Human Lactobacillus Strains from the Intestine can Suppress IgE-Mediated Degranulation of Rat Basophilic Leukaemia (RBL-2H3) Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Preparation
2.2. Cell Culture
2.3. Analysis of the β-Hexosaminidase Release
2.4. Binding of IgE
2.5. Cytokine Production
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Inhibition of IgE-Mediated Degranulation by Lactobacilli
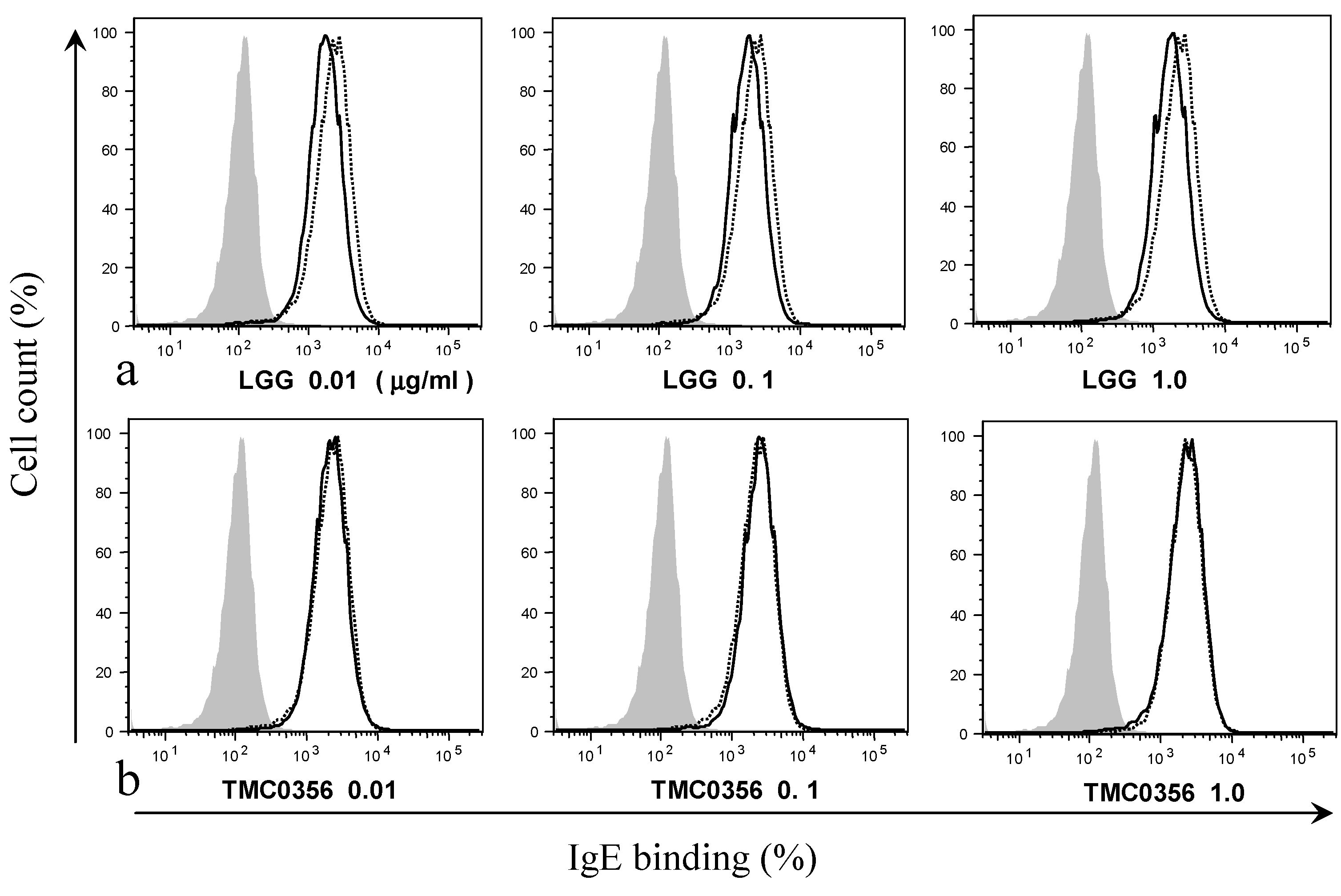
3.2. Effect of LGG and TMC0356 on Binding of IgE to the Surface of RBL-2H3 Cells
3.3. Effect of LGG and TMC0356 on Cytokine Production
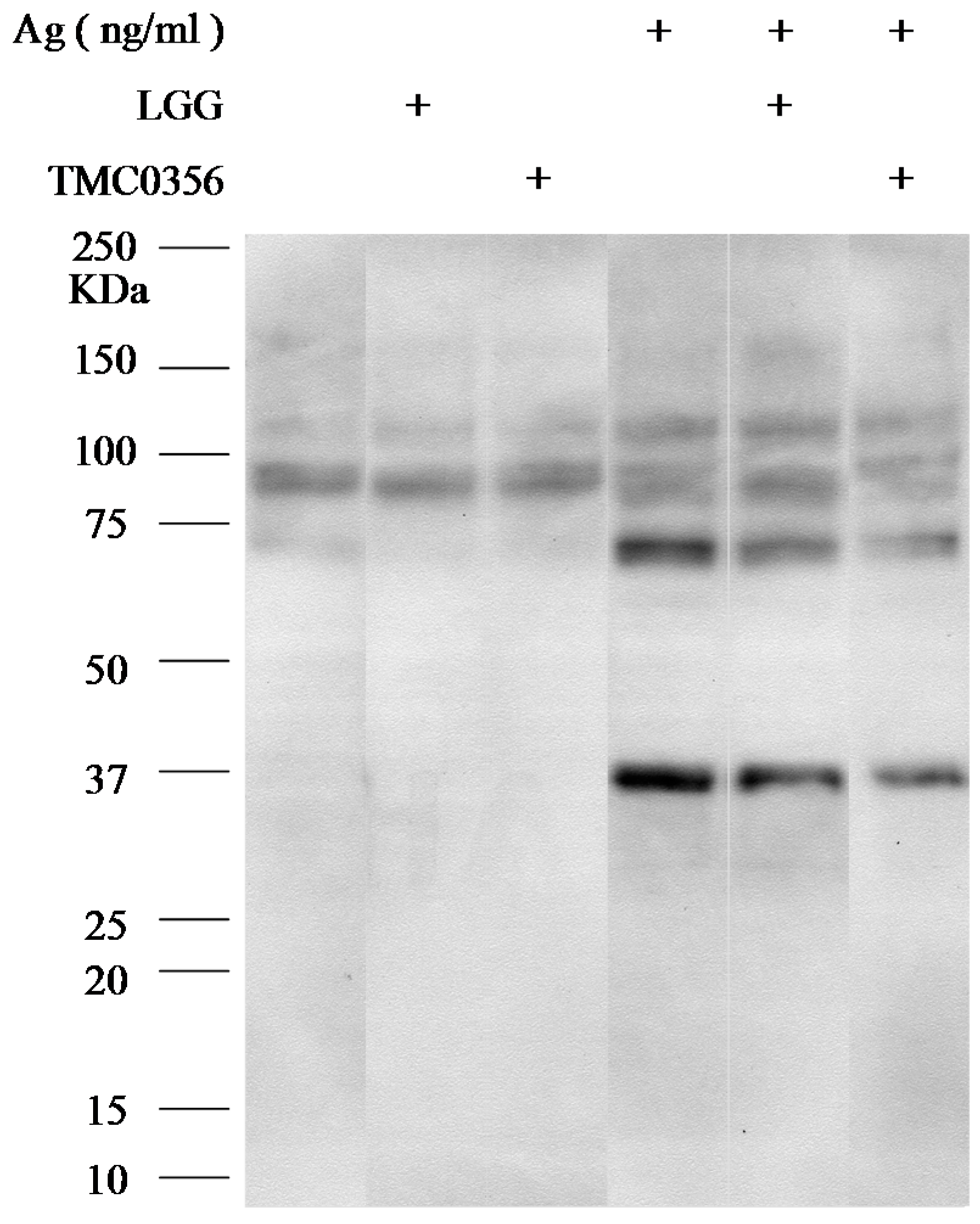
3.4. Effect of LGG and TMC0356 on Intracellular Signalling
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Strachan, D.P. Hay fever, hygiene, and household size. Br. Med. J. 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Strachan, D.P. Family size, infection and atopy: The first decade of the “hygiene hypothesis”. Thorax 2000, 55, S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Rautava, S.; Ruuskanen, O.; Salminen, S.; Isolauri, E. The Hygiene Hypothesis of Atopic Disease—An Extanded Version. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Stobberigh, E.E.; van den Brandt, P.A.; Thijs, C. The role of the intestinal microbiota in the development of atopic disorders. Allergy 2007, 62, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- Björkstén, B.; Naaber, P.; Sepp, E.; Mikelsaar, M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin. Exp. Allergy 1999, 29, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Björkstén, B. The intestinal microflora in allergic patients. Biosci. Microflora 2002, 20, 135–140. [Google Scholar]
- Ouwehand, A.C. Antiallergic effects of probiotics. J. Nutr. 2007, 137, 794–797. [Google Scholar]
- Abbas, A.K.; Lichtman, A.H. Basic immunology: Functions and disorders of the immune system. Am. J. Epidemiol. 2001, 2, 185–186. [Google Scholar]
- Kobayashi, S.; Tanabe, S. Evaluation of the anti-allergic activity of citrus unshiu using rat basophilic leukemia RBL-2H3 cells as well as basophils of patients with seasonal allergic rhinitis to pollen. Int. J. Molecul. Medical 2006, 17, 511–515. [Google Scholar] [CrossRef]
- Sugiura, Y.; Takeuchi, Y.; Kakinuma, M.; Amano, H. Infibitory effects of seaweeds on histamine release from rat basophile leukemia cells (RBL-2H3). Fishers Sci. 2006, 72, 1286–1291. [Google Scholar] [CrossRef]
- Kanda, T.; Akiyama, H.; Yanagida, A.; Tanabe, M.; Goda, Y.; Toyoda, M.; Teshima, R.; Saito, Y. Inhibitory effects of apple polyphenol on induced histamine release from RBL-2H3 cells and rat mast cells. Biosci. Biotechnol. Biochem. 1998, 62, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, B.S.; Zheng, P.Y.; Liao, X.Q.; Chong, J.; Tang, S.G.; Yang, P.C. Fc gamma receptor signaling in mast cells links microbial stimulation to mucosal immune inflammation in the intestine. Am. J. Pathol. 2008, 73, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Magerl, M.; Lammel, V.; Siebenhaar, F.; Zuberbier, T.; Metz, M.; Maurer, M. Non-pathogenic commensal Escherichia coli bacteria can inhibit degranulation of mast cells. Exp. Dermatol. 2008, 17, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Krämer, S.; Sellge, G.; Lorentz, A.; Krueger, D.; Schemann, M.; Feilhauer, K.; Gunzer, F.; Bischoff, S.C. Selective activation of human intestinal mast cells by Escherichia coli hemolysin. J. Immunol. 2008, 181, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Kasakura, K.; Takahashi, K.; Aizawa, T.; Hosono, A.; Kaminogawa, S. A TLR 2 ligand suppresses allergic inflammatory reactions by acting directly on mast cells. Int. Arch. Allergy Immunol. 2009, 150, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; He, F.; Kawase, M.; Harata, G.; Hiramatsu, M.; Salminen, S.; Iino, H. Lactobacillus strains stabilize intestinal microbiota in Japanese cedar pollinosis patients. Microbiol. Immunol. 2009, 53, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Hiramatsu, M.; Saito, H.; Ishii, T.; Yasueda, H.; Akiyama, K. Effect of fermented milk prepared with two probiotic strains on Japanese cedar pollinosis in a double-blind placebo-controlled clinical study. Int. J. Food Microbiol. 2009, 128, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Harata, G.; He, F.; Takahashi, K.; Hosono, A.; Kawase, M.; Kubota, A.; Hiramatsu, M.; Kaminogawa, S. Bifidobacterium suppresses IgE-mediated degranulation of rat basophilic leulemia (RBL-2H3) cells. Microbiol. Immunol. 2010, 54, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomized placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef]
- Kalliomaki, M.; Kirjavainen, P.; Eerola, E.; Kero, P.; Salminen, S.; Isolauri, E. Distinct patterns of neonatal gut microflora in infants developing or not developing atopy. J. Allergy Clin. Immunol. 2001, 107, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Salminen, S.; Poussa, T.; Arvilommi, H.; Isolauri, E. Probiotics and prevention of atopic disease: 4-Year follow-up of a randomized placebo-controlled trial. Lancet 2003, 361, 1869–1871. [Google Scholar] [CrossRef]
- Viljanen, M.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: A double-blind placebo-controlled trial. Allergy 2005, 60, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, M.; Kuitunen, M.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Savilahti, E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatric Allergy Immunol. 2005, 16, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Helin, T.; Haahtela, S.; Haahtela, T. No effect of oral treatment with an intestinal bacterial strain, Lactobacillus rhamnosus (ATCC53103), on birch-pollen allergy: A placebo-contralled double-blind study. Allergy 2002, 57, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, M.; Hashimoto, H.; He, F.; Yamazaki, K.; Hosono, A. Inhibitory effects of fecal Lactobacilli and Bifidobacteria on the mutagenicities of Trp-P-2 and IQ. Milchwissenschaft 1998, 53, 309–313. [Google Scholar]
- Morita, H.; He, F.; Fuse, T.; Ouwehand, A.C.; Hashimoto, H.; Hosoda, M. Adhesion of lactic acid bacteria to Caco-2 cells and their effect on cytokine secretion. Microbiol. Immnol. 2002, 46, 293–297. [Google Scholar] [CrossRef]
- Morita, H.; He, F.; Fuse, T.; Ouwehand, A.C.; Hashimoto, H.; Hosoda, M.; Mizumachi, K.; Kurisaki, J. Cytokine production by the murine macrophage cell line J774.1 after exposure to Lactobacilli. Biosci. Biotechnol. Biochem. 2002, 66, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Harata, G.; Kubota, A.; Mizumachi, K.; Hiramatsu, M. Characterization of inhibitory effects of Lactobacilli against immunoglobulin E production in vitro and in vivo. Int. J. Probiotics Prebiotics 2007, 2, 29–38. [Google Scholar]
- Morita, H.; He, F.; Kawase, M.; Kubuta, A.; Hiramatsu, M.; Kurisaki, J.; Salminen, S. Preliminary human study for possible alteration of serum immunoglobulin production in perennial allergic rhinitis with fermented milk prepared with Lactobacillus gasseri TMC0356. Microbiol. Immunol. 2006, 50, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Hata, J.; Kobayakawa, S.; Hiramatsu, M. Inhibitory effect of Lactobacillus gasseri TMC0356 andLGG on enhanced vascular permeability of nasal mucosa in experimental allergic rhinitis of rats. Biosci. Biotechnol. Biochem. 2006, 70, 3025–3030. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Harata, G.; Hiramatsu, M. Orally administrated Lactobacillus gasseri TMC0356 and Lactobacillus GG alleviated nasal blockage of guinea pig with allergic rhinitis. Microbiol. Immunol. 2007, 51, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Oksaharju, A.; Kankainen, M.; Kekkonen, R.; Lindstedt, K.; Kovanen, P.; Korpela, R.; Miettinen, M. Probiotic Lactobacillus rhamnosus downregulates FCER1 and HRH4 expression in human cells. World J. Gastroentero. 2011, 17, 750–759. [Google Scholar] [CrossRef] [PubMed]
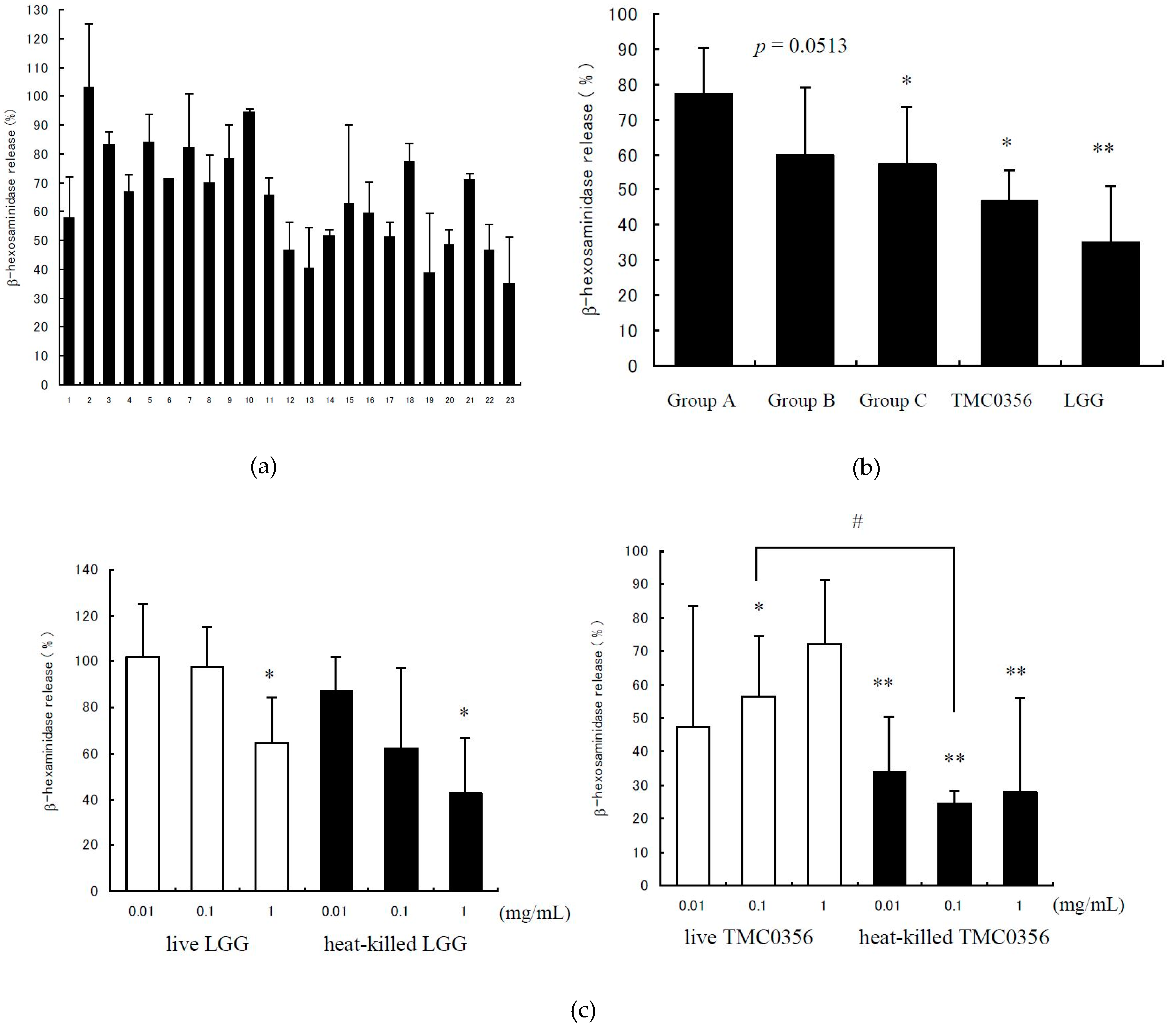
| No. | Microorganism |
|---|---|
| 1 | Lactobacillus brevis JCM1059 T |
| 2 | Lactobacillus reuteri JCM1112 T |
| 3 | Lactobacillus gasseri JCM1131 T |
| 4 | Lactobacillus acidophilus JCM1132 T |
| 5 | Lactobacillus casei subsp. Casei JCM1134 T |
| 6 | Lactobacillus casei subsp. Rhamnosus JCM1136 T |
| 7 | Lactobacillus plantarum JCM1149 T |
| 8 | Lactobacillus salivarius subsp. Salivarius JCM1231 T |
| 9 | Lactobacillus johnsonii JCM2012 T |
| 10–16 | Lactobacilli isolated from fermented milk/yoghurt |
| 17–21 | Lactobacilli isolated from human fecal |
| 22 | Lactobacillus gasseri TMC0356 |
| 23 | Lactobacillus rhamnosus GG |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harata, G.; He, F.; Takahashi, K.; Hosono, A.; Miyazawa, K.; Yoda, K.; Hiramatsu, M.; Kaminogawa, S. Human Lactobacillus Strains from the Intestine can Suppress IgE-Mediated Degranulation of Rat Basophilic Leukaemia (RBL-2H3) Cells. Microorganisms 2016, 4, 40. https://doi.org/10.3390/microorganisms4040040
Harata G, He F, Takahashi K, Hosono A, Miyazawa K, Yoda K, Hiramatsu M, Kaminogawa S. Human Lactobacillus Strains from the Intestine can Suppress IgE-Mediated Degranulation of Rat Basophilic Leukaemia (RBL-2H3) Cells. Microorganisms. 2016; 4(4):40. https://doi.org/10.3390/microorganisms4040040
Chicago/Turabian StyleHarata, Gaku, Fang He, Kyoko Takahashi, Akira Hosono, Kenji Miyazawa, Kazutoyo Yoda, Masaru Hiramatsu, and Shuichi Kaminogawa. 2016. "Human Lactobacillus Strains from the Intestine can Suppress IgE-Mediated Degranulation of Rat Basophilic Leukaemia (RBL-2H3) Cells" Microorganisms 4, no. 4: 40. https://doi.org/10.3390/microorganisms4040040
APA StyleHarata, G., He, F., Takahashi, K., Hosono, A., Miyazawa, K., Yoda, K., Hiramatsu, M., & Kaminogawa, S. (2016). Human Lactobacillus Strains from the Intestine can Suppress IgE-Mediated Degranulation of Rat Basophilic Leukaemia (RBL-2H3) Cells. Microorganisms, 4(4), 40. https://doi.org/10.3390/microorganisms4040040





