Effect of Stool Sampling on a Routine Clinical Method for the Quantification of Six Short Chain Fatty Acids in Stool Using Gas Chromatography–Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Method
2.1. Chemicals
2.2. Preparation of Stool Samples
2.3. SCFA Extraction and Derivatization Method
2.4. GC-MS Conditions
2.5. Method Validation
2.5.1. Extraction Recovery (ER%) and Matrix Effect (ME%)
2.5.2. Linearity and Sensitivity (LOD, LOQ)
2.5.3. Precision and Accuracy
2.5.4. Stability
2.6. Statistical Analysis
3. Results and Discussion
3.1. Method Validation
3.2. Comparison of Sample Storage
3.3. Impact of Lyophilization and Glycerol Addition
3.4. Impact of Freeze–Thawing and +4 °C Stool Storage
3.5. Stability of SCFA after Stool Collection
3.6. Post-Extraction and Derivatization Stability
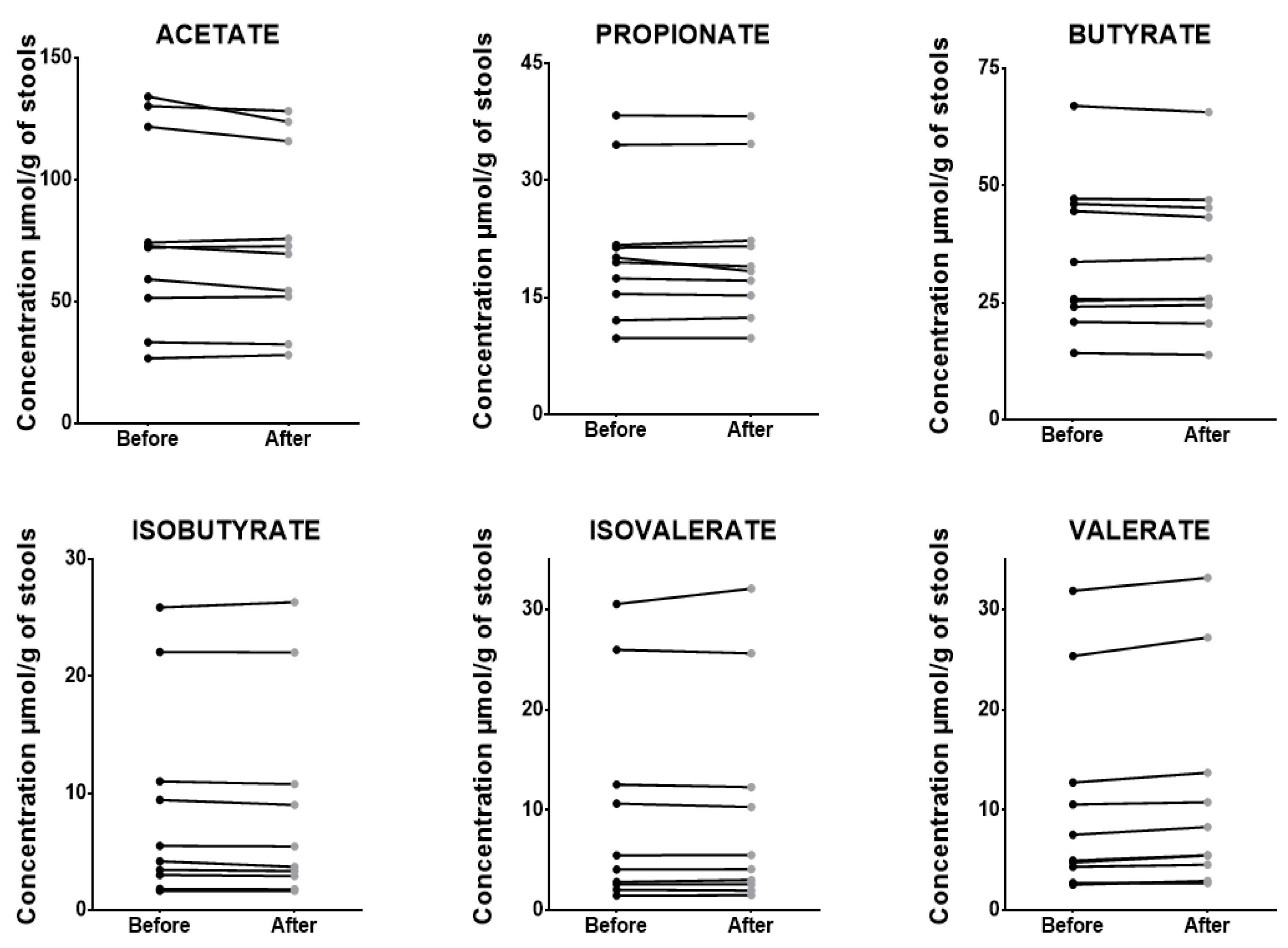
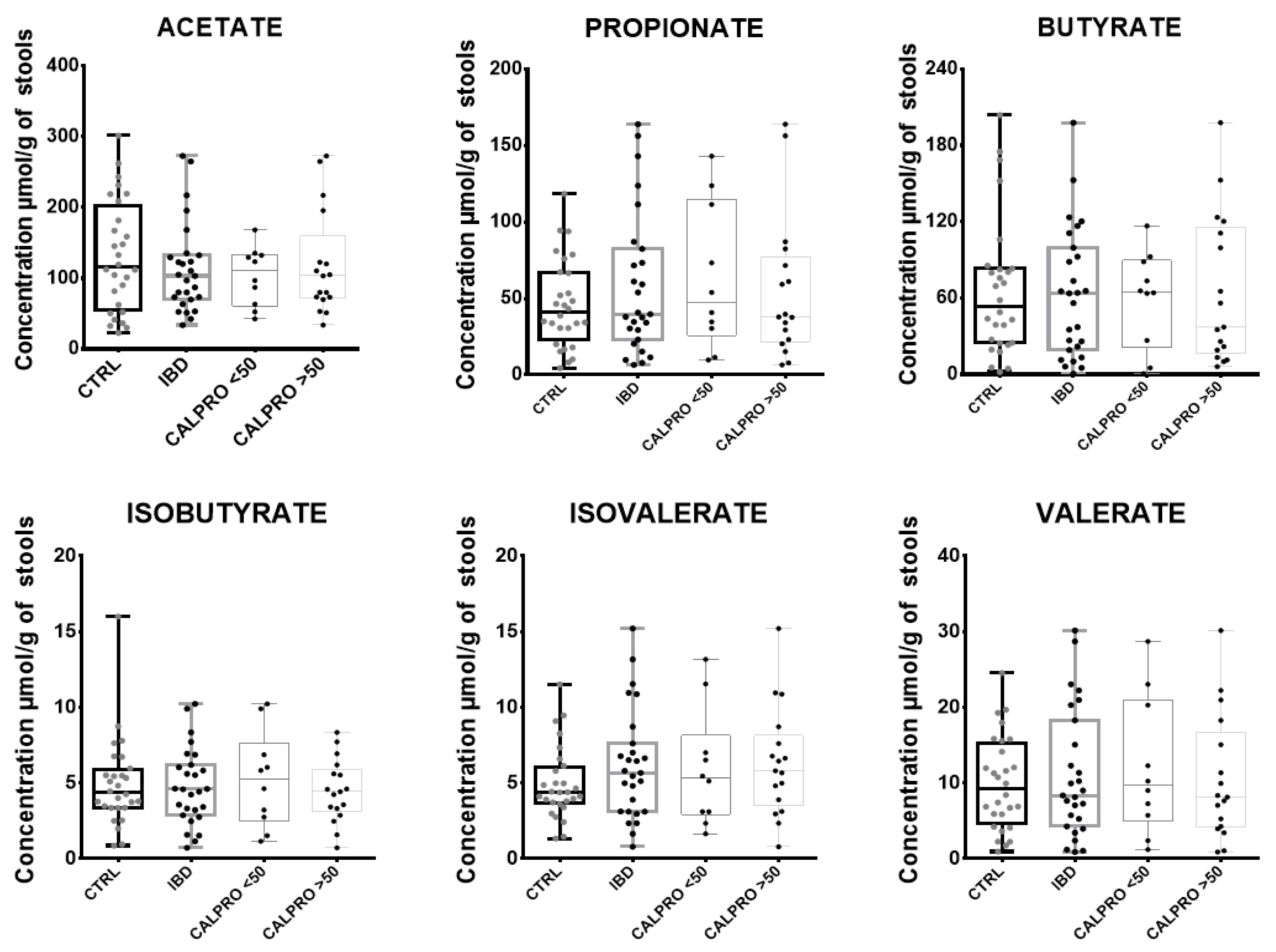
3.7. Application with Clinical Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Albenberg, L.G.; Wu, G.D. Diet and the Intestinal Microbiome: Associations, Functions, and Implications for Health and Disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Food, Immunity, and the Microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef]
- Nyangale, E.P.; Mottram, D.S.; Gibson, G.R. Gut Microbial Activity, Implications for Health and Disease: The Potential Role of Metabolite Analysis. J. Proteome Res. 2012, 11, 5573–5585. [Google Scholar] [CrossRef]
- Turroni, S.; Brigidi, P.; Cavalli, A.; Candela, M. Microbiota–Host Transgenomic Metabolism, Bioactive Molecules from the Inside. J. Med. Chem. 2018, 61, 47–61. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Sellin, C. Review Article: Short Chain Fatty Acids in Health and Disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Primec, M.; Mičetić-Turk, D.; Langerholc, T. Analysis of Short-Chain Fatty Acids in Human Feces: A Scoping Review. Anal. Biochem. 2017, 526, 9–21. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Velázquez, O.C.; Lederer, H.M.; Rombeau, J.L. Butyrate and the Colonocyte. Production, Absorption, Metabolism, and Therapeutic Implications. Adv. Exp. Med. Biol. 1997, 427, 123–134. [Google Scholar]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Al-Qadami, G.H.; Secombe, K.R.; Subramaniam, C.B.; Wardill, H.R.; Bowen, J.M. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms 2022, 10, 2048. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory Role of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Cell Commun. Signal. 2022, 20, 64. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Q.; Luo, M.; Xiong, L. Gut Microbiota-Derived Metabolites in Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 729346. [Google Scholar] [CrossRef]
- Olsson, A.; Gustavsen, S.; Nguyen, T.D.; Nyman, M.; Langkilde, A.R.; Hansen, T.H.; Sellebjerg, F.; Oturai, A.B.; Bach Søndergaard, H. Serum Short-Chain Fatty Acids and Associations With Inflammation in Newly Diagnosed Patients With Multiple Sclerosis and Healthy Controls. Front. Immunol. 2021, 12, 661493. [Google Scholar] [CrossRef]
- Trend, S.; Leffler, J.; Jones, A.P.; Cha, L.; Gorman, S.; Brown, D.A.; Breit, S.N.; Kermode, A.G.; French, M.A.; Ward, N.C.; et al. Associations of Serum Short-Chain Fatty Acids with Circulating Immune Cells and Serum Biomarkers in Patients with Multiple Sclerosis. Sci. Rep. 2021, 11, 5244. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A Decrease of the Butyrate-Producing Species Roseburia Hominis and Faecalibacterium Prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Hu, Y.; Le Leu, R.K.; Christophersen, C.T.; Somashekar, R.; Conlon, M.A.; Meng, X.Q.; Winter, J.M.; Woodman, R.J.; McKinnon, R.; Young, G.P. Manipulation of the Gut Microbiota Using Resistant Starch Is Associated with Protection against Colitis-Associated Colorectal Cancer in Rats. Carcinogenesis 2016, 37, 366–375. [Google Scholar] [CrossRef]
- Toden, S.; Lockett, T.J.; Topping, D.L.; Scherer, B.L.; Watson, E.-J.L.; Southwood, J.G.; Clarke, J.M. Butyrylated Starch Affects Colorectal Cancer Markers Beneficially and Dose-Dependently in Genotoxin-Treated Rats. Cancer Biol. Ther. 2014, 15, 1515–1523. [Google Scholar] [CrossRef]
- Song, H.E.; Lee, H.Y.; Kim, S.J.; Back, S.H.; Yoo, H.J. A Facile Profiling Method of Short Chain Fatty Acids Using Liquid Chromatography-Mass Spectrometry. Metabolites 2019, 9, 173. [Google Scholar] [CrossRef]
- McKay, M.J.; Castaneda, M.; Catania, S.; Charles, K.A.; Shanahan, E.; Clarke, S.J.; Engel, A.; Varelis, P.; Molloy, M.P. Quantification of Short-Chain Fatty Acids in Human Stool Samples by LC-MS/MS Following Derivatization with Aniline Analogues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2023, 1217, 123618. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Ecker, J.; Roth, S.; Schweizer, S.; Öttl, V.; Schött, H.F.; Yoon, H.; Haller, D.; Holler, E.; Burkhardt, R.; et al. Quantification of Fecal Short Chain Fatty Acids by Liquid Chromatography Tandem Mass Spectrometry—Investigation of Pre-Analytic Stability. Biomolecules 2019, 9, 121. [Google Scholar] [CrossRef]
- Eberhart, B.L.; Wilson, A.S.; O’Keefe, S.J.D.; Ramaboli, M.C.; Nesengani, L.T. A Simplified Method for the Quantitation of Short-Chain Fatty Acids in Human Stool. Anal. Biochem. 2021, 612, 114016. [Google Scholar] [CrossRef]
- Scortichini, S.; Boarelli, M.C.; Silvi, S.; Fiorini, D. Development and Validation of a GC-FID Method for the Analysis of Short Chain Fatty Acids in Rat and Human Faeces and in Fermentation Fluids. J. Chromatogr. B 2020, 1143, 121972. [Google Scholar] [CrossRef]
- Furuhashi, T.; Sugitate, K.; Nakai, T.; Jikumaru, Y.; Ishihara, G. Rapid Profiling Method for Mammalian Feces Short Chain Fatty Acids by GC-MS. Anal. Biochem. 2018, 543, 51–54. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; You, Y.; Yin, M.; Ren, C.; Zhan, J.; Huang, W. A Fast and Accurate Way to Determine Short Chain Fatty Acids in Mouse Feces Based on GC–MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1099, 73–82. [Google Scholar] [CrossRef]
- Hoving, L.R.; Heijink, M.; van Harmelen, V.; van Dijk, K.W.; Giera, M. GC-MS Analysis of Short-Chain Fatty Acids in Feces, Cecum Content, and Blood Samples. In Clinical Metabolomics; Giera, M., Ed.; Humana Press: New York, NY, USA, 2018; pp. 247–256. [Google Scholar]
- Ueyama, J.; Oda, M.; Hirayama, M.; Sugitate, K.; Sakui, N.; Hamada, R.; Ito, M.; Saito, I.; Ohno, K. Freeze-Drying Enables Homogeneous and Stable Sample Preparation for Determination of Fecal Short-Chain Fatty Acids. Anal. Biochem. 2020, 589, 113508. [Google Scholar] [CrossRef]
- Van Eijk, H.M.H.; Bloemen, J.G.; Dejong, C.H.C. Application of Liquid Chromatography-Mass Spectrometry to Measure Short Chain Fatty Acids in Blood. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 719–724. [Google Scholar] [CrossRef] [PubMed]
- ISO 15189; Medical Laboratories—Requirements for Quality and Competence. ISO: Geneva, Switzerland, 2022.
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of New Methods. Forensic Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef]
- Mochizuki, A.; Ieki, K.; Kamimori, H.; Nagao, A.; Nakai, K.; Nakayama, A.; Nanba, E. Proposal for Risk-Based Scientific Approach on Full and Partial Validation for General Changes in Bioanalytical Method. Bioanalysis 2018, 10, 577–586. [Google Scholar] [CrossRef]
- Blume, H.; Brendel, E.; Brudny-Klöppel, M.; Grebe, S.; Lausecker, B.; Rohde, G.; Siethoff, C. Workshop/Conference Report on EMA Draft Guideline on Validation of Bioanalytical Methods. Eur. J. Pharm. Sci. 2011, 42, 300–305. [Google Scholar] [CrossRef] [PubMed]
- FDA; ICH. Bioanalytical Method Validation; FDA (International Council for Harmonization of Technical Requirements for Pharmaceuticlas for Human Use): Silver Spring, MD, USA, 2019. Available online: https://www.fda.gov/media/128343/download (accessed on 9 April 2024).
- Reygner, J.; Charrueau, C.; Delannoy, J.; Mayeur, C.; Robert, V.; Cuinat, C.; Meylheuc, T.; Mauras, A.; Augustin, J.; Nicolis, I.; et al. Freeze-Dried Fecal Samples Are Biologically Active after Long-Lasting Storage and Suited to Fecal Microbiota Transplantation in a Preclinical Murine Model of Clostridioides Difficile Infection. Gut Microbes 2020, 11, 1405–1422. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Qiu, Y.; Zhong, W.; Baxter, S.; Su, M.; Li, Q.; Xie, G.; Ore, B.M.; Qiao, S.; Spencer, M.D.; et al. A Targeted Metabolomic Protocol for Short-Chain Fatty Acids and Branched-Chain Amino Acids. Metabolomics 2013, 9, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The Impact of the Level of the Intestinal Short Chain Fatty Acids in Inflammatory Bowel Disease Patients Versus Healthy Subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Lemay, J.-A.; Yamamoto, M.; Kroezen, Z.; Shanmuganathan, M.; Ly, R.; Hart, L.; Pai, N.; Britz-McKibbin, P. Lyophilized Fecal Short-Chain Fatty Acid and Electrolyte Determination by Capillary Electrophoresis with Indirect UV Detection for Assessment of Pediatric Inflammatory Bowel Disease. J. Pharm. Biomed. Anal. 2021, 192, 113658. [Google Scholar] [CrossRef] [PubMed]
- Soubières, A.A.; Poullis, A. Emerging Biomarkers for the Diagnosis and Monitoring of Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Piątek-Guziewicz, A.; Zagrodzki, P.; Mach, T.; Zwolińska-Wcisło, M. Fecal Levels of Lactic, Succinic and Short-Chain Fatty Acids in Patients with Ulcerative Colitis and Crohn Disease: A Pilot Study. J. Clin. Med. 2021, 10, 4701. [Google Scholar] [CrossRef]
- Kaczmarczyk, O.; Dąbek-Drobny, A.; Woźniakiewicz, M.; Paśko, P.; Dobrowolska-Iwanek, J.; Woźniakiewicz, A.; Targosz, A.; Ptak-Belowska, A.; Piątek-Guziewicz, A.; Wcisło, K.; et al. Association between Fecal Levels of Short-Chain Fatty Acids and Serum Pro- and Anti-Inflammatory Cytokines in Patients with Inflammatory Bowel Disease. Folia Med. Cracov. 2022, 62, 43–55. [Google Scholar] [CrossRef]

| Internal Standard | Monitor Ion (M/Z) | Retention Time (min) | Calibration Range (nmol) | LOD (nmol) | LOQ (nmol) | Slope | Intercept | R² | |
|---|---|---|---|---|---|---|---|---|---|
| Acetate | Acetate D4 | 56.1 | 3.86 | 12.89–1500 | 3.87 | 12.89 | 1.424 × 10−4 | 5.580 × 10−4 | 0.991 |
| Propionate | Propianate D5 | 57.1 | 6.64 | 0.76–1500 | 0.2 | 0.76 | 4.922 × 10−4 | 6.517 × 10−4 | 0.996 |
| Butyrate | Butyrate D8 | 71.1 | 9.80 | 18.64–1500 | 6.08 | 18.64 | 2.656 × 10−4 | 5.391 × 10−4 | 0.999 |
| Isobutyrate | Butyrate D8 | 71.1 | 8.30 | 1.86–1500 | 0.54 | 1.86 | 4.481 × 10−4 | 5.908 × 10−4 | 0.999 |
| Valerate | 3-Methyl pentanoic acid | 85.1 | 13.25 | 4.9–1500 | 1.9 | 4.9 | 3.051 × 10−4 | 3.630 × 10−4 | 0.995 |
| Isovalerate | 3-Methyl pentanoic acid | 85.1 | 11.60 | 3.09–1500 | 1.2 | 3.09 | 3.623 × 10−4 | 1.043 × 10−4 | 0.994 |
| Acetate D4 | 60.1 | 3.80 | |||||||
| Propionate D5 | 62.1 | 6.55 | |||||||
| Butyrate D8 | 78.2 | 9.95 | |||||||
| 3-Methyl pentanoic acid | 99.1 | 15.15 |
| Stools | Concentration Intraday (µmol/g) | SD Intraday (µmol/g) | CV Intraday (%) | Concentration Interday (µmol/g) | SD Interday (µmol/g) | CV Interday (µmol/g) | |
|---|---|---|---|---|---|---|---|
| Acetate | 1 | 46.22 | 4.59 | 10% | 45.32 | 0.98 | 2% |
| 2 | 52.78 | 3.69 | 7% | 49.16 | 2.47 | 5% | |
| 3 | 105.41 | 3.86 | 4% | 107.08 | 2.82 | 3% | |
| Propionate | 1 | 13.47 | 0.85 | 6% | 12.29 | 0.18 | 2% |
| 2 | 19.74 | 0.68 | 3% | 19.42 | 0.35 | 2% | |
| 3 | 24.23 | 0.89 | 4% | 23.06 | 0.64 | 3% | |
| Butyrate | 1 | 10.93 | 0.68 | 6% | 9.63 | 0.12 | 1% |
| 2 | 24.23 | 0.84 | 3% | 23.97 | 0.14 | 1% | |
| 3 | 45.31 | 1.84 | 4% | 42.97 | 0.63 | 1% | |
| Isobutyrate | 1 | 2.83 | 0.2 | 7% | 2.62 | 0.06 | 2% |
| 2 | 1.71 | 0.08 | 5% | 1.68 | 0.05 | 3% | |
| 3 | 3.3 | 0.19 | 6% | 3.14 | 0.13 | 4% | |
| Valerate | 1 | 2.97 | 0.19 | 6% | 2.99 | 0.05 | 2% |
| 2 | 2.47 | 0.09 | 4% | 2.81 | 0.05 | 2% | |
| 3 | 4.67 | 0.24 | 5% | 4.95 | 0.22 | 4% | |
| Isovalerate | 1 | 3.3 | 0.25 | 8% | 2.8 | 0.05 | 2% |
| 2 | 1.57 | 0.07 | 5% | 1.52 | 0.03 | 2% | |
| 3 | 2.74 | 0.19 | 7% | 2.53 | 0.06 | 3% |
| Stools | Spiked (nmol) | Matrix Effect | Extraction Recovery | |
|---|---|---|---|---|
| Acetate | Stool 1 | 167 | 95% | 113% |
| 18.5 | 106% | 91% | ||
| Stool 2 | 167 | 109% | 81% | |
| 18.5 | 113% | 87% | ||
| Propionate | Stool 1 | 167 | 91% | 115% |
| 18.5 | 105% | 88% | ||
| Stool 2 | 167 | 93% | 100% | |
| 18.5 | 107% | 91% | ||
| Butyrate | Stool 1 | 167 | 101% | 111% |
| 18.5 | 107% | 87% | ||
| Stool 2 | 167 | 108% | 94% | |
| 18.5 | 112% | 88% | ||
| Isobutyrate | Stool 1 | 167 | 100% | 124% |
| 18.5 | 106% | 97% | ||
| Stool 2 | 167 | 93% | 121% | |
| 18.5 | 108% | 98% | ||
| Valerate | Stool 1 | 167 | 112% | 127% |
| 18.5 | 116% | 96% | ||
| Stool 2 | 167 | 109% | 119% | |
| 18.5 | 123% | 96% | ||
| Isovalerate | Stool 1 | 167 | 115% | 126% |
| 18.5 | 121% | 98% | ||
| Stool 2 | 167 | 109% | 129% | |
| 18.5 | 129% | 96% |
| AA | SD(AA) | PA | SD(PA) | BA | SD(BA) | IBA | SD(IBA) | IVA | SD(IVA) | VA | SD(VA) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
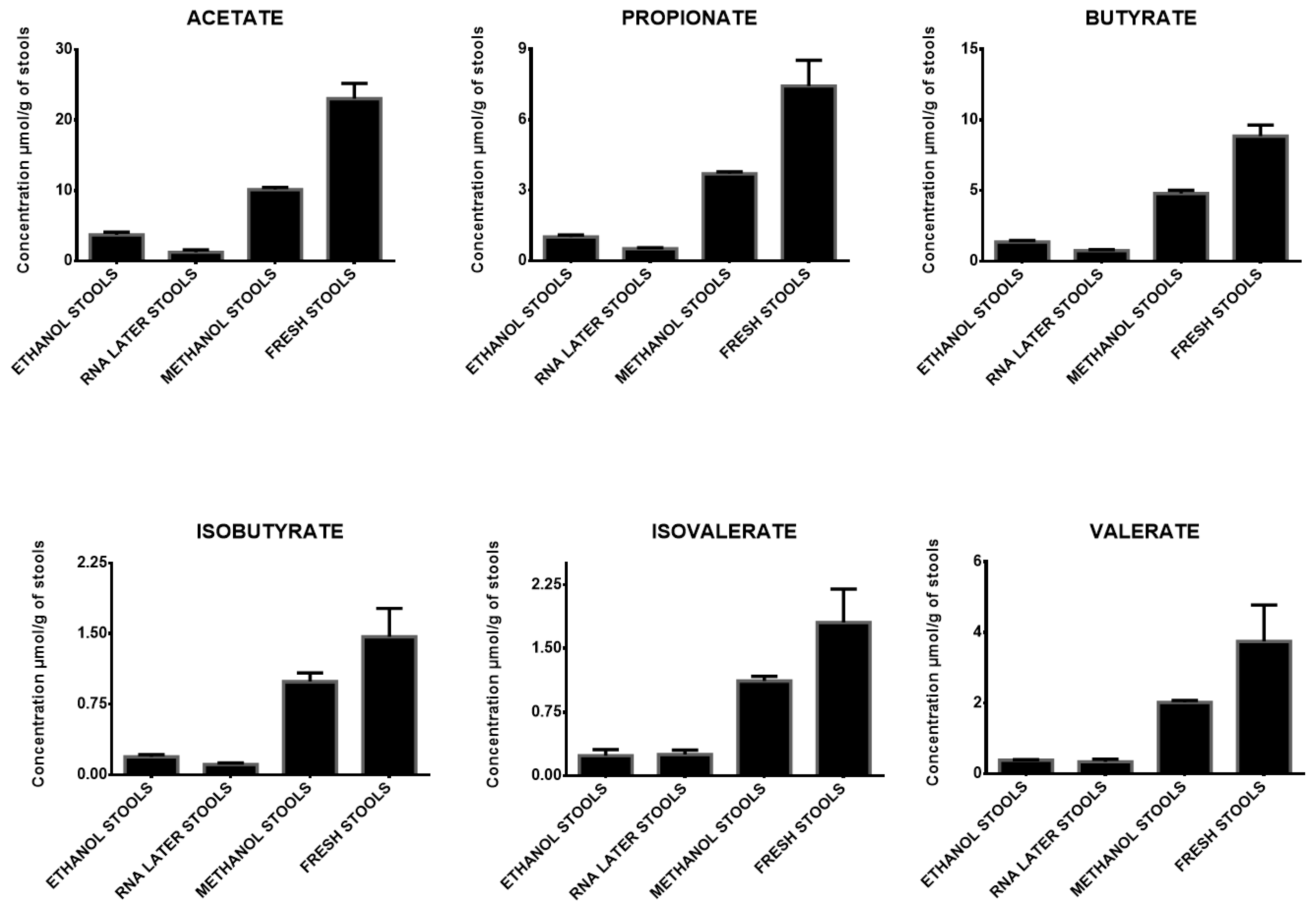
| Mean concentrations in ethanol stools | 3.71 | 0.38 | 1.01 | 0.05 | 1.36 | 0.10 | 0.19 | 0.02 | 0.24 | 0.07 | 0.38 | 0.01 |
| Mean percentages in ethanol stools | 16% | 14% | 15% | 13% | 13% | 10% | ||||||
| Mean concentrations in RNA later stools | 1.26 | 0.31 | 0.52 | 0.03 | 0.76 | 0.05 | 0.11 | 0.02 | 0.25 | 0.05 | 0.34 | 0.07 |
| Mean percentages in RNA later stools | 5% | 7% | 9% | 8% | 14% | 9% | ||||||
| Mean concentrations in methanol stools | 10.13 | 0.33 | 3.70 | 0.08 | 4.80 | 0.23 | 0.99 | 0.09 | 1.12 | 0.05 | 2.02 | 0.05 |
| Mean percentages in methanol stools | 44% | 50% | 54% | 68% | 62% | 54% | ||||||
| Mean concentrations in fresh stools | 23.04 | 2.17 | 7.44 | 0.63 | 8.85 | 0.79 | 1.47 | 0.17 | 1.81 | 0.39 | 3.75 | 1.03 |
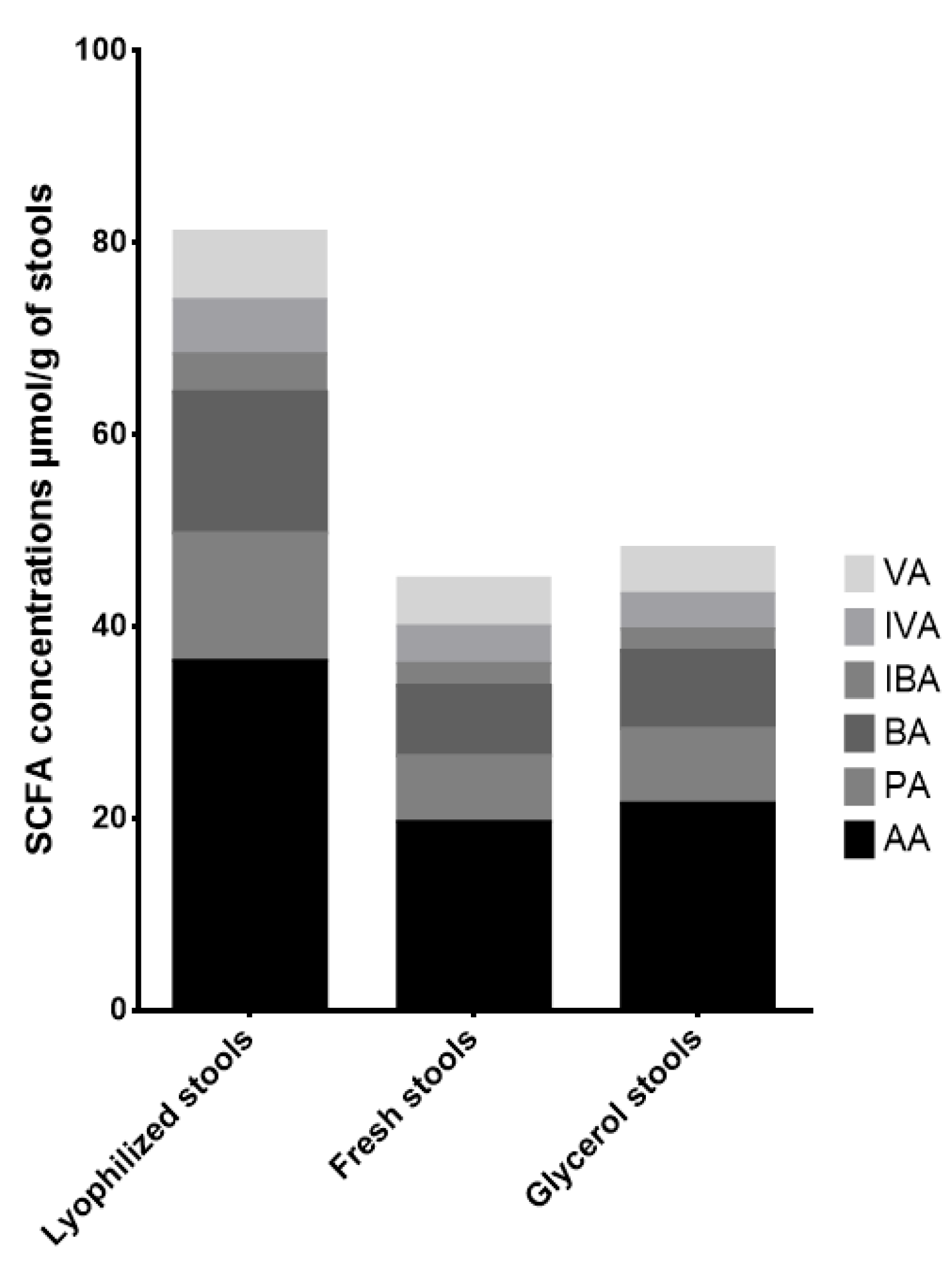
| Lyophilized Stools | Glycerol Stools | ||
|---|---|---|---|
| Acetate | STOOL A | 54% | 0.4% |
| STOOL B | 84% | −0.3% | |
| STOOL C | 117% | 25% | |
| Propionate | STOOL A | 34% | −0.3% |
| STOOL B | 108% | −9% | |
| STOOL C | 134% | 42% | |
| Butyrate | STOOL A | 0.9% | −0.3% |
| STOOL B | 94% | −33% | |
| STOOL C | 165% | 37% | |
| Isobutyrate | STOOL A | 30% | 0.3% |
| STOOL B | 96% | −25% | |
| STOOL C | 100% | −0.1% | |
| Isovalerate | STOOL A | 10% | 0.6% |
| STOOL B | 84% | −35% | |
| STOOL C | 74% | −0.1% | |
| Valerate | STOOL A | −0.7% | 0.2% |
| STOOL B | 70% | −43% | |
| STOOL C | 94% | 14% | |
| Concentration (µmol/g of Stools) | Variation (µmol/g of Stools) | Variation % | ||
|---|---|---|---|---|
| Acetate | fresh stool | 25.55 | ||
| fresh stool 2FT | 21.81 | −3.74 | −15% | |
| fresh stool 5FT | 22.78 | −2.77 | −11% | |
| lyoph stool | 73.29 * | |||
| lyoph stool 2FT | 74.04 * | 0.75 * | 1% | |
| lyoph stool 5FT | 64.28 * | −9.01 * | −12% | |
| Propionate | fresh stool | 8.56 | ||
| fresh stool 2FT | 6.37 | −2.19 | −26% | |
| fresh stool 5FT | 6.25 | −2.31 | −27% | |
| lyoph stool | 30.55 * | |||
| lyoph stool 2FT | 28.27 * | −2.28 * | −7% | |
| lyoph stool 5FT | 23.58 * | −6.97 * | −23% | |
| Butyrate | fresh stool | 9.63 | ||
| fresh stool 2FT | 8.05 | −1.58 | −16% | |
| fresh stool 5FT | 8 | −1.63 | −17% | |
| lyoph stool | 42.92 * | |||
| lyoph stool 2FT | 44.07 * | 1.15 * | 3% | |
| lyoph stool 5FT | 30.56 * | −12.36 * | −29% | |
| Isobutyrate | fresh stool | 1.66 | ||
| fresh stool 2FT | 1.12 | −0.54 | −33% | |
| fresh stool 5FT | 0.93 | −0.73 | −44% | |
| lyoph stool | 7.84 * | |||
| lyoph stool 2FT | 5.36 * | −2.48 * | −32% | |
| lyoph stool 5FT | 3.89 * | −3.95 * | −50% | |
| Valerate | fresh stool | 4.28 | ||
| fresh stool 2FT | 2.56 | −1.72 | −40% | |
| fresh stool 5FT | 2.12 | −2.16 | −50% | |
| lyoph stool | 20.27 * | |||
| lyoph stool 2FT | 13.1 * | −7.17 * | −35% | |
| lyoph stool 5FT | 10.37 * | −9.9 * | −49% | |
| Isovalerate | fresh stool | 2.02 | ||
| freshstool 2FT | 1.35 | −0.67 | −33% | |
| fresh stool 5FT | 1.01 | −1.01 | −50% | |
| lyoph stool | 8.29 * | |||
| lyoph stool 2FT | 7.63 * | −0.66 * | −8% | |
| lyoph stool 5FT | 4.35 * | −3.94 * | −48% |
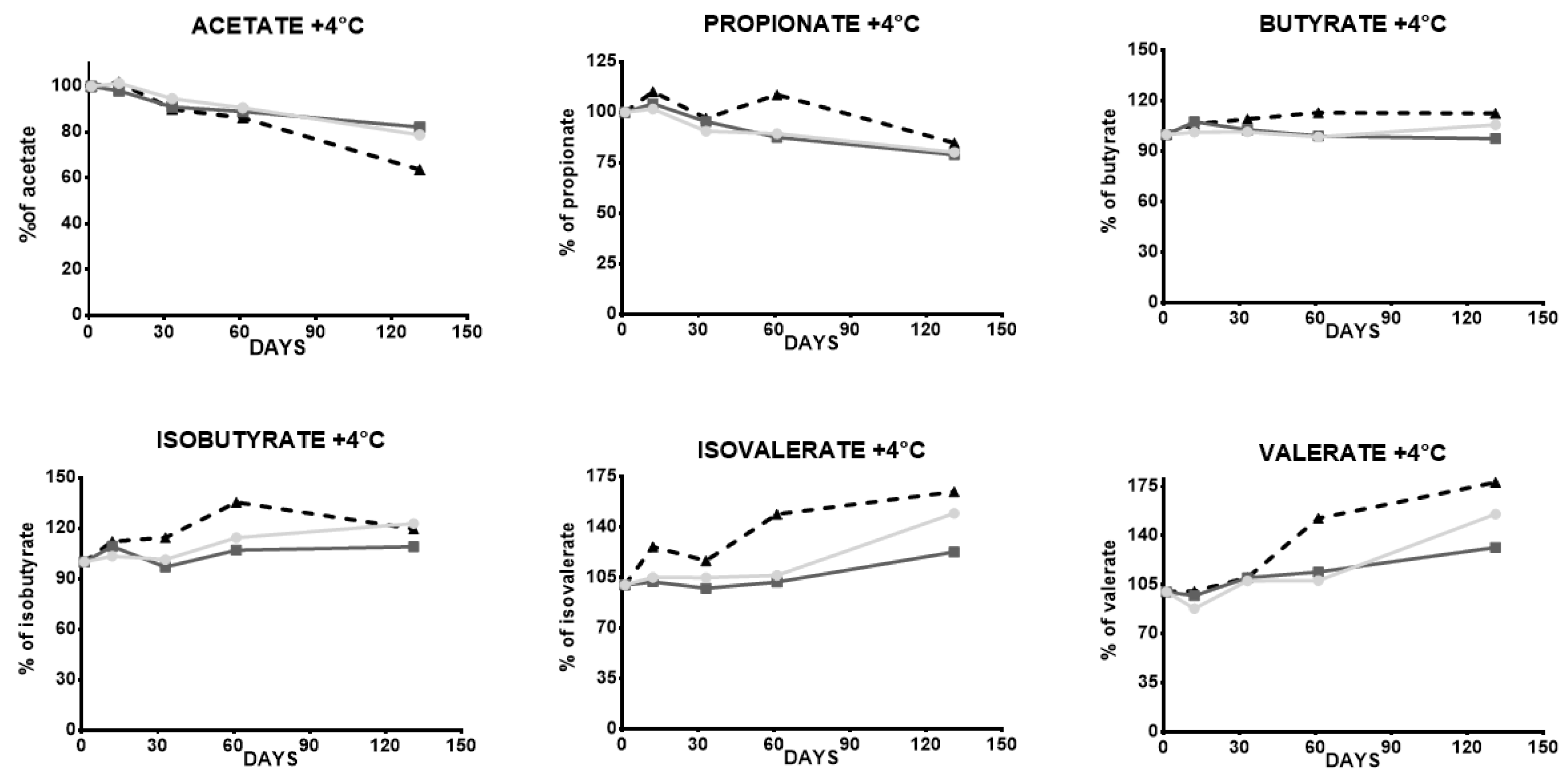
| 12 Days | 33 Days | 60 Days | 130 Days | ||
|---|---|---|---|---|---|
| Acetate | STOOL 1 | 2% | −5% | −9% | −21% |
| STOOL 2 | −2% | −9% | −11% | −18% | |
| STOOL 3 | 2% | −10% | −14% | −36% | |
| Propionate | STOOL 1 | 1% | −9% | −11% | −20% |
| STOOL 2 | 4% | −4% | −12% | −21% | |
| STOOL 3 | 10% | −3% | 9% | −15% | |
| Butyrate | STOOL 1 | 1% | 2% | −1% | 6% |
| STOOL 2 | 8% | 3% | −1% | −2% | |
| STOOL 3 | 6% | 9% | 13% | 12% | |
| Isobutyrate | STOOL 1 | 3% | 1% | 15% | 23% |
| STOOL 2 | 9% | −3% | 7% | 9% | |
| STOOL 3 | 12% | 14% | 35% | 20% | |
| Isovalerate | STOOL 1 | 5% | 5% | 6% | 50% |
| STOOL 2 | 2% | −2% | 2% | 23% | |
| STOOL 3 | 26% | 17% | 49% | 64% | |
| Valerate | STOOL 1 | −12% | 8% | 8% | 56% |
| STOOL 2 | −3% | 10% | 14% | 31% | |
| STOOL 3 | 1% | 10% | 52% | 78% |
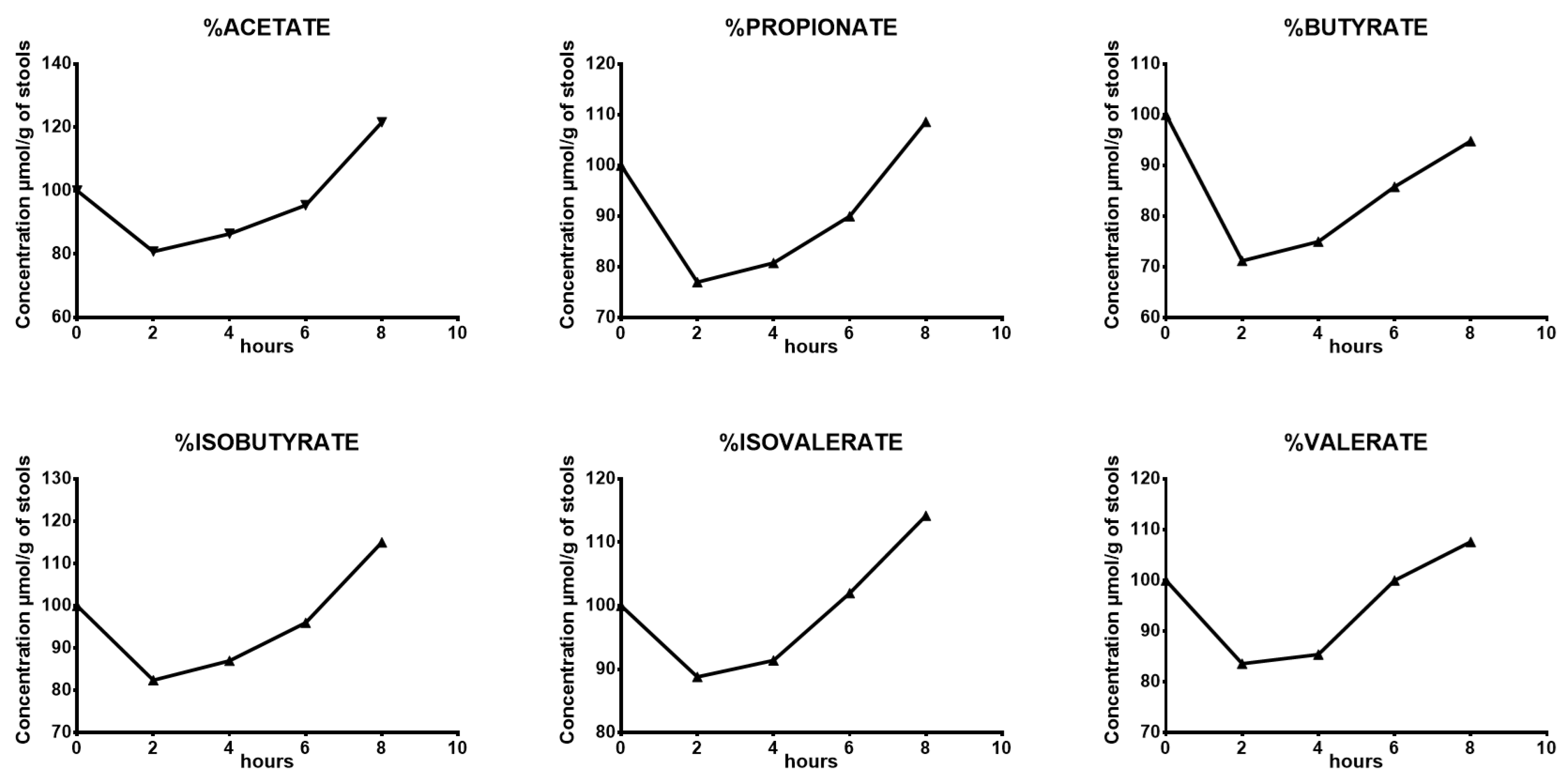
| 2 Hours | 4 Hours | 6 Hours | 8 Hours | |
|---|---|---|---|---|
| Acetate | −19% | −14% | −5% | 21% |
| Propionate | −23% | −19% | −10% | 9% |
| Butyrate | −29% | −25% | −14% | −5% |
| Isobutyrate | −17% | −13% | −4% | 15% |
| Isovalerate | −11% | −9% | 2% | 14% |
| Valerate | −16% | −15% | 0% | 8% |
| AA | PA | BA | IBA | IVA | VA | |
|---|---|---|---|---|---|---|
| Mean concentrations in lyophilized stool before storage | 73.29 | 30.55 | 42.92 | 7.84 | 8.29 | 20.27 |
| Mean concentrations in lyophilized stool after storage | 69.14 | 26.66 | 39.57 | 7.01 | 7.72 | 20.88 |
| Mean variation percentages in lyophilized stool after storage | −6% | −13% | −8% | −11% | −7% | 3% |
| Mean concentrations in fresh stool before storage | 25.55 | 8.56 | 9.63 | 1.66 | 2.02 | 4.28 |
| Mean concentrations in fresh stool after storage | 23.77 | 7.39 | 8.86 | 1.62 | 2.05 | 4.40 |
| Mean variation percentages in fresh stool after storage | −7% | −14% | −8% | −2% | 2% | 3% |
| SCFA (µmol/g of Stools) | CTRL | SD of CTRL | Range of CTRL | IBD | SD of IBD | Range of IBD |
|---|---|---|---|---|---|---|
| Acetate | 151.2 | 84.3 | 22.6–301.6 | 163.7 | 94.0 | 45.1–364 |
| Propionate | 77.5 | 61.6 | 4.6–118.6 | 58.5 | 37.3 | 8.8–219 |
| Isobutyrate | 6.3 | 3.4 | 0.9–16 | 6.5 | 4.2 | 1–13.6 |
| Butyrate | 84.2 | 68.0 | 1.9–203.7 | 81.3 | 59.4 | 0.8–263.8 |
| Isovalérate | 8.2 | 4.8 | 1.3–11.5 | 6.5 | 3.5 | 1–20.3 |
| Valérate | 14.7 | 11.3 | 0.9–24.5 | 12.7 | 8.6 | 1.2–40.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdi, T.; Desmons, A.; Krasniqi, P.; Lacorte, J.-M.; Kapel, N.; Lamazière, A.; Fourati, S.; Eguether, T. Effect of Stool Sampling on a Routine Clinical Method for the Quantification of Six Short Chain Fatty Acids in Stool Using Gas Chromatography–Mass Spectrometry. Microorganisms 2024, 12, 828. https://doi.org/10.3390/microorganisms12040828
Mahdi T, Desmons A, Krasniqi P, Lacorte J-M, Kapel N, Lamazière A, Fourati S, Eguether T. Effect of Stool Sampling on a Routine Clinical Method for the Quantification of Six Short Chain Fatty Acids in Stool Using Gas Chromatography–Mass Spectrometry. Microorganisms. 2024; 12(4):828. https://doi.org/10.3390/microorganisms12040828
Chicago/Turabian StyleMahdi, Tarek, Aurore Desmons, Pranvera Krasniqi, Jean-Marc Lacorte, Nathalie Kapel, Antonin Lamazière, Salma Fourati, and Thibaut Eguether. 2024. "Effect of Stool Sampling on a Routine Clinical Method for the Quantification of Six Short Chain Fatty Acids in Stool Using Gas Chromatography–Mass Spectrometry" Microorganisms 12, no. 4: 828. https://doi.org/10.3390/microorganisms12040828





