Comparative Genomics Reveals Genetic Diversity and Variation in Metabolic Traits in Fructilactobacillus sanfranciscensis Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Genome Sequencing and Assembly
2.2. Genomic Characteristics Prediction and Pan-Genome and Core Genes Analysis
2.3. Phylogenetic Analysis and Determination of the Average Nucleotide Identity (ANI) Value
2.4. Genotypic and Phenotypic Analysis of Carbohydrate Metabolism
2.5. Genotypic Analysis of EPS-Producing Strains
2.6. Genotypic and Phenotypic Analysis of Antibiotic Resistance
2.7. CRISPR Identification and Bacteriocin-Derived Genes Analysis
3. Results
3.1. Genome Characteristics of F. sanfranciscensis
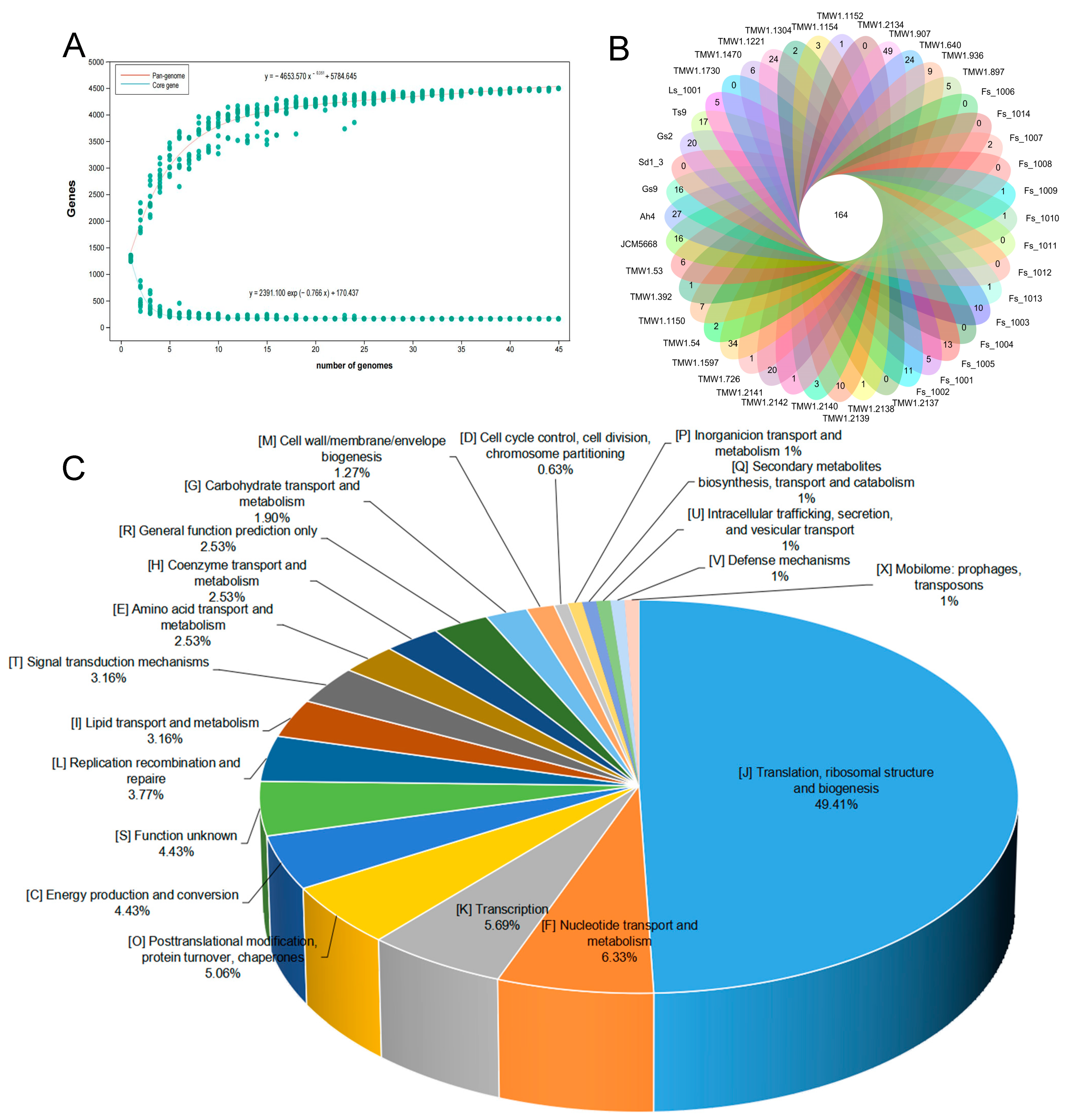
3.2. Pan-Genome and Core Genes of F. sanfranciscensis
3.3. ANI and Phylogenetic Analysis of F. sanfranciscensis
3.4. Genotypic and Phenotypic Analysis of Carbohydrate Metabolism
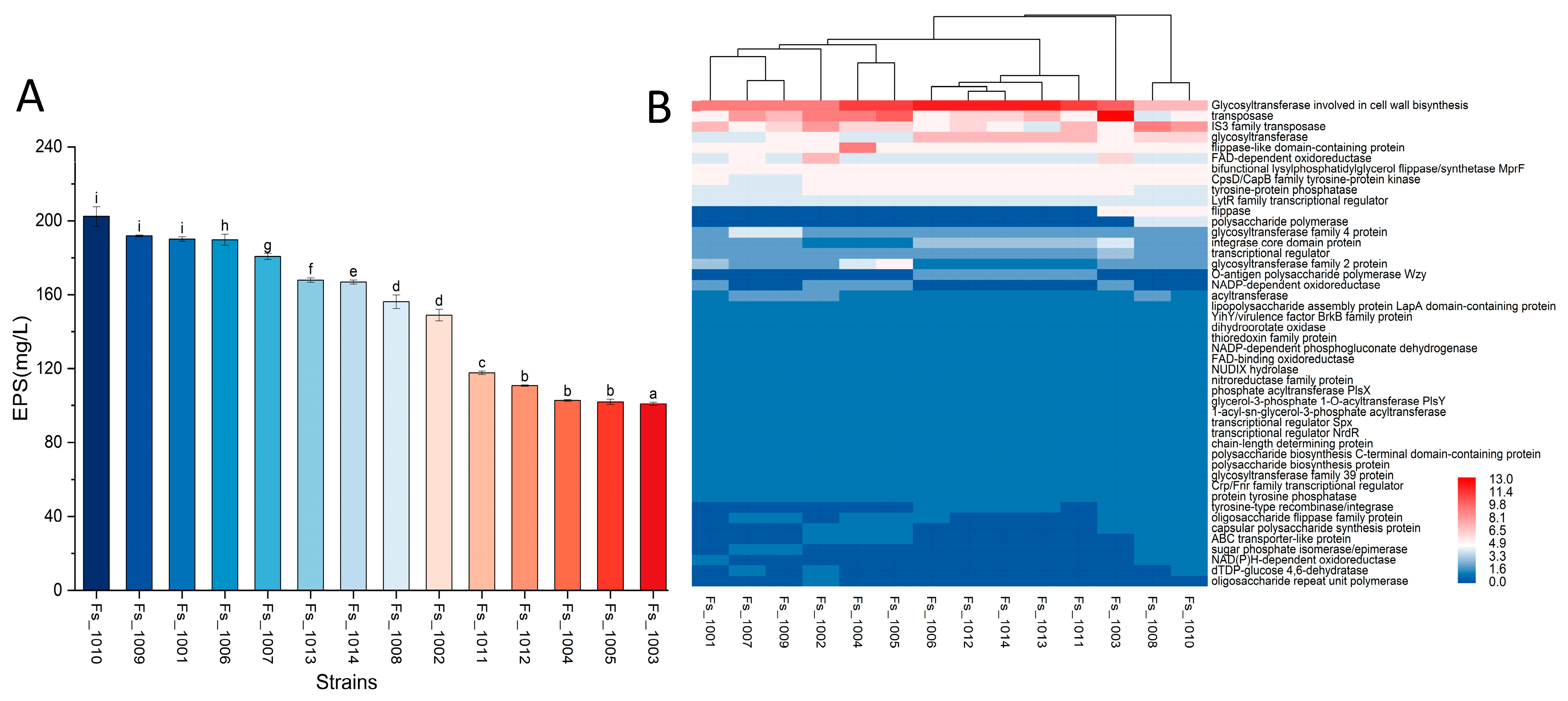
3.5. Genotypic Analysis of EPS
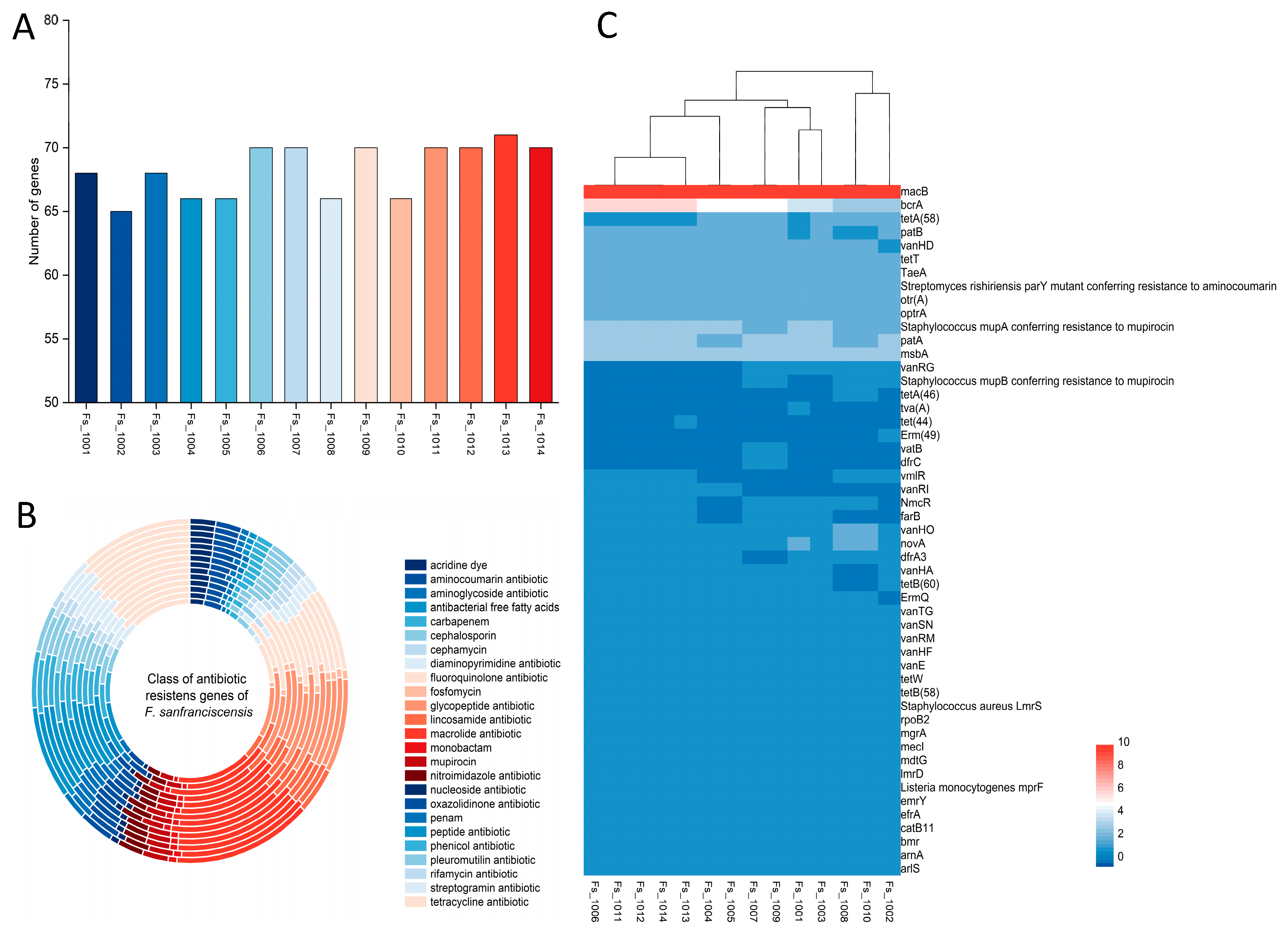
3.6. Phenotypic and Genotypic Analysis of Antibiotic Resistance
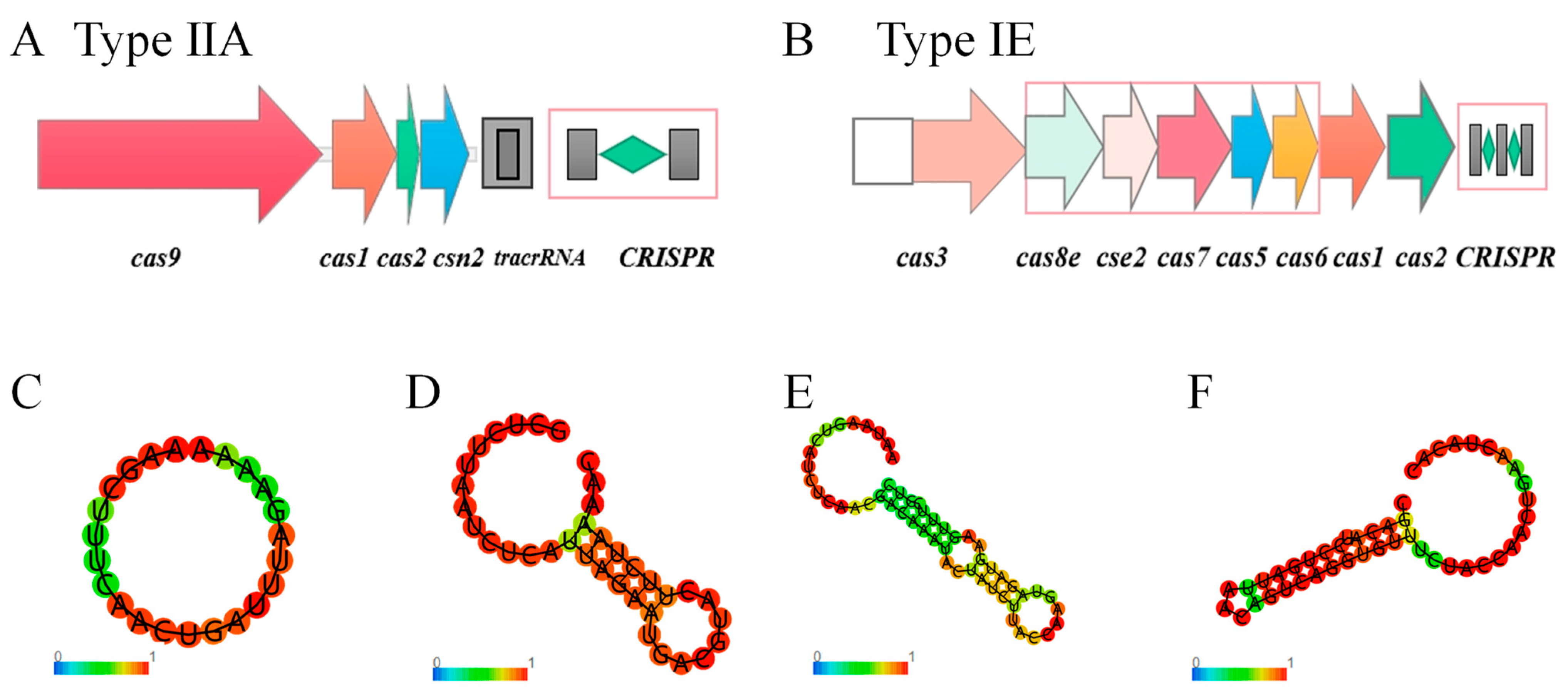
3.7. CRISPR Identification of F. sanfranciscensis
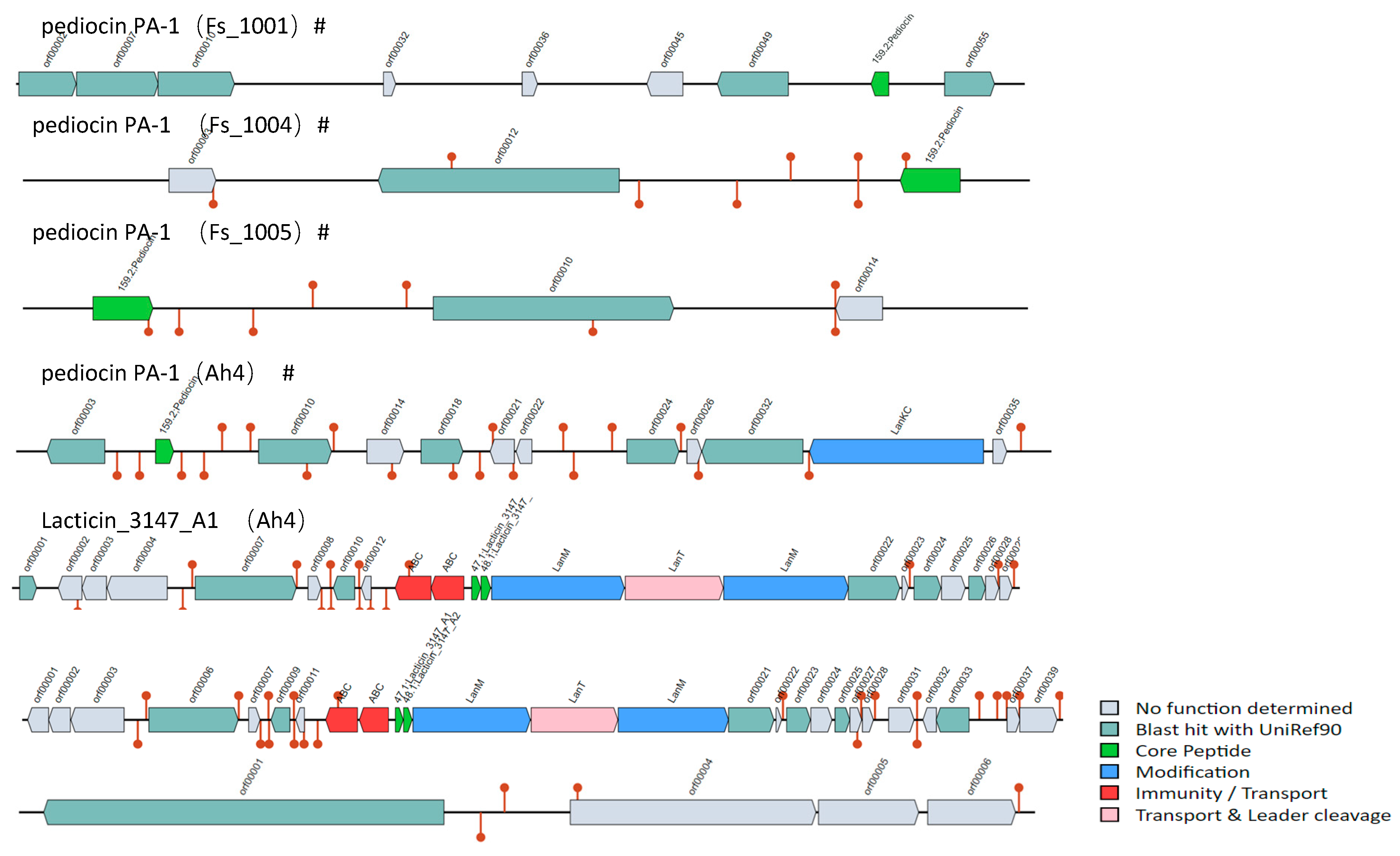
3.8. Bacteriocin Operons Analysis in F. sanfranciscensis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains—A major source of sustainable protein for health. Nutr. Res. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef]
- China’s Rising Bakery Sector. Available online: https://www.fas.usda.gov/data/china-s-rising-bakery-sector (accessed on 3 April 2023).
- Zhang, G.H.; Tu, J.; Sadiq, F.A.; Zhang, W.Z.; Wang, W. Prevalence, genetic diversity, and technological functions of the Lactobacillus sanfranciscensis in sourdough: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1209–1226. [Google Scholar] [CrossRef]
- Samuel, D. Bread in archaeology. Civilisations 2002, 49, 27–36. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Van Kerrebroeck, S.; Comasio, A.; Harth, H.; De Vuyst, L. Impact of starter culture, ingredients, and flour type on sourdough bread volatiles as monitored by selected ion flow tube-mass spectrometry. Food Res. Int. 2018, 106, 254–262. [Google Scholar] [CrossRef]
- De Vuyst, L.; Harth, H.; Van Kerrebroeck, S.; Leroy, F. Yeast diversity of sourdoughs and associated metabolic properties and functionalities. Int. J. Food Microbiol. 2016, 239, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Landis, E.A.; Oliverio, A.M.; McKenney, E.A.; Nichols, L.M.; Kfoury, N.; Biango-Daniels, M.; Shell, L.K.; Madden, A.A.; Shapiro, L.; Sakunala, S.; et al. The diversity and function of sourdough starter microbiomes. Elife 2021, 10, e61644. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Huangfu, X.Y.; Zhao, M.Y.; Zhao, R.Y. Chinese traditional sourdough steamed bread made by retarded sponge-dough method: Microbial dynamics, metabolites changes and bread quality during continuous propagation. Food Res. Int. 2023, 163, 112145. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alvarado, O.; Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; Vinderola, G.; García-Cayuela, T. Role of lactic acid bacteria and yeasts in sourdough fermentation during breadmaking: Evaluation of postbiotic-like components and health benefits. Front. Microbiol. 2022, 13, 969460. [Google Scholar] [CrossRef]
- Rogalski, E.; Ehrmann, M.A.; Vogel, R.F. Intraspecies diversity and genome-phenotype-associations in Fructilactobacillus sanfranciscensis. Microbiol. Res. 2020, 243, 126625. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, T.J.; Zhang, G.H.; Chen, J.C.; Gu, J.S.; Yuan, L.; He, G.Q. Genotyping of Lactobacillus sanfranciscensis isolates from Chinese traditional sourdoughs by multilocus sequence typing and multiplex RAPD-PCR. Int. J. Food Microbiol. 2017, 258, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Korakli, M.; Rossmann, A.; Gänzle, M.G.; Vogel, R.F. Sucrose metabolism and exopolysaccharide production in wheat and rye sourdoughs by Lactobacillus sanfranciscensis. J. Agric. Food Chem. 2001, 49, 5194–5200. [Google Scholar] [CrossRef]
- Zapparoli, G.; Torriani, S.; Dellaglio, F. Differentiation of Lactobacillus sanfranciscensis strains by randomly amplified polymorphic DNA and pulsed-field gel electrophoresis. FEMS Microbiol. Lett. 1998, 166, 325–332. [Google Scholar] [CrossRef]
- Kitahara, M.; Sakata, S.; Benno, Y. Biodiversity of Lactobacillus sanfranciscensis strains isolated from five sourdoughs. Lett. Appl. Microbiol. 2005, 40, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, E.; Vogel, R.F.; Ehrmann, M.A. Monitoring of Lactobacillus sanfranciscensis strains during wheat and rye sourdough fermentations by CRISPR locus length polymorphism PCR. Int. J. Food Microbiol. 2020, 316, 108475. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, E.; Ehrmann, M.A.; Vogel, R.F. Strain-specific interaction of Fructilactobacillus sanfranciscensis with yeasts in the sourdough fermentation. Eur. Food Res. Technol. 2021, 247, 1437–1447. [Google Scholar] [CrossRef]
- Boudaoud, S.; Aouf, C.; Devillers, H.; Sicard, D.; Segond, D. Sourdough yeast-bacteria interactions can change ferulic acid metabolism during fermentation. Food Microbiol. 2021, 98, 103790. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.K.; Chen, S.L.; Xu, X.P.; Lin, X.Z.; Hu, F.L. Isolation and identification of bacteria from pollen and bee bread. J. Zhejiang Univ. Sci. B 2001, 27, 627–630. Available online: https://www.zjujournals.com/agr/CN/Y2001/V27/I6/627 (accessed on 6 April 2024).
- Corsetti, A.; Gobbetti, M.; Smacchi, E. Antibacterial activity of sourdough lactic acid bacteria isolation of a bacteriocin like inhibitory substance from Lactobacillus sanfrancisco. Food Microbiol. 1996, 13, 447–456. [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhang, W.Z.; Liang, W.L.; Wang, W.; Tu, J. Studies on identification and characterization of a high-yield exopolysaccharides lactic acid bacteria. J. Chin. Inst. Food Sci. Technol. 2020, 20, 229–238. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, G.H.; Zhang, W.Z.; Sun, L.J.; Sadiq, F.A.; Yang, Y.K.; Gao, J.; Sang, Y.X. Preparation screening, production optimization and characterization of exopolysaccharides produced by Lactobacillus sanfranciscensis Ls-1001 isolated from Chinese traditional sourdough. Int. J. Biol. Macromol. 2019, 139, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Tovar, L.E.R.; Gänzle, M.G. Degradation of wheat germ agglutinin during sourdough fermentation. Foods 2021, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.J.; Lyu, F.Z.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional efficacy of probiotic Lactobacillus sanfranciscensis in apple, orange and tomato juices with special reference to storage stability and in vitro gastrointestinal survival. Beverages 2020, 6, 13. [Google Scholar] [CrossRef]
- Foschino, R.; Arrigoni, C.; Picozzi, C.; Mora, D.; Galli, A. Phenotypic and genotypic aspects of Lactobacillus sanfranciscensis strains isolated from sourdoughs in Italy. Food Microbiol. 2001, 18, 277–285. [Google Scholar] [CrossRef]
- Baek, H.W.; Bae, J.H.; Lee, Y.G.; Kim, S.A.; Min, W.; Shim, S.; Han, N.S.; Seo, J.H. Dynamic interactions of lactic acid bacteria in Korean sourdough during back-slopping process. J. Appl. Microbiol. 2021, 131, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.B.; Liu, B.H.; Xie, Y.L.; Li, Z.Y.; Huang, W.H.; Yuan, J.Y.; He, G.Z.; Chen, Y.X.; Pan, Q.; Liu, Y.J.; et al. SOAPdenovo2 an empirically improved memory-efficient short-read de novo assembler. GigaScience 2012, 1, 2047-2217X-1-2018, Erratum in GigaScience 2015, 4, s13742-015-0069-2. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, K.D.; Tatusova, T.; Maglott, D.R. NCBI reference sequences (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007, 35, D61–D65. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Wu, J.Y.; Yang, J.H.; Sun, S.X.; Xiao, J.F.; Yu, J. PGAP: Pan-genomes analysis pipeline. Bioinformatics 2012, 28, 416–418. [Google Scholar] [CrossRef]
- Yu, L.L.; Zang, X.J.; Chen, Y.; Gao, Y.H.; Pei, Z.M.; Yang, B.; Zhang, H.; Narbad, A.; Tian, F.W.; Zhai, Q.X.; et al. Phenotype-genotype analysis of Latilactobacills curvatus from different niches: Carbohydrate metabolism, antibiotic resistance, bacteriocin, phage fragments and linkages with CRISPR-Cas systems. Food Res. Int. 2022, 160, 111640. [Google Scholar] [CrossRef]
- Kelleher, P.; Bottacini, F.; Mahony, J.; Kilcawley, K.N.; Van Sinderen, D. Comparative and functional genomics of the Lactococcus lactis taxon; insights into evolution and niche adaptation. BMC Genom. 2017, 18, 267. [Google Scholar] [CrossRef]
- Lin, G.P.; Liu, Q.; Wang, L.Y.; Li, H.T.; Zhao, J.X.; Zhang, H.; Wang, G.; Chen, W. The comparative analysis of genomic diversity and genes involved in carbohydrate metabolism of eighty-eight Bifidobacterium pseudocatenulatum isolates from different niches of China. Nutrients 2022, 14, 2347. [Google Scholar] [CrossRef]
- Mailund, T.; Brodal, G.S.; Fagerberg, R.; Pedersen, C.N.; Phillips, D. Recrafting the neighbor-joining method. BMC Bioinf. 2006, 7, 29. [Google Scholar] [CrossRef]
- Andriani, D.; Hasan, P.N.; Utami, T.; Suroto, D.A.; Rachma, W.; Rahayu, E.S. Genotypic and phenotypic analyses of antibiotic resistance in indonesian indigenous Lactobacillus probiotics. Appl. Food Biotechnol. 2021, 8, 267–274. [Google Scholar] [CrossRef]
- Grissa, I.; Vergnaud, G.; Pourcel, C. The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats. BMC Bioinf. 2007, 8, 172. [Google Scholar] [CrossRef]
- Kline, L.; Sugihara, T.F. Microorganisms of the San Francisco sourdough bread process: II. Isolation and characterization of undescribed bacterial species responsible for the souring activity. Appl. Microbiol. 1971, 21, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Stolz, P.; Vogel, R.F.; Hammes, W.P. Utilization of electron acceptors by lactobacilli isolated from sourdough. Eur. Food Res. Technol. 1995, 201, 91–96. [Google Scholar] [CrossRef]
- Böcker, G.; Stolz, P.; Hammes, W.P. Neue Erkenntnisse zum Ökosystem Sauerteig und zur Physiologie der sauerteigtypischen Stämme Lactobacillus sanfrancisco aund Lactobacillus pontis. Getreide Mehl Brot 1995, 49, 370–374. Available online: https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=2933309 (accessed on 3 April 2024).
- Ehrmann, M.A.; Vogel, R.F. Characterisation of IS153, an IS3-family insertion sequence isolated from Lactobacillus sanfranciscensis and its use for strain differentiation. Syst. Appl. Microbiol. 2001, 24, 443–450. [Google Scholar] [CrossRef]
- Liske, R.B.; Niessen, L.; Vogel, R.F. Potential of lactic acid bacteria to reduce the growth of Fusarium culmorum in the malting process. Mycotoxin Res. 2000, 16 (Suppl. S1), 62–65. [Google Scholar] [CrossRef]
- Rogalski, E.; Ehrmann, M.A.; Vogel, R.F. Role of Kazachstania humilis and Saccharomyces cerevisiae in the strain-specific assertiveness of Fructilactobacillus sanfranciscensis strains in rye sourdough. Eur. Food Res. Technol. 2020, 246, 1817–1827. [Google Scholar] [CrossRef]
- Vogel, R.F.; Pavlovic, M.; Ehrmann, M.A.; Wiezer, A.; Liesegang, H.; Offschanka, S.; Voget, S.; Angelov, A.; Böcker, G.; Liebl, W. Genomic analysis reveals Lactobacillus sanfranciscensis as stable element in traditional sourdoughs. Microb. Cell Fact. 2011, 10 (Suppl. S1), S6. [Google Scholar] [CrossRef]
- De Angelis, M.; Di Cagno, R.; Gallo, G.; Curci, M.; Siragusa, S.; Crecchio, C.; Parente, E.; Gobbetti, M. Molecular and functional characterization of Lactobacillus sanfranciscensis strains isolated from sourdoughs. Int. J. Food Microbiol. 2007, 114, 69–82. [Google Scholar] [CrossRef]
- Chan, J.Z.; Halachev, M.R.; Loman, N.J.; Constantinidou, C.; Pallen, M.J. Defining bacterial species in the genomic era: Insights from the genus Acinetobacter. BMC Microbiol. 2012, 12, 302. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Zheng, J. Lifestyles of sourdough lactobacilli-Do they matter for microbial ecology and bread quality? Int. J. Food Microbiol. 2019, 302, 15–23. [Google Scholar] [CrossRef]
- Giovannoni, S.J.; Cameron Thrash, J.; Temperton, B. Implications of streamlining theory for microbial ecology. ISME J. 2014, 8, 1553–1565. [Google Scholar] [CrossRef]
- Yang, H.Y.; Sadiq, F.A.; Liu, T.J.; Zhang, G.H.; He, G.Q. Use of physiological and transcriptome analysis to infer the interactions between Saccharomyces cerevisiae and Lactobacillus sanfranciscensis isolated from Chinese traditional sourdoughs. LWT–Food Sci. Technol. 2020, 126, 109268. [Google Scholar] [CrossRef]
- Stolz, P.; Böcker, G.; Vogel, R.F.; Hammes, W.P. Utilisation of maltose and glucose by lactobacilli isolated from sourdough. FEMS Microbiol. Lett. 1993, 109, 237–242. [Google Scholar] [CrossRef]
- Zheng, J.S.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris HM, B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Wu, J.; Han, X.; Ye, M.; Li, Y.; Wang, X.; Zhong, Q. Exopolysaccharides synthesized by lactic acid bacteria: Biosynthesis pathway, structure-function relationship, structural modification and applicability. Crit. Rev. Food Sci. Nutr. 2022, 63, 7043–7064. [Google Scholar] [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J. Food Prot. 1998, 61, 1636–1643. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, R.; Tian, X.; Zhou, X.; Pan, X.; Wong, A. Assessing the risk of probiotic dietary supplements in the context of antibiotic resistance. Front. Microbiol. 2017, 8, 908. [Google Scholar] [CrossRef]
- Anisimova, E.A.; Yarullina, D.R. Antibiotic resistance of Lactobacillus strains. Curr. Microbiol. 2019, 76, 1407–1416. [Google Scholar] [CrossRef]
- Scaltriti, E.; Carminati, D.; Cortimiglia, C.; Ramoni, R.; Sørensen, K.I.; Giraffa, G.; Zago, M. Survey on the CRISPR arrays in Lactobacillus helveticus genomes. Lett. Appl. Microbiol. 2019, 68, 394–402. [Google Scholar] [CrossRef]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Koonin, E.V. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2019, 18, 67–83. [Google Scholar] [CrossRef]
- Sun, Z.; Harris, H.M.B.; Mccann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffery, I.B.; Cooney, J.C.; Kagawa, T.F. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015, 6, 8322. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.G.; Lai, Z.W.; Tan, J.S. Bacteriocins from lactic acid bacteria: Purification strategies and applications in food and medical industries: A review. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 51. [Google Scholar] [CrossRef]
- Yang, E.; Fan, L.; Jiang, Y.; Doucette, C.; Fillmore, S. Antimicrobial activity of bacteriocin-producing lactic acid bacteria isolated from cheeses and yogurts. AMB Express 2012, 2, 48. [Google Scholar] [CrossRef]


| Organism | Strain | Accession No. | Isolation Source | Genome Size (bp) | GC Content (%) | CDS Coding (Total) | References |
|---|---|---|---|---|---|---|---|
| Fructilactobacillus sanfranciscensis | DSM20541; TMW 1.53 | MIYJ00000000 | Sourdough, USA; San Francisco sourdough | 1,295,539 | 34.76 | 1221 | [36] |
| Fructilactobacillus sanfranciscensis | TMW 1.54 | NZ_MIYE01000000 | Rye sourdough, Germany | 1,308,584 | 34.62 | 1225 | [37] |
| Fructilactobacillus sanfranciscensis | TMW 1.392 | NZ_MIYH01000000 | Sourdough, Freising Germany | 1,262,093 | 34.49 | 1185 | [38] |
| Fructilactobacillus sanfranciscensis | TMW 1.640 | SCEZ00000000 | Wheat sourdough, Switzerland | 1,297,108 | 34.85 | 1243 | [39] |
| Fructilactobacillus sanfranciscensis | TMW 1.726 | NZ_MIYD01000000 | Sourdough, Italy | 1,253,149 | 34.67 | 1184 | [40] |
| Fructilactobacillus sanfranciscensis | TMW 1.897 | SCEP00000000 | Sourdough, Greece; Athens | 1,249,687 | 34.60 | 1209 | [41] |
| Fructilactobacillus sanfranciscensis | TMW 1.907 | SCEY00000000 | Sourdough, Greece; Athens | 1,281,500 | 34.69 | 1257 | [16] |
| Fructilactobacillus sanfranciscensis | TMW 1.936 | SCEX00000000 | Sourdough, Greece; Athens | 1,242,512 | 34.74 | 1190 | [16] |
| Fructilactobacillus sanfranciscensis | TMW 1.1150 | NZ_MIYG01000000 | Sourdough, Germany | 1,281,791 | 34.69 | 1195 | [16] |
| Fructilactobacillus sanfranciscensis | TMW 1.1152 | SCEV00000000 | Sourdough, USA | 1,253,076 | 34.58 | 1186 | [16] |
| Fructilactobacillus sanfranciscensis | TMW 1.1154 | SCEU00000000 | Sourdough, USA | 1,242,516 | 34.52 | 1191 | [16] |
| Fructilactobacillus sanfranciscensis | TMW 1.1221 | SCET00000000 | Sourdough, France | 1,256,830 | 34.48 | 1203 | [16] |
| Fructilactobacillus sanfranciscensis | TMW 1.1304 | SCES00000000 | Rye sourdough, Germany | 1,309,936 | 34.59 | 1280 | [42] |
| Fructilactobacillus sanfranciscensis | TMW 1.1470 | SCER00000000 | Sourdough, Russia | 1,264,120 | 34.55 | 1211 | [16] |
| Fructilactobacillus sanfranciscensis | TMW 1.1597 | NZ_MIYF01000000 | Rye sourdough, Germany | 1,318,230 | 34.81 | 1232 | [16] |
| Fructilactobacillus sanfranciscensis | TMW 1.1730 | SCEQ00000000 | Sourdough, Germany | 1,307,874 | 34.60 | 1279 | [16] |
| Fructilactobacillus sanfranciscensis | TMW 1.2137 | NZ_MIXX01000000 | Sourdough, Italy | 1,272,753 | 34.73 | 1210 | [43] |
| Fructilactobacillus sanfranciscensis | TMW 1.2138 | NZ_MIXY01000000 | Sourdough, Italy | 1,252,720 | 34.68 | 1190 | [43] |
| Fructilactobacillus sanfranciscensis | TMW 1.2139 | NZ_MIXZ01000000 | Sourdough, Italy | 1,329,228 | 34.77 | 1256 | [43] |
| Fructilactobacillus sanfranciscensis | TMW 1.2140 | NZ_MIYA01000000 | Sourdough, Italy | 1,293,144 | 34.71 | 1201 | [43] |
| Fructilactobacillus sanfranciscensis | TMW 1.2141 | NZ_MIYB01000000 | Sourdough, Italy | 1,309,594 | 34.71 | 1241 | [43] |
| Fructilactobacillus sanfranciscensis | TMW 1.2142 | NZ_MIYC01000000 | Sourdough, Italy | 1,278,409 | 34.71 | 1212 | [43] |
| Fructilactobacillus sanfranciscensis | TMW 1.2134 | SCEW00000000.1 | Rye sourdough, Germany | 1,254,138 | 34.46 | 1230 | [16] |
| Fructilactobacillus sanfranciscensis | LS451 | NZ_CP045563.1 | San Francisco sourdough; South Korea | 1,310,991 | 35.15 | 1318 | (Korea University; 3 November 2019) |
| Fructilactobacillus sanfranciscensis | JCM 5668 | NZ_QRFO00000000 | San Francisco sourdough | 1,267,448 | 34.82 | 1318 | (ChunLab; 2 August 2018) |
| Fructilactobacillus sanfranciscensis | Ls-1001 | NZ_RPFX00000000 | Sourdough, Shanxi China | 1,349,331 | 34.49 | 1386 | (our lab; 20 May 2018) |
| Fructilactobacillus sanfranciscensis | Ah4 | NZ_QFCR00000000.1 | Sourdough, Anhui, China | 1,368,476 | 34.68 | 1408 | (our lab; 20 May 2018) |
| Fructilactobacillus sanfranciscensis | Gs2 | NZ_QGEE00000000.1 | Sourdough, Gansu China | 1,373,332 | 34.53 | 1401 | (our lab; 20 May 2018) |
| Fructilactobacillus sanfranciscensis | Gs9 | NZ_QGEF00000000.1 | Sourdough, Gansu China | 1,365,822 | 34.43 | 1400 | (our lab; 20 May 2018) |
| Fructilactobacillus sanfranciscensis | Ts9 | GCA_006334515.1 | Sourdough, Shanxi China | 1,325,807 | 34.78 | 1386 | (our lab; 20 May 2018) |
| Fructilactobacillus sanfranciscensis | Sd1_3 | NZ_QGHM00000000.1 | Sourdough, Shandong China | 1,304,535 | 34.55 | 1335 | (our lab; 20 May 2018) |
| Fructilactobacillus sanfranciscensis | Fs_1001 | SAMN32652484 | Sourdough, China | 1,341,618 | 34.72 | 1367 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1002 | SAMN32652485 | Sourdough, China | 1,318,495 | 34.61 | 1342 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1003 | SAMN32652486 | Sourdough, China | 1,328,174 | 34.69 | 1368 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1004 | SAMN32652487 | Sourdough, China | 1,333,883 | 34.75 | 1353 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1005 | SAMN32652488 | Sourdough, China | 1,351,179 | 34.77 | 1374 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1006 | SAMN32652489 | Sourdough, China | 1,299,074 | 34.57 | 1336 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1007 | SAMN32652490 | Sourdough, China | 1,293,766 | 34.60 | 1315 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1008 | SAMN32652491 | Sourdough, China | 1,305,974 | 34.70 | 1329 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1009 | SAMN32652492 | Sourdough, China | 1,292,330 | 34.59 | 1315 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1010 | SAMN32652493 | Sourdough, China | 1,302,254 | 34.70 | 1324 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1011 | SAMN32652494 | Sourdough, China | 1,300,226 | 34.58 | 1336 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1012 | SAMN32652495 | Sourdough, China | 1,302,328 | 34.59 | 1339 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1013 | SAMN32652496 | Sourdough, China | 1,302,216 | 34.62 | 1338 | (our lab; this study; 25 January 2023) |
| Fructilactobacillus sanfranciscensis | Fs_1014 | SAMN32652497 | Sourdough, China | 1,300,972 | 34.58 | 1332 | (our lab; this study; 25 January 2023) |
| Predicted Gene | NR Hit | NR Description |
|---|---|---|
| epsA | EKK20066.1 | Cell envelope-associated transcriptional attenuator LytR-CpsA-Psr |
| POH10471.1 | LytR family transcriptional regulator | |
| epsB | WP_041817972.1 | CpsD/CapB family tyrosine-protein kinase |
| AEN99639.1 | Putative tyrosine-protein kinase capB | |
| epsD | MVF15937.1 | tyrosine-protein phosphatase |
| wzx | WP_103429181.1 | bifunctional lysylphosphatidylglycerol flippase/synthetase MprF |
| WP_046041031.1 | flippase | |
| WP_014082292.1 | flippase-like domain-containing protein | |
| WP_014081504.1 | oligosaccharide flippase family protein | |
| wzy | WP_139571209.1 | polysaccharide polymerase |
| WP_198988114.1 | O-antigen polysaccharide polymerase Wzy | |
| rgpI, waaB | WP_139571129.1 | Glycosyltransferase |
| Antibiotics | Aminoglycolsides | Sulfa-Mido | Tetra- Cycline | Penicillins | Chloram-Phenicol | Linco- Samides | Macro Lide | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | CN | K | S | SXT | TE | OXO | AMP | C | CD | E | |
| Fs_1001 | R | R | R | R | IR | IR | S | S | S | S | |
| Fs_1002 | R | R | R | R | IR | S | S | S | S | S | |
| Fs_1003 | R | R | R | R | R | S | S | S | S | S | |
| Fs_1004 | R | R | R | R | IR | IR | S | S | S | S | |
| Fs_1005 | R | R | R | R | R | S | S | S | S | S | |
| Fs_1006 | R | R | R | R | R | S | S | S | S | S | |
| Fs_1007 | R | R | R | R | R | R | S | S | S | S | |
| Fs_1008 | R | R | R | R | R | IR | S | S | S | S | |
| Fs_1009 | R | R | R | R | R | S | S | S | S | S | |
| Fs_1010 | R | R | R | R | IR | S | S | S | S | S | |
| Fs_1011 | R | R | R | R | R | S | S | S | S | S | |
| Fs_1012 | R | R | R | R | R | S | S | S | S | S | |
| Fs_1013 | R | R | R | R | IR | IR | S | S | S | S | |
| Fs_1014 | R | R | R | R | R | IR | S | S | S | S | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Yu, Y.; Kemperman, R.; Jimenez, L.; Ahmed Sadiq, F.; Zhang, G. Comparative Genomics Reveals Genetic Diversity and Variation in Metabolic Traits in Fructilactobacillus sanfranciscensis Strains. Microorganisms 2024, 12, 845. https://doi.org/10.3390/microorganisms12050845
He X, Yu Y, Kemperman R, Jimenez L, Ahmed Sadiq F, Zhang G. Comparative Genomics Reveals Genetic Diversity and Variation in Metabolic Traits in Fructilactobacillus sanfranciscensis Strains. Microorganisms. 2024; 12(5):845. https://doi.org/10.3390/microorganisms12050845
Chicago/Turabian StyleHe, Xiaxia, Yujuan Yu, Rober Kemperman, Luciana Jimenez, Faizan Ahmed Sadiq, and Guohua Zhang. 2024. "Comparative Genomics Reveals Genetic Diversity and Variation in Metabolic Traits in Fructilactobacillus sanfranciscensis Strains" Microorganisms 12, no. 5: 845. https://doi.org/10.3390/microorganisms12050845




