Evidence for Immunity against Tetanus, Diphtheria, and Pertussis through Natural Infection or Vaccination in Adult Solid Organ Transplant Recipients: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Full Search Strategy
2.4. Data Extraction and Risk of Bias
3. Results
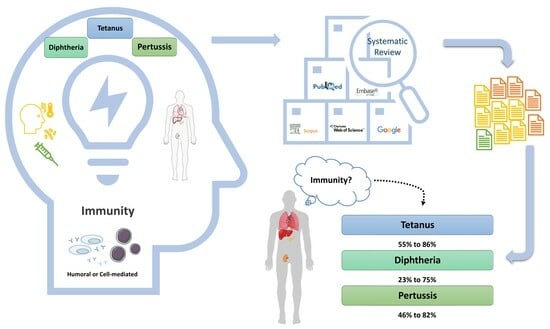
3.1. What Is the Percentage of Adult SOT Recipients Who Are Immune to Tetanus, Diphtheria, and Pertussis?
3.1.1. Tetanus
| First Author/Publication Year/Country in Which the Study Was Conducted | Study Design | Population Description | Antibody Cut-Offs | Limitations |
|---|---|---|---|---|
| Boey/2021/Belgium [20] | Cross-sectional study | Six groups of patients with chronic diseases (1052 patients), including 230 heart or lung transplant recipients, were investigated. SOT recipients Female: 73/230 (32%) Median age (range): 59 (19–87) years Median (range) time from transplantation: 7 (1–29) years | Diphtheria Seronegative: Anti-DT < 0.01 IU/mL Seroprotective: Anti-DT ≥ 0.1 IU/mL Tetanus Seronegative: Anti-TT titers < 0.01 IU/mL Seroprotective: Anti-TT titers ≥ 0.1 IU/mL Pertussis Seropositive: Anti-PT, anti-FHA (filamentous hemagglutinin), and anti-Prn (pertactin) titers ≥ 5 IU/mL. Pertussis infection or vaccination in the past two years: Anti-PT titers ≥ 50 IU/mL Recent infection or vaccination: Anti-PT titers ≥ 100 IU/mL | Single-center study; lack of documented vaccination history for all the patients |
| Blanchard-Rohner/2019/Switzerland [21] | Cross-sectional study | This study included two groups of transplant recipients, and anti-TT antibodies were measured on transplantation day. Group 1: Sixty-five (29 liver (±kidney), 25 kidney, one lung, four heart, and six pancreas/Langerhans islets) SOT recipients who were transplanted during 2013 and before the implementation of a systematic vaccination approach. Female: 21/65 (32%) Median (IQR) age: 53 (46–61) Group 2: A systematic vaccination approach was introduced in 2014, and 219 SOT candidates were included from January 2014 to November 2015. Fifty-four (27 Liver (±kidney), 11 kidney, eight lung, six heart, and two pancreas/Langerhans islets) out of 219 were transplanted during the study. Female: 14/54 (26%) Median (IQR) age, years: 56 (46–63) | Tetanus A vaccine was offered if Anti–TT < 500 IU/L (<0.5 IU/mL) Seroprotective: Anti–TT titers > 100 IU/L L (<0.1 IU/mL) | Lack of documented vaccination history for all the SOT recipients; lack of follow-ups after transplantation |
| Rohde/2014/United States [18] | Cross-sectional study | Seventy-five lung transplant recipients and 36 healthy individuals were included. Serum samples were collected from 2004 to 2008. Lung transplant recipients: Female: 41/75 (55%) Median (IQR) age, years: 57 (50–65) * Median (range) time from transplantation: 5.3 (0.17–16.6) years Controls: Female: 17/36 (47%) Median (IQR) age, years: 46 (37–53) * | Diphtheria Seroprotective: Anti-DT ≥ 0.1 IU/mL Tetanus Seroprotective: Anti-TT titers ≥ 0.15 IU/mL | Only the most recent Td or Tdap vaccination is reported, not any prior vaccinations. Lack of documented vaccination history for last Td or Tdap for 20% of the SOT recipients.; lack of regular follow-ups after transplantation |
| Broeders/2013/Australia [10] | Cohort study | Ninety-four kidney transplant recipients with a functional graft and 49 healthy hospital workers were included in this study. Anti-TT antibodies were measured on transplantation day and one year later. Kidney transplant recipients: Female: 40/94 (43%) Median (IQR) age, years: 46 (33–59) ** Controls: Female: 24/49 (49%) Median (IQR) age, years: 42 (32–52) ** | Tetanus Seroprotective: Anti-TT titers ≥ 0.1 IU/mL | No information about previous vaccination |
| Puissant-Lubrano/2010/France [22] | Cross-sectional study | The immune response to tetanus vaccination was investigated before and one month after vaccination in 39 kidney transplant recipients who received different immunosuppressive agents, including antiproliferative agents and/or calcineurin inhibitors plus steroids. Group 1: Thirteen out of the thirty-nine kidney transplant recipients received rituximab with a median (IQR) of 9 (4–11.5) months before vaccination. Female: 3/13 (23%) Median (IQR) age, years: 55 (40–64) Median (range) time from transplantation, years: 6.9 (2.5–15.3) *** Group 2: Twenty-six out of the thirty-nine kidney transplant recipients did not receive rituximab. Female: 11/26 (42%) Median (IQR) age, years: 48 (36–59) Median (range) time from transplantation, years: 2.3 (2–4) *** Controls: Serum specimens from 30 healthy blood donors were used to compare the antibody response before vaccination. | Diphtheria Seroprotective: Anti-DT ≥ 0.1 IU/mL Tetanus Seroprotective: Anti-TT titers ≥ 0.15 IU/mL A 4-fold increase in anti-TT after vaccination was considered as significant response to vaccination. | Lack of detailed vaccination records |
| Struijk/2010/The Netherlands [17] | Randomized controlled trial | This study was a sub-study of an open, randomized, multicenter trial. Thirty-six stable kidney transplant recipients and 13 age- and sex-matched healthy persons were included as controls. Kidney transplant recipients were assigned into 3 groups (12 per group). Kidney transplant recipients were not vaccinated against TT in the previous five years, and were within the 2nd year after transplantation and received double immunosuppressive maintenance therapy consisting of prednisolone with CsA, MPA, or everolimus from 6 months after transplantation. The controls were excluded if they received immunosuppressants or were vaccinated against tetanus within the previous five years. Group 1 (Prednisolone + CsA) Female: 2/12 (17%) Median (IQR) age, years: 58 (34–72) Median (range) time from transplantation, years: Group 2: (Prednisolone + MPA) Female: 3/12 (25%) Median (IQR) age, years: 60 (30–70) Median (range) time from transplantation, years: Group 3: (Prednisolone + everolimus) Female: 4/12 (33%) Median (IQR) age, years: 50 (27–68) Controls: Female: 5/13 (38%) Median (IQR) age, years: 55 (42–63) | Participants were vaccinated with three vaccines simultaneously. Immunocyanin (Immucothel, Biosyn Arzneimittel GmbH, Fellbach, Germany), Tetanus toxoid (Aventis Pasteur MSD Brussels, Belgium), and Polyvalent Pneumococcal vaccine (Pneumovax, Merck Sharp and Dohme, Haarlem, The Netherlands). Blood specimens were collected before and 14 days after vaccination. Anti-TT antibody concentrations were measured using ELISA, and TT-specific cellular responses were measured using ELISPOT assay. | |
| Chesi/2009/Germany [19] | Cross-sectional study | Four hundred sixty-four adult SOT recipients (267 liver and 197 kidney transplants) were included. Liver transplant recipients: Female: 112/267 (42%) Median (IQR) age, years: 57 (20–79) Median (range) time from transplantation, years: 5.6 (0.5–20) Immunosuppressive therapy: 51%, 42%, and 7.1% received one, two, and three or more immunosuppressive drugs Kidney transplant recipients Female: 97/197 (49%) Median (IQR) age, years: 51 (18–79) Median (range) time from transplantation, years: 3.8 (0.5–31) Kidney transplant recipients were younger, with a shorter time from transplantation (p < 0.005). Immunosuppressive therapy: 0.5%, 35%, and 65% received one, two, and three or more immunosuppressive drugs. | Anti-TT and anti-DT IgG antibodies were measured by ELISA (Virion Serion, Wurzburg, Germany) on a Behring ELISA Processor, BEP III. Diphtheria Seroprotective: Anti-DT ≥ 0.1 IU/mL Tetanus Seroprotective: Anti-TT titers ≥ 0.1 IU/mL | Patients reported vaccination status; only 159 patients (34.3%) possessed a vaccination certificate, more frequently kidney transplant recipients than liver transplant recipients (43.5% vs. 27.3%; p < 0.005). Immunosuppressive therapy is only reported as 1, 2, or 3 or more drugs. |
| Goldfarb/2001/United States [23] | Cohort study | Sixty-seven lung transplant recipients were grouped by IgG level. Low IgG Female: 11/25 (44%) Median (IQR) age: 50 (21–60) Moderately low IgG Female 11/22 (50%) Median (IQR) age: 51 (17–61) Normal IgG Female 11/20 (55%) Median (IQR) age: 37 (11–59) | Hypogammaglobulinemia was defined as an IgG level of <600 mg/dL. Low IgG levels were defined as less than 400 mg/dL. Moderately low IgG was defined as levels between 400 and 600 mg/dL. Normal IgG levels were above 600 mg/dL. | Single-center study; only 59 of 130 on-site transplants followed in the study; different pre and post-transplant amount of participants in humoral immune surveys |
| Krüger/2001/Germany [9] | Cohort study | Seventy-one anti-TT and anti-DT seronegative patients on hemodialysis were vaccinated simultaneously against tetanus and diphtheria and followed for five years. Anti-TT and anti-DT antibodies were measured one and five years after vaccination. Thirty-five patients were transplanted within five years of follow-up, and antibody concentrations were available for fifteen alive kidney transplant recipients in the fifth year. The kidney transplant recipients’ characteristics have not been reported. No re-vaccination was performed within the last five years. | Diphtheria Seroprotective: Anti-DT ≥ 0.1 IU/mL Tetanus Seroprotective: Anti-TT titers ≥ 0.1 IU/mL | Loss of follow-up for a considerable proportion of the transplant recipients: the kidney transplant recipients’ characteristics were not reported. |
| Huzly/1997/Germany [24] | Cohort study | One hundred sixty-four kidney transplant recipients and 106 healthy volunteers were included. Kidney transplant recipients Female: 58/164 (59.9%) Median (IQR) age: 43 (16–66) Median time sice renal transplant (IQR) yr: 2 (1–24) Immunosuppressive therapy: 83 (50.6%) received cyclosporine, azathioprine and prednisone. Twenty-nine (17.7%) received azathioprine and prednisone. Twenty-seven (16.4%) received cyclosporine and prednisone. Sixteen (9.8%) received cyclosporine and azathioprine. Nine (5.5%) received only cyclosporine Controls: Female: 67/106 (63.2%) Median (IQR) age: 42 (18–68) | Diphtheria Relative protective: Anti-DT ≥ 0.01 IU/mL Seroprotective: Anti-DT ≥ 0.1 IU/mL Tetanus Seroprotective: Anti-TT titers ≥ 0.01 IU/mL | Only 55 of 164 patients were part of the 12-month follow-up. |
| Girndt/1995/Germany [25] | Cohort study | Fifty-seven anti-TT seronegative patients with chronic kidney disease, including seven kidney transplant recipients and fifteen controls from the outpatient hypertension clinic Kidney transplant recipients Female: 0/7 (0%) Mean (SD) age: 47.8 (8.4) Median time since kidney transplantation (IQR), years: 2 (1–24) Mean (SD) serum creatinine (mg/dL): 1.39 (0.30) All received prednisolone, cyclosporine, and azathioprine as immunosuppressive treatment. Controls Female: 8/15 (0%) Mean (SD) age: 51.3 (13.3) Mean (SD) serum creatinine (mg/dL): 0.97 (0.25) | Tetanus Seroprotective: Anti-TT titers ≥ 0.01 IU/mL | A limited number of participants; only patients vaccinated for tetanus more than ten years ago were tested for seronegativity. |
3.1.2. Diphtheria
3.1.3. Pertussis
3.2. Do Adult SOT Recipients Elicit an Antibody Response When Vaccinated against DTP?
3.3. What Is the Antibody Decay Profile after Vaccination against DTP in Adult SOT Recipients?
3.4. Gender and Age Differences in Antibody Response
3.5. Is There a Difference in the Antibody Response When Vaccine Boosters Are Administered before versus after Transplantation?
3.6. Immunosuppressive Combinations and Immune Response to Vaccination
3.7. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fishman, J.A. Infection in Organ Transplantation. Am. J. Transplant. 2017, 17, 856–879. [Google Scholar] [CrossRef]
- Walti, L.N.; Mugglin, C.; Mombelli, M.; Manuel, O.; Hirsch, H.H.; Khanna, N.; Mueller, N.J.; Berger, C.; Boggian, K.; Garzoni, C.; et al. Vaccine-Preventable Infections Among Solid Organ Transplant Recipients in Switzerland. JAMA Netw. Open 2023, 6, e2310687. [Google Scholar] [CrossRef]
- Lima, E.Q.; Silva, R.G.; Fernandes, I.M.M.; Abbud-Filho, M.; Burdmann, E.A. Tetanus-Induced Acute Kidney Injury in a Renal Transplant Recipient. Am. J. Trop. Med. Hyg. 2007, 77, 400–402. [Google Scholar] [CrossRef]
- de La Chapelle, A.; Lavabre, O.; Pinsard, M.; Delamonica, J.; Relyveld, E.H. Tetanus in a Renal Transplant Recipient Exhibiting the Presence of Circulating Antitetanus Antibodies Determined by ELISA. Biomed. Pharmacother. 2002, 56, 208–210. [Google Scholar] [CrossRef]
- Hovel, E.M.; Pease, R.C.; Scarano, A.J.; Chen, D.J.; Saddler, C.M. Bordetella Pertussis in a Four-Time Kidney Transplant Recipient: A Call for Immunization Programs at Transplant Centers. Transpl. Infect. Dis. 2019, 21, e13120. [Google Scholar] [CrossRef]
- Garbiras, M.; Shabaka, A.; Calvo, N.; Martin, L.; Moreno, M.A.; Lopez de la Manzanara, V.; Sanchez-Fructuoso, A.I. Whooping Cough in a Renal Transplant Recipient. Transpl. Infect. Dis. 2016, 18, 280–283. [Google Scholar] [CrossRef]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. Executive Summary: 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef]
- Danziger-Isakov, L.; Kumar, D. Vaccination of Solid Organ Transplant Candidates and Recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13563. [Google Scholar] [CrossRef]
- Krüger, S.; Müller-Steinhardt, M.; Kirchner, H.; Kreft, B. A 5-Year Follow-up on Antibody Response after Diphtheria and Tetanus Vaccination in Hemodialysis Patients. Am. J. Kidney Dis. 2001, 38, 1264–1270. [Google Scholar] [CrossRef]
- Broeders, E.N.; Wissing, K.M.; Ghisdal, L.; Lemy, A.; Hoang, A.D.; Vereerstraeten, P.; Mascart, F.; Abramowicz, D. Large Decrease of Anti-Tetanus Anatoxin and Anti-Pneumococcal Antibodies at One Year after Renal Transplantation. Clin. Nephrol. 2013, 79, 313–317. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Evidence for Immunity via Natural Infection or Vaccination against Diphtheria, Tetanus, and Pertussis in Adult Solid Organ Transplant Recipients: A Systematic Review. Available online: https://osf.io/evcwt (accessed on 10 August 2023).
- Covidence Systematic Review Software; Veritas Health Innovation: Melbourne, Australia; Available online: https://www.covidence.org/ (accessed on 10 August 2023).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, 14898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- McGuinness, L.A. Robvis: An R Package and Web Application for Visualising Risk-of-Bias Assessments. Available online: https://github.com/mcguinlu/robvis (accessed on 7 April 2021).
- Struijk, G.H.; Minnee, R.C.; Koch, S.D.; Zwinderman, A.H.; Van Donselaar-Van Der Pant, K.A.M.I.; Idu, M.M.; Ten Berge, I.J.M.; Bemelman, F.J. Maintenance Immunosuppressive Therapy with Everolimus Preserves Humoral Immune Responses. Kidney Int. 2010, 78, 934–940. [Google Scholar] [CrossRef]
- Rohde, K.A.; Cunningham, K.C.; Henriquez, K.M.; Nielsen, A.R.; Worzella, S.L.; Hayney, M.S. A Cross-Sectional Study of Tetanus and Diphtheria Antibody Concentrations Post Vaccination among Lung Transplant Patients Compared with Healthy Individuals. Transpl. Infect. Dis. 2014, 16, 871–877. [Google Scholar] [CrossRef]
- Chesi, C.; Günther, M.; Huzly, D.; Neuhaus, R.; Reinke, P.; Engelmann, H.B.; Mockenhaupt, F.P.; Bienzle, U. Immunization of Liver and Renal Transplant Recipients: A Seroepidemiological and Sociodemographic Survey. Transpl. Infect. Dis. 2009, 11, 507–512. [Google Scholar] [CrossRef]
- Boey, L.; Bosmans, E.; Ferreira, L.B.; Heyvaert, N.; Nelen, M.; Smans, L.; Tuerlinckx, H.; Roelants, M.; Claes, K.; Derdelinckx, I.; et al. Seroprevalence of Antibodies against Diphtheria, Tetanus and Pertussis in Adult At-Risk Patients. Vaccines 2021, 9, 18. [Google Scholar] [CrossRef]
- Blanchard-Rohner, G.; Enriquez, N.; Lemaître, B.; Cadau, G.; Combescure, C.; Giostra, E.; Hadaya, K.; Meyer, P.; Gasche-Soccal, P.M.; Berney, T.; et al. Usefulness of a Systematic Approach at Listing for Vaccine Prevention in Solid Organ Transplant Candidates. Am. J. Transplant. 2019, 19, 512–521. [Google Scholar] [CrossRef]
- Puissant-Lubrano, B.; Rostaing, L.; Kamar, N.; Abbal, M.; Fort, M.; Blancher, A. Impact of Rituximab Therapy on Response to Tetanus Toxoid Vaccination in Kidney-Transplant Patients. Exp. Clin. Transpl. 2010, 8, 19–28. [Google Scholar]
- Goldfarb, N.S.; Avery, R.K.; Goormastic, M.; Mehta, A.C.; Schilz, R.; Smedira, N.; Pien, L.; Haug, M.T.; Gordon, S.M.; Hague, L.K.; et al. Hypogammaglobulinemia in lung transplant recipients. Transplantation 2001, 27, 242–246. [Google Scholar] [CrossRef]
- Huzly, D.; Neifer, S.; Reinke, P.; Schröder, K.; Schönfeld, C.; Hofmann, T.; Bienzle, U. Routine immunizations in adult renal transplant recipients. Transplantation 1997, 63, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Girndt, M.; Pietsch, M.; Köhler, H. Tetanus Immunization and Its Association to Hepatitis B Vaccination in Patients with Chronic Renal Failure. Am. J. Kidney Dis. 1995, 26, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Muhoza, P.; Danovaro-Holliday, M.C.; Diallo, M.S.; Murphy, P.; Sodha, S.V.; Requejo, J.H.; Wallace, A.S. Routine Vaccination Coverage—Worldwide, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Enke, B.U.; Bökenkamp, A.; Offner, G.; Bartmann, P.; Brodehl, J. Response to Diphtheria and Tetanus Booster Vaccination in Pediatric Renal Transplant Recipients. Transplantation 1997, 64, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzi, C.; Ghio, L.; Balloni, A.; Panuccio, A.; Foti, M.; Edefonti, A.; Assael, B.M. Duration of Immunity to Diphtheria and Tetanus in Young Kidney Transplant Patients. Pediatr. Transplant. 1999, 3, 109–114. [Google Scholar] [CrossRef]
- Cortes Villalobos, A.M.P.; Mercado, J.J.C.; Pedraza, G.; Buenrostro, L.E.M.; Ruiz-Palacios, G.; Cuellar-Rodríguez, J. Immune Response to Bordetella Pertussis Vaccine in Candidates and Receptors of Solid Organ Transplant. Open Forum Infect. Dis. 2017, 4, S717–S718. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenzing, E.; Harboe, Z.B.; Sørensen, S.S.; Rasmussen, A.; Nielsen, S.D.; Rezahosseini, O. Evidence for Immunity against Tetanus, Diphtheria, and Pertussis through Natural Infection or Vaccination in Adult Solid Organ Transplant Recipients: A Systematic Review. Microorganisms 2024, 12, 847. https://doi.org/10.3390/microorganisms12050847
Lenzing E, Harboe ZB, Sørensen SS, Rasmussen A, Nielsen SD, Rezahosseini O. Evidence for Immunity against Tetanus, Diphtheria, and Pertussis through Natural Infection or Vaccination in Adult Solid Organ Transplant Recipients: A Systematic Review. Microorganisms. 2024; 12(5):847. https://doi.org/10.3390/microorganisms12050847
Chicago/Turabian StyleLenzing, Emil, Zitta Barrella Harboe, Søren Schwartz Sørensen, Allan Rasmussen, Susanne Dam Nielsen, and Omid Rezahosseini. 2024. "Evidence for Immunity against Tetanus, Diphtheria, and Pertussis through Natural Infection or Vaccination in Adult Solid Organ Transplant Recipients: A Systematic Review" Microorganisms 12, no. 5: 847. https://doi.org/10.3390/microorganisms12050847