Effects of Elevated Hydrostatic Pressure against Mesophilic Background Microflora and Habituated Salmonella Serovars in Orange Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Inoculum Preparation
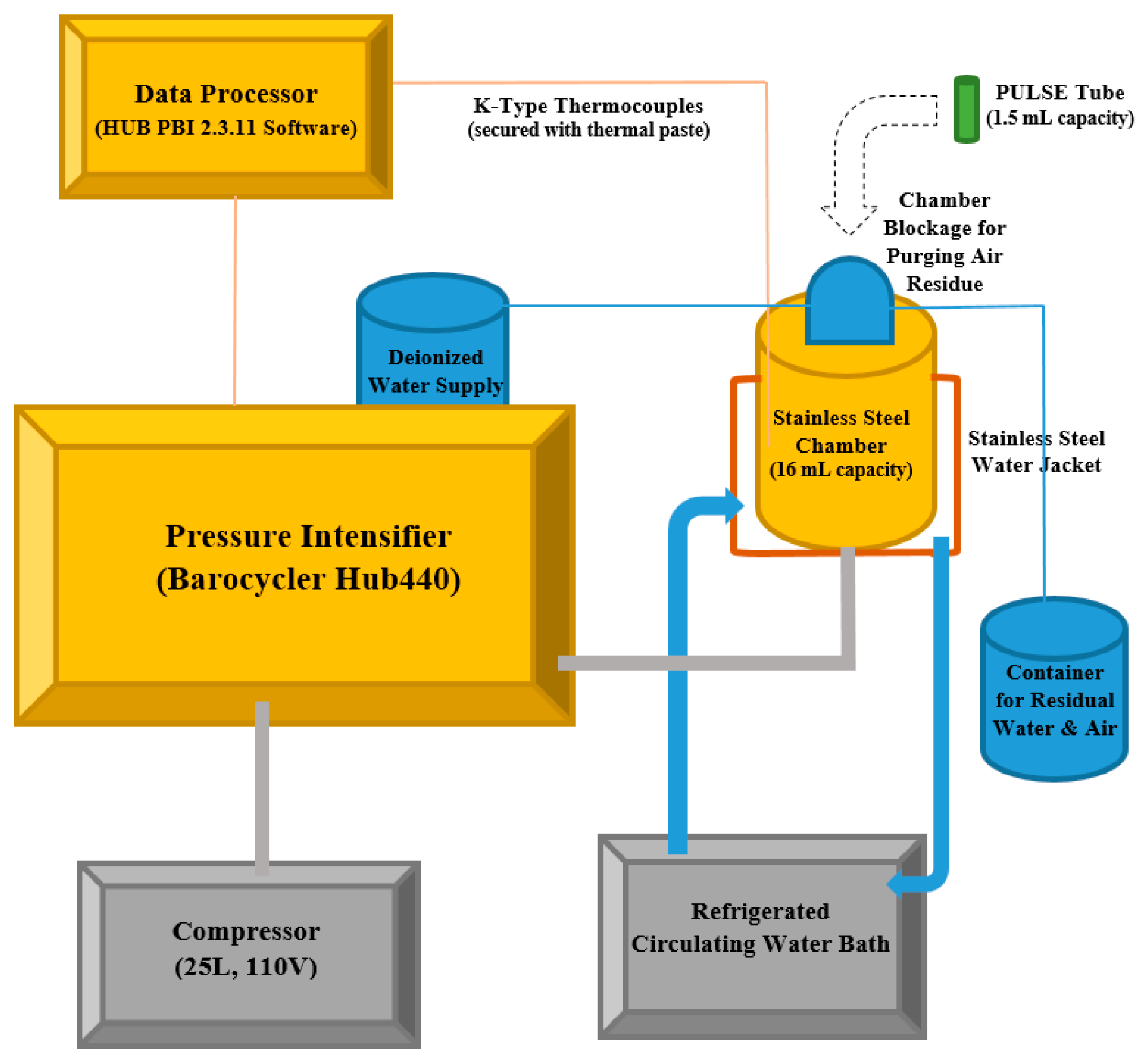
2.2. High Pressure Processing Treatment
2.3. Microbiological and pH Analyses
2.4. Experimental Design and Statistical Analyses
3. Results and Discussion
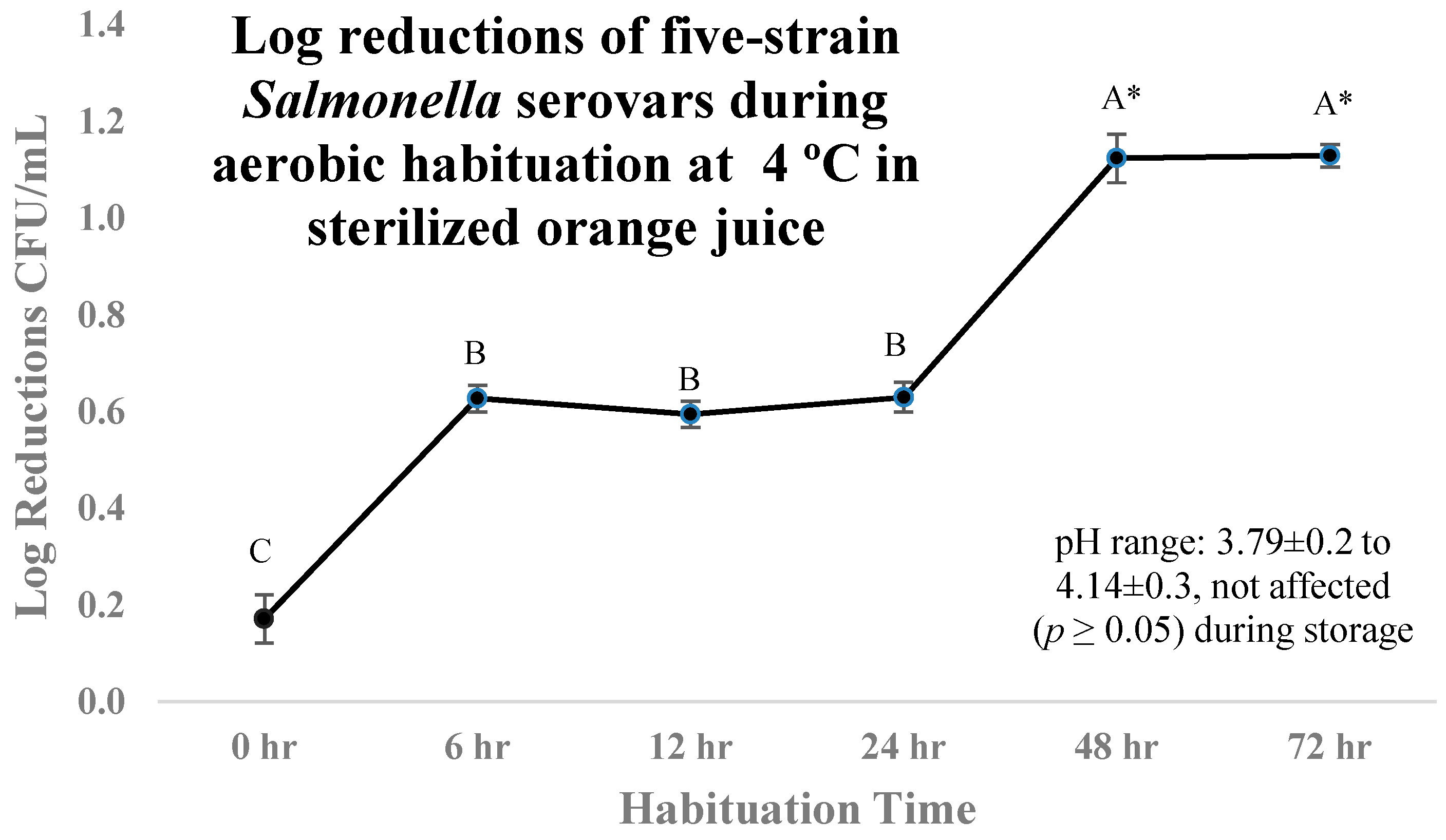
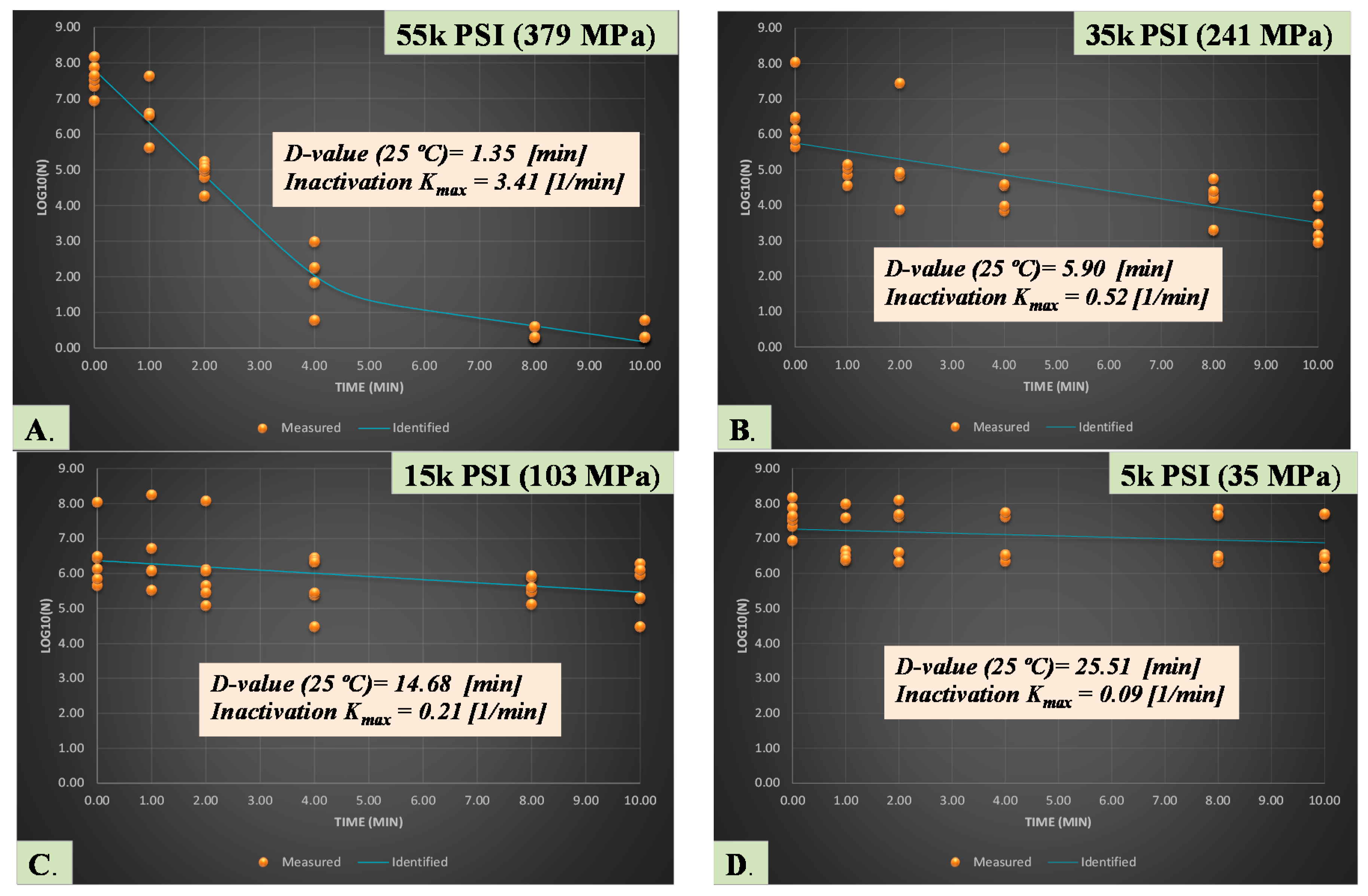
3.1. Sensitivity and Inactivation of Salmonella Serovars
3.2. Sensitivity and Inactivation of Mesophilic Background Microflora
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fouladkhah, A. The Need for Evidence-Based Outreach in the Current Food Safety Regulatory Landscape. Commentary section. J. Ext. 2017, 55, 2COM1. [Google Scholar]
- Bajpai, V.K.; Baek, K.H.; Kang, S.C. Control of Salmonella in foods by using essential oils: A review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Foodborne Outbreak Online Database (FOOD Tool). Available online: https://wwwn.cdc.gov/foodborneoutbreaks/ (accessed on 7 December 2017).
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Mahon, B.E.; Jones, T.F.; Griffin, P.M. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol. Infect. 2015, 143, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Sivapalasingam, S.; Friedman, C.R.; Cohen, L.; Tauxe, R.V. Fresh produce: A growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 2004, 67, 2342–2353. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). 21 Code of Federal Regulations 120; CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=120 (accessed on 7 December 2017).
- Danyluk, M.D.; Goodrich-Schneider, R.M.; Schneider, K.R.; Harris, L.J.; Worobo, R.W. Outbreaks of Foodborne Disease Associated with Fruit and Vegetable Juices, 1922–2010. EDIS Publication FSHN12-04. Available online: http://ucfoodsafety.ucdavis.edu/files/223883.pdf (accessed on 8 March 2018).
- Foley, D.M.; Pickett, K.; Varon, J.; Lee, J.; Mln, D.B.; Caporaso, R.; Prakash, A. Pasteurization of fresh orange juice using gamma irradiation: Microbiological, flavor, and sensory analyses. J. Food Sci. 2002, 67, 1495–1501. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Nguyen, L.T.; Jiang, B.; Balasubramaniam, V.M. Improvement in texture of pressure-assisted thermally processed carrots by combined pretreatment using response surface methodology. Food Bioprocess. Technol. 2010, 3, 762–771. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Barbosa-Cánovas, G.V.; Lelieveld, H.L. High Pressure Processing of Food: Principles, Technology and Applications, 1st ed.; Springer Science & Business Media: New York, NY, USA, 2016; ISBN 978-1-4939-3234-4. [Google Scholar]
- Pinto, C.; Moreira, S.A.; Fidalgo, L.G.; Santos, M.D.; Delgadillo, I.; Saraiva, J.A. Shelf-life extension of watermelon juice preserved by hyperbaric storage at room temperature compared to refrigeration. LWT-Food Sci. Technol. 2016, 72, 78–80. [Google Scholar] [CrossRef]
- Pressure BioScience Inc. (PBI). High Pressure Processing. Available online: https://d1io3yog0oux5.cloudfront.net/_57b4ee00ca1d2b0fef872a3c158b2b76/pressurebiosciences/db/399/3062/pdf/Investor+Presentation+Oct+2017.pdf (accessed on 7 December 2017).
- Wang, C.Y.; Huang, H.W.; Hsu, C.P.; Yang, B.B. Recent advances in food processing using high hydrostatic pressure technology. Crit. Rev. Food Sci. Nutr. 2016, 56, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Fouladkhah, A.; Geornaras, I.; Yang, H.; Sofos, J.N. Lactic acid resistance of Shiga toxin-producing Escherichia coli and multidrug-resistant and susceptible Salmonella Typhimurium and Salmonella Newport in meat homogenate. Food Microbiol. 2013, 36, 260–266. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture-Food Safety Inspection Service (USDA-FSIS). Serotypes Profile of Salmonella Isolates from Meat and Poultry Products January 1998 through December 2014. 2014. Available online: https://www.fsis.usda.gov/wps/wcm/connect/3866026a-582d-4f0e-a8ce-851b39c7390f/Salmonella-Serotype-Annual-2014.pdf?MOD=AJPERES (accessed on 7 December 2017).
- Fouladkhah, A.; Geornaras, I.; Sofos, J.N. Effects of Reheating against Listeria monocytogenes Inoculated on Cooked Chicken Breast Meat Stored Aerobically at 7 degree C. Food Prot. Trends 2012, 32, 697–704. [Google Scholar]
- Manu, D.; Mendoca, A.; Daraba, A.; Dickson, J.; Sebranek, J.; Shaw, A.; Wang, F.; White, S. Antimicrobial efficacy of cinnamaldehyde against Escherichia coli O157: H7 and Salmonella enterica in carrot juice and mixed berry juice held at 4 C and 12 C. Foodborne Pathog. Dis. 2017, 14, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Geeraerd, A.H.; Valdramidis, V.P.; Van Impe, J.F. GInaFiT, A freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Estrada, R.M.; Hernández-Herrero, M.M.; López-Pedemonte, T.J.; Briñez-Zambrano, W.J.; Guamis-López, B.; Roig-Sagués, A.X. Inactivation of Listeria monocytogenes and Salmonella enterica serovar Senftenberg 775W inoculated into fruit juice by means of ultra-high pressure homogenisation. Food Control 2011, 22, 313–317. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Sofos, J.N. Comparative acid stress response of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella Typhimurium after habituation at different pH conditions. Lett. Appl. Microbiol. 2004, 38, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, A.S. Thermal inactivation of stationary-phase and acid-adapted Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes in fruit juices. J. Food Prot. 2001, 64, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, N.; Giaouris, E.; Grigoraki, I.; Skandamis, P.; Nychas, G.J. Effect of acid tolerance response (ATR) on attachment of Listeria monocytogenes Scott A to stainless steel under extended exposure to acid or/and salt stress and resistance of sessile cells to subsequent strong acid challenge. Int. J. Food Microbiol. 2011, 145, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lee, H.Y.; Ahn, J. High pressure inactivation kinetics of Salmonella enterica and Listeria monocytogenes in milk, orange juice, and tomato juice. Food Sci. Biotechnol. 2009, 18, 861–866. [Google Scholar]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology, 7th ed.; Springer Science & Business Media: New York, NY, USA, 2006; ISBN 978-0-387-23413-7. [Google Scholar]
- Buchanan, R.L.; Golden, M.H.; Whiting, R.C. Differentiation of the effects of pH and lactic or acetic acid concentration on the kinetics of Listeria monocytogenes inactivation. J. Food Prot. 1993, 56, 474–478. [Google Scholar] [CrossRef]
- Bull, M.K.; Zerdin, K.; Howe, E.; Goicoechea, D.; Paramanandhan, P.; Stockman, R.; Sellahewa, J.; Szabo, E.A.; Johnson, R.L.; Stewart, C.M. The effect of high pressure processing on the microbial, physical and chemical properties of Valencia and Navel orange juice. Innov. Food Sci. Emerg. Technol. 2004, 5, 135–149. [Google Scholar] [CrossRef]
- Polydera, A.C.; Stoforos, N.G.; Taoukis, P.S. Comparative shelf life study and vitamin C loss kinetics in pasteurised and high pressure processed reconstituted orange juice. J. Food Eng. 2003, 60, 21–29. [Google Scholar] [CrossRef]
- De Ancos, B.; Sgroppo, S.; Plaza, L.; Cano, M.P. Possible nutritional and health-related value promotion in orange juice preserved by high-pressure treatment. J. Sci. Food Agric. 2002, 82, 790–796. [Google Scholar] [CrossRef]
- Velázquez-Estrada, R.M.; Hernández-Herrero, M.M.; Rüfer, C.E.; Guamis-López, B.; Roig-Sagués, A.X. Influence of ultra-high pressure homogenization processing on bioactive compounds and antioxidant activity of orange juice. Innov. Food Sci. Emerg. Technol. 2013, 18, 89–94. [Google Scholar] [CrossRef]
- Fouladkhah, A.; Geornaras, I.; Nychas, G.J.; Sofos, J.N. Antilisterial Properties of Marinades during Refrigerated Storage and Microwave Oven Reheating against Post-Cooking Inoculated Chicken Breast Meat. J. Food Sci. 2013, 78, M285–M289. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.; Chowdhury, S.; Fouladkhah, A. Biofilm formation and decontamination of wild-type and pressure-stressed Cronobacter sakazakii and Salmonella serovars. Health and Medical Sciences Section. In Proceedings of the 127th Meeting of Tennessee Academy of Science, University of Tennessee at Martin, Martin, TN, USA, 17 November 2017. [Google Scholar]
- Daryaei, H.; Balasubramaniam, V.M. Kinetics of Bacillus coagulans spore inactivation in tomato juice by combined pressure–heat treatment. Food Control 2013, 30, 168–175. [Google Scholar] [CrossRef]
- Parish, M.E. High pressure inactivation of Saccharomyces cerevisiae, endogenous microflora and pectinmethylesterase in orange juice. J. Food Saf. 1998, 18, 57–65. [Google Scholar] [CrossRef]
- Yue, T.; Zhang, J.; Yuan, Y. Spoilage by Alicyclobacillus bacteria in juice and beverage products: Chemical, physical, and combined control methods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 771–797. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allison, A.; Daniels, E.; Chowdhury, S.; Fouladkhah, A. Effects of Elevated Hydrostatic Pressure against Mesophilic Background Microflora and Habituated Salmonella Serovars in Orange Juice. Microorganisms 2018, 6, 23. https://doi.org/10.3390/microorganisms6010023
Allison A, Daniels E, Chowdhury S, Fouladkhah A. Effects of Elevated Hydrostatic Pressure against Mesophilic Background Microflora and Habituated Salmonella Serovars in Orange Juice. Microorganisms. 2018; 6(1):23. https://doi.org/10.3390/microorganisms6010023
Chicago/Turabian StyleAllison, Abimbola, Edward Daniels, Shahid Chowdhury, and Aliyar Fouladkhah. 2018. "Effects of Elevated Hydrostatic Pressure against Mesophilic Background Microflora and Habituated Salmonella Serovars in Orange Juice" Microorganisms 6, no. 1: 23. https://doi.org/10.3390/microorganisms6010023



