J.D.; Peralta-Yahya, P. Microbial synthesis of pinene. ACS Synth. Biol. 2014, 3, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Tippmann, S.; Anfelt, J.; David, F.; Rand, J.M.; Siewers, V.; Uhlen, M.; Nielsen, J.; Hudson, E.P. Affibody scaffolds improve sesquiterpene production in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Rasool, A.; Liu, H.; Lv, B.; Chang, P.; Song, H.; Wang, Y.; Li, C. Engineering Saccharomyces cerevisiae for high yield production of α-amyrin via synergistic remodeling of α-amyrin synthase and expanding the storage pool. Metab. Eng. 2020, 62, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, J.; Wang, Z.; Li, Y.; Meng, X.; Shen, Y.; Liu, W. Improved production of germacrene A, a direct precursor of elemene, in engineered Saccharomyces cerevisiae by expressing a cyanobacterial germacrene A synthase. Microb. Cell. Fact. 2021, 20, 1–15. [Google Scholar] [CrossRef]
- Yoshikuni, Y.; Ferrin, T.E.; Keasling, J.D. Designed divergent evolution of enzyme function. Nature 2006, 20, 1078–1082. [Google Scholar] [CrossRef]
- Zha, W.; Zhang, F.; Shao, J.; Ma, X.; Zhu, J.; Sun, P.; Wu, R.; Zi, J. Rationally engineering santalene synthase to readjust the component ratio of sandalwood oil. Nat. Commun. 2022, 13, 2508. [Google Scholar] [CrossRef]
- Kampranis, S.C.; Ioannidis, D.; Purvis, A.; Mahrez, W.; Ninga, E.; Katerelos, N.A.; Anssour, S.; Dunwell, J.M.; Degenhardt, J.; Makris, A.M.; et al. Rational conversion of substrate and product specificity in a Salvia monoterpene synthase: Structural insights into the evolution of terpene synthase function. Plant Cell 2007, 19, 1994–2005. [Google Scholar] [CrossRef]
- Furubayashi, M.; Ikezumi, M.; Kajiwara, J.; Iwasaki, M.; Fujii, A.; Li, L.; Saito, K.; Umeno, D. A high-throughput colorimetric screening assay for terpene synthase activity based on substrate consumption. PLoS ONE 2014, 9, e93317. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhong, X.; Hu, M.; Lu, L.; Deng, Z.; Liu, T. In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli. Biotechnol. Bioeng. 2014, 111, 1396–1405. [Google Scholar] [CrossRef]
- Tsang, A.; Seidle, H.; Jawaid, S.; Zhou, W.; Smith, C.; Couch, R.D. Francisella tularensis 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase: Kinetic characterization and phosphoregulation. PLoS ONE 2011, 6, e20884. [Google Scholar] [CrossRef] [PubMed]
- Bitok, J.K.; Meyers, C.F. 2C-Methyl-d-erythritol 4-phosphate enhances and sustains cyclodiphosphate synthase IspF activity. ACS Chem. Biol. 2012, 7, 1702–1710. [Google Scholar] [CrossRef]
- Zhou, K.; Zou, R.; Stephanopoulos, G.; Too, H.P. Metabolite profiling identified methylerythritol cyclodiphosphate efflux as a limiting step in microbial isoprenoid production. PLoS ONE 2012, 7, e47513. [Google Scholar] [CrossRef]
- Liu, C.L.; Bi, H.R.; Bai, Z.; Fan, L.H.; Tan, T.W. Engineering and manipulation of a mevalonate pathway in Escherichia coli for isoprene production. Appl. Microbiol. Biotechnol. 2019, 103, 239–250. [Google Scholar] [CrossRef]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef]
- Alonso-Gutierrez, J.; Chan, R.; Batth, T.S.; Adams, P.D.; Keasling, J.D.; Petzold, C.J.; Lee, T.S. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 2013, 19, 33–41. [Google Scholar] [CrossRef]
- Bao, S.H.; Zhang, D.Y.; Meng, E. Improving biosynthetic production of pinene through plasmid recombination elimination and pathway optimization. Plasmid 2019, 105, 102431. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Harada, H.; Yamasaki, K.; Okamoto, S.; Hirase, S.; Tanaka, Y.; Misawa, N.; Utsumi, R. Isolation and functional characterization of a beta-eudesmol synthase, a new sesquiterpene synthase from Zingiber zerumbet Smith. FEBS Lett. 2008, 582, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Park, J.E.; Choi, E.S.; Kim, S.W. Farnesol production in Escherichia coli through the construction of a farnesol biosynthesis pathway—Application of PgpB and YbjG phosphatases. Biotechnol. J. 2016, 11, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, C.; Zhou, J.; Kim, S.W. Combinatorial engineering of hybrid mevalonate pathways in Escherichia coli for protoilludene production. Microb. Cell. Fact. 2016, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Kim, S.K.; Yoon, P.K.; Kang, Y.; Kim, B.S.; Fu, Y.; Sung, B.H.; Jung, H.C.; Lee, D.H.; Kim, S.W.; et al. Fermentative production and direct extraction of (−)-α-bisabolol in metabolically engineered Escherichia coli. Microb. Cell. Fact. 2016, 15, 185. [Google Scholar] [CrossRef] [PubMed]
- Friehs, K. Plasmid Copy Number and Plasmid Stability. New Trends and Developments in Biochemical Engineering; Springer: Cham, Switzerland, 2004; pp. 47–82. [Google Scholar]
- Ou, B.; Garcia, C.; Wang, Y.; Zhang, W.; Zhu, G. Techniques for chromosomal integration and expression optimization in Escherichia coli. Biotechnol. Bioeng. 2018, 115, 2467–2478. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, C.; Bi, C.; Li, Q.; Zhang, X. Combinatory optimization of chromosomal integrated mevalonate pathway for β-carotene production in Escherichia coli. Microb. Cell. Fact. 2016, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gutierrez, J.; Koma, D.; Hu, Q.; Yang, Y.; Chan, L.G.; Petzold, C.J.; Adams, P.D.; Vickers, C.E.; Nielsen, L.K.; Keasling, J.D.; et al. Toward industrial production of isoprenoids in Escherichia coli: Lessons learned from CRISPR-Cas9 based optimization of a chromosomally integrated mevalonate pathway. Biotechnol. Bioeng. 2018, 115, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.J.; Pitera, D.J.; Withers, S.T.; Newman, J.D.; Keasling, J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 2003, 21, 796–802. [Google Scholar] [CrossRef]
- Ohto, C.; Muramatsu, M.; Obata, S.; Sakuradani, E.; Shimizu, S. Overexpression of the gene encoding HMG-CoA reductase in Saccharomyces cerevisiae for production of prenyl alcohols. Appl. Microbiol. Biotechnol. 2009, 82, 837. [Google Scholar] [CrossRef]
- Jackson, B.E.; Hart-Wells, E.A.; Matsuda, S.P. Metabolic engineering to produce sesquiterpenes in yeast. Org. Lett. 2003, 5, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Yao, M.; Wang, Y.; Zhou, L.; Song, T.; Liu, H.; Xiao, W.; Yuan, Y. Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae. Metab. Eng. 2017, 41, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Siemon, T.; Wang, Z.; Bian, G.; Seitz, T.; Ye, Z.; Lu, Y.; Cheng, S.; Ding, Y.; Huang, Y.; Deng, Z.; et al. Semisynthesis of plant-derived englerin A enabled by microbe engineering of guaia-6,10(14)-diene as building block. J. Am. Chem. Soc. 2020, 142, 2760–2765. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wei, L.; Lv, Y.-B.; Chen, J.; Hua, Q. Elevating limonene production in oleaginous yeast Yarrowia lipolytica via genetic engineering of limonene biosynthesis pathway and optimization of medium composition. Biotechnol. Bioprocess. Eng. 2019, 24, 500–506. [Google Scholar] [CrossRef]
- Jung, J.; Lim, J.; Kim, S.; Im, D.; Seok, J.; Lee, S.; Oh, M.; Jung, G. Precise precursor rebalancing for isoprenoids production by fine control of gapA expression in Escherichia coli. Metab. Eng. 2016, 38, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, Y.; Ramos, K.R.M.; Nisola, G.M.; Valdehuesa, K.N.G.; Lee, W.K.; Park, S.J.; Chung, W.J. Combination of entner-doudoroff pathway with MEP increases isoprene production in engineered Escherichia coli. PLoS ONE 2013, 8, e83290. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Y.; Tang, Q.; Kong, W.; Chung, W.J.; Lu, T. MEP pathway-mediated isopentenol production in metabolically engineered Escherichia coli. Microb. Cell Fact. 2014, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zeng, W.; Xu, S.; Zhou, J. Metabolism and strategies for enhanced supply of acetyl-CoA in Saccharomyces cerevisiae. Bioresour. Technol. 2021, 342, 125978. [Google Scholar] [CrossRef]
- Kong, X.; Wu, Y.; Yu, W.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Efficient synthesis of limonene in Saccharomyces cerevisiae using combinatorial metabolic engineering strategies. J. Agric. Food Chem. 2023, 71, 7752–7764. [Google Scholar] [CrossRef]
- Fordjour, E.; Liu, C.; Hao, Y.; Sackey, I.; Yang, Y.; Liu, X.; Li, Y.; Tan, T.; Bai, Z. Engineering Escherichia coli BL21 (DE3) for high-yield production of germacrene A, a precursor of β-elemene via combinatorial metabolic engineering strategies. Biotechnol. Bioeng. 2023, 120, 3039–3056. [Google Scholar] [CrossRef]
- Rodriguez, S.; Denby, C.M.; Van Vu, T.; Baidoo, E.E.; Wang, G.; Keasling, J.D. ATP citrate lyase mediated cytosolic acetyl-CoA biosynthesis increases mevalonate production in Saccharomyces cerevisiae. Microb. Cell Fact. 2016, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, M.A.; Maury, J.; Patil, K.R.; Schalk, M.; Clark, A.; Nielsen, J. Enhancing sesquiterpene production in Saccharomyces cerevisiae through in silico driven metabolic engineering. Metab. Eng. 2009, 11, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lin, Y.C.; Guo, J.J.; Du, M.M.; Tao, X.; Gao, B.; Zhao, M.; Ma, Y.; Wang, F.Q.; Wei, D.Z. High-level production of sesquiterpene patchoulol in Saccharomyces cerevisiae. ACS Synth. Biol. 2021, 10, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, X.; Lai, Y.; Mo, Q.; Yuan, J. High-yielding terpene-based biofuel production in Rhodobacter capsulatus. ACS Synth. Biol. 2021, 10, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, L.; Wang, C.; Choi, E.S.; Kim, S.W. Enhanced performance of the methylerythritol phosphate pathway by manipulation of redox reactions relevant to IspC, IspG, and IspH. J. Biotechnol. 2017, 248, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, T.; Zhang, W.; Xu, J. Cofactor engineering for efficient production of α-farnesene by rational modification of NADPH and ATP regeneration pathway in Pichia pastoris. Int. J. Mol. Sci. 2023, 24, 1767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, C.; Yang, L.; Choi, E.S.; Kim, S.W. Geranyl diphosphate synthase: An important regulation point in balancing a recombinant monoterpene pathway in Escherichia coli. Enzym. Microb. Technol. 2015, 68, 50–55. [Google Scholar] [CrossRef]
- Liu, W.; Xu, X.; Zhang, R.; Cheng, T.; Cao, Y.; Li, X.; Guo, J.; Liu, H.; Xian, M. Engineering Escherichia coli for high-yield geraniol production with biotransformation of geranyl acetate to geraniol under fed-batch culture. Biotechnol. Biofuels 2016, 9, 58. [Google Scholar]
- Peng, B.; Nielsen, L.K.; Kampranis, S.C.; Vickers, C.E. Engineered protein degradation of farnesyl pyrophosphate synthase is an effective regulatory mechanism to increase monoterpene production in Saccharomyces cerevisiae. Metab. Eng. 2018, 47, 83–93. [Google Scholar] [CrossRef]
- Peng, B.; Plan, M.R.; Chrysanthopoulos, P.; Hodson, M.P.; Nielsen, L.K.; Vickers, C.E. A squalene synthase protein degradation method for improved sesquiterpene production in Saccharomyces cerevisiae. Metab. Eng. 2017, 39, 209–219. [Google Scholar] [CrossRef]
- Paramasivan, K.; Mutturi, S. Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit. Rev. Biotechnol. 2017, 37, 974–989. [Google Scholar] [CrossRef]
- Jin, K.; Xia, H.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Compartmentalization and transporter engineering strategies for terpenoid synthesis. Microb. Cell Fact. 2022, 21, 92. [Google Scholar] [CrossRef]
- Dusséaux, S.; Wajn, W.T.; Liu, Y.; Ignea, C.; Kampranis, S.C. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc. Natl. Acad. Sci. USA 2020, 117, 31789–31799. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.L.; Xu, J.Z.; Zhang, W.G. Dual regulation of cytoplasm and peroxisomes for improved α-farnesene production in recombinant Pichia pastoris. ACS Synth. Biol. 2021, 10, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shi, T.Q.; Peng, Q.Q.; Sun, X.M.; Ji, X.J.; Huang, H. Harnessing Yarrowia lipolytica peroxisomes as a subcellular factory for α-humulene overproduction. J. Agric. Food Chem. 2021, 69, 13831–13837. [Google Scholar] [CrossRef]
- Galdieri, L.; Zhang, T.; Rogerson, D.; Lleshi, R.; Vancura, A. Protein acetylation and acetyl coenzyme a metabolism in budding yeast. Eukaryot. Cell 2014, 13, 1472–1483. [Google Scholar] [CrossRef]
- Farhi, M.; Marhevka, E.; Masci, T.; Marcos, E.; Eyal, Y.; Ovadis, M.; Abeliovich, H.; Vainstein, A. Harnessing yeast subcellular compartments for the production of plant terpenoids. Metab. Eng. 2011, 13, 474–481. [Google Scholar] [CrossRef]
- Yee, D.A.; DeNicola, A.B.; Billingsley, J.M.; Creso, J.G.; Subrahmanyam, V.; Tang, Y. Engineered mitochondrial production of monoterpenes in Saccharomyces cerevisiae. Metab. Eng. 2019, 55, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Cao, X.; Liu, W.; Yu, H.; Ye, L. High-level production of linalool by engineered Saccharomyces cerevisiae harboring dual mevalonate pathways in mitochondria and cytoplasm. Enzym. Microb. Technol. 2020, 134, 109462. [Google Scholar] [CrossRef]
- Fordjour, E.; Mensah, E.O.; Hao, Y.; Yang, Y.; Liu, X.; Li, Y.; Liu, C.; Bai, Z. Toward improved terpenoids biosynthesis: Strategies to enhance the capabilities of cell factories. Bioresour. Bioprocess. 2022, 9, 1–33. [Google Scholar] [CrossRef]
- Rea, P.A. Plant ATP-binding cassette transporters. Annu. Rev. Plant Biol. 2007, 58, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Demissie, Z.A.; Tarnowycz, M.; Adal, A.M.; Sarker, L.S.; Mahmoud, S.S. A lavender ABC transporter confers resistance to monoterpene toxicity in yeast. Planta 2019, 249, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Weston, N.; Sharma, P.; Ricci, V.; Piddock, L.V. Regulation of the AcrAB-TolC efflux pump in Enterobacteriaceae. Res. Microbiol. 2018, 169, 425–431. [Google Scholar] [CrossRef]
- Shah, A.A.; Wang, C.; Chung, Y.R.; Kim, J.Y.; Choi, E.S.; Kim, S.W. Enhancement of geraniol resistance of Escherichia coli by MarA overexpression. J. Biosci. Bioeng. 2013, 115, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.C.; Turner, C.D.; Krömer, J.O.; Nielsen, L.K. Alleviating monoterpene toxicity using a two-phase extractive fermentation for the bioproduction of jet fuel mixtures in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 2513–2522. [Google Scholar] [CrossRef] [PubMed]
- Schewe, H.; Mirata, M.A.; Schrader, J. Bioprocess engineering for microbial synthesis and conversion of isoprenoids. Adv. Biochem. Eng. Biotechnol. 2015, 148, 251–286. [Google Scholar]
- Wu, T.; Liu, J.; Li, M.; Zhang, G.; Liu, L.; Li, X.; Men, X.; Xian, M.; Zhang, H. Improvement of sabinene tolerance of Escherichia coli using adaptive laboratory evolution and omics technologies. Biotechnol. Biofuels 2020, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Cao, L.; Wang, K.; Ledesma-Amaro, R.; Ji, X.J. Engineering Yarrowia lipolytica to produce advanced biofuels: Current status and perspectives. Bioresour. Technol. 2021, 341, 125877. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Liu, N.; Eser, B.E.; Guo, Z.; Jensen, P.R.; Stephanopoulos, G. Synthesis of high-titer alka(e)nes in Yarrowia lipolytica is enabled by a discovered mechanism. Nat. Commun. 2020, 11, 6198. [Google Scholar] [CrossRef]
- Godara, A.; Kao, K.C. Adaptive laboratory evolution of β-caryophyllene producing Saccharomyces cerevisiae. Microb. Cell Fact. 2021, 20, 106. [Google Scholar] [CrossRef]
- Yao, Z.; Guo, Y.; Wang, H.; Chen, Y.; Wang, Q.; Nielsen, J.; Dai, Z. A highly efficient transcriptome-based biosynthesis of non-ethanol chemicals in Crabtree negative Saccharomyces cerevisiae. Biotechnol. Biofuels Bioprod. 2023, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Mavrommati, M.; Daskalaki, A.; Papanikolaou, S.; Aggelis, G. Adaptive laboratory evolution principles and applications in industrial biotechnology. Biotechnol. Adv. 2022, 54, 107795. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Lin, M.; Han, P.; Yao, G.; Jiang, H. Biosynthesis Progress of High-Energy-Density Liquid Fuels Derived from Terpenes. Microorganisms 2024, 12, 706. https://doi.org/10.3390/microorganisms12040706
Liu J, Lin M, Han P, Yao G, Jiang H. Biosynthesis Progress of High-Energy-Density Liquid Fuels Derived from Terpenes. Microorganisms. 2024; 12(4):706. https://doi.org/10.3390/microorganisms12040706
Chicago/Turabian StyleLiu, Jiajia, Man Lin, Penggang Han, Ge Yao, and Hui Jiang. 2024. "Biosynthesis Progress of High-Energy-Density Liquid Fuels Derived from Terpenes" Microorganisms 12, no. 4: 706. https://doi.org/10.3390/microorganisms12040706





