Novel Effector Protein EspY3 of Type III Secretion System from Enterohemorrhagic Escherichia coli Is Localized in Actin Pedestals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Medium
2.2. Primers Design, PCR Amplification and EspY3 Cloning
2.3. Detection of Recombinant Secreted Effectors to the Culture Supernatant
2.4. Translocation Assay and Fluorescence Confocal Microscopy
3. Results
3.1. Bioinformatic Analysis of EspY3
3.2. Expression and Secretion of Recombinant EspY3 Protein by T3SS
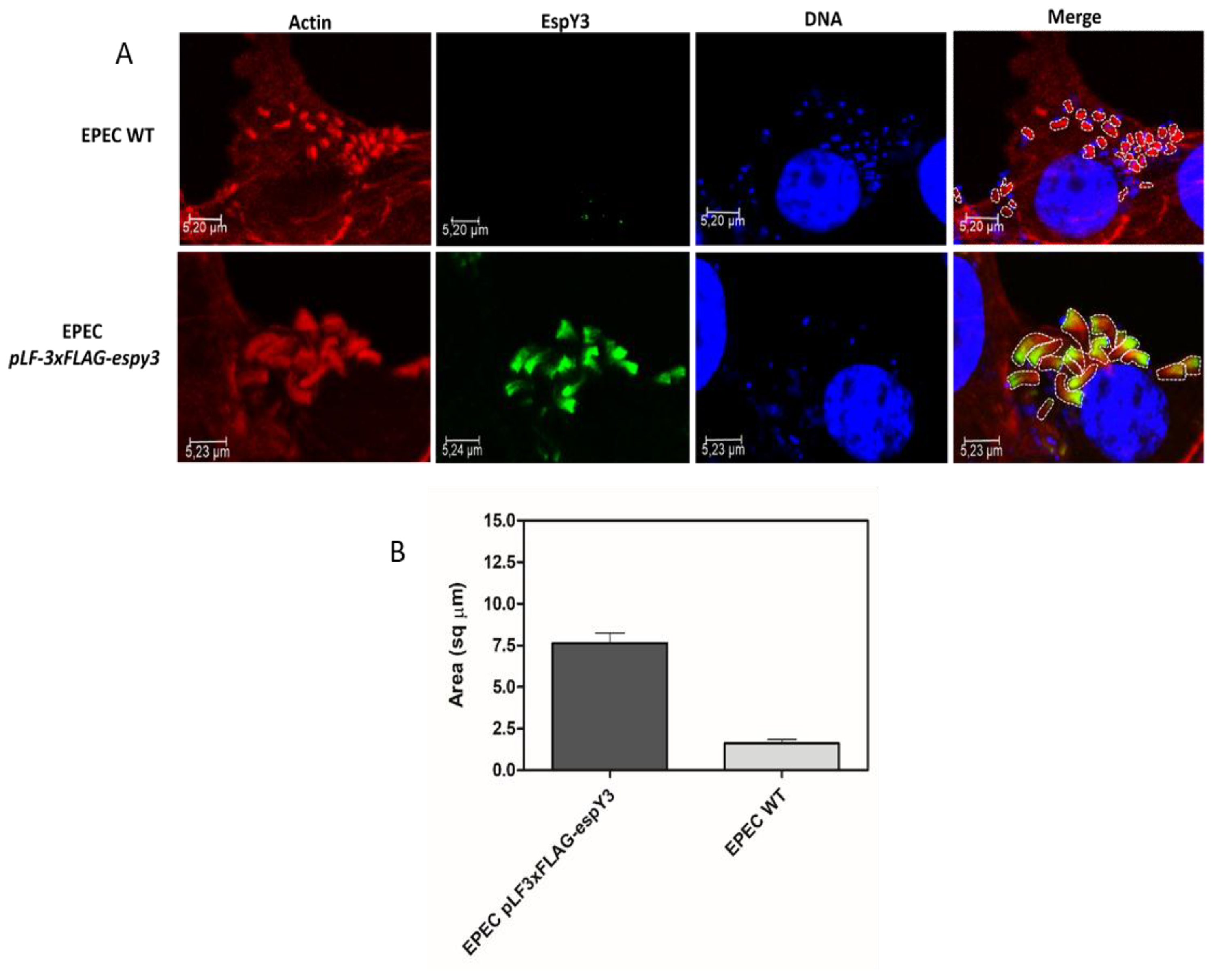
3.3. Subcellular Localization of EspY3 by Confocal Microscopy
3.4. Elongation of Polymerized Actin Pedestals by EspY3
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garmendia, J.; Frankel, G.; Crepin, V.F. Enteropathogenic and enterohemorrhagic Escherichia coli infections: Translocation, translocation, translocation. Infect. Immun. 2005, 73, 2573–2585. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Griffin, P.M.; Ostroff, S.M.; Tauxe, R.V.; Greene, K.D.; Wells, J.G.; Lewis, J.H.; Blake, P.A. Illnesses associated with Escherichia coli O157:H7 infections: A broad clinical spectrum. Ann. Intern. Med. 1988, 109, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Donnenberg, M.S. Pathogenic strategies of enteric bacteria. Nature 2000, 406, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; de Hoog, C.L.; Yu, H.B.; Li, Y.; Croxen, M.A.; Thomas, N.A.; Puente, J.L.; Foster, L.J.; Finlay, B.B. A comprehensive proteomic analysis of the type III secretome of Citrobacter rodentium. J. Biol. Chem. 2010, 285, 6790–6800. [Google Scholar] [CrossRef] [PubMed]
- Campellone, K.G.; Rankin, S.; Pawson, T.; Kirschner, M.W.; Tipper, D.J.; Leong, J.M. Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly. J. Cell. Biol. 2004, 164, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, B.; Finlay, B.B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-gamma1. Infect. Immun. 1997, 65, 2528–2536. [Google Scholar] [PubMed]
- Gruenheid, S.; DeVinney, R.; Bladt, F.; Goosney, D.; Gelkop, S.; Gish, G.D.; Pawson, T.; Finlay, B.B. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell. Biol. 2001, 3, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.; Weiner, O.D.; Goosney, D.L.; Sedat, J.W.; Finlay, B.B.; Abo, A.; Bishop, J.M. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat. Cell. Biol. 1999, 1, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Arbeloa, A.; Bulgin, R.R.; MacKenzie, G.; Shaw, R.K.; Pallen, M.J.; Crepin, V.F.; Berger, C.N.; Frankel, G. Subversion of actin dynamics by EspM effectors of attaching and effacing bacterial pathogens. Cell Microbiol. 2008, 10, 1429–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, K.S.; Mousnier, A.; Hemrajani, C.; Fairweather, N.; Berger, C.N.; Frankel, G. The enteropathogenic Escherichia coli effector NleH inhibits apoptosis induced by Clostridium difficile toxin B. Microbiology 2010, 156, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Sham, H.P.; Shames, S.R.; Croxen, M.A.; Ma, C.; Chan, J.M.; Khan, M.A.; Wickham, M.E.; Deng, W.; Finlay, B.B.; Vallance, B.A. Attaching and effacing bacterial effector NleC suppresses epithelial inflammatory responses by inhibiting NF-κB and p38 mitogen-activated protein kinase activation. Infect. Immun. 2011, 79, 3552–3562. [Google Scholar] [CrossRef] [PubMed]
- Newton, H.J.; Pearson, J.S.; Badea, L.; Kelly, M.; Lucas, M.; Holloway, G.; Wagstaff, K.M.; Dunstone, M.A.; Sloan, J.; Whisstock, J.C.; et al. The type III effectors NleE and NleB from enteropathogenic E. coli and OspZ from shigella block nuclear translocation of NF-κB p65. PLoS Pathog. 2010, 6, e1000898. [Google Scholar] [CrossRef] [PubMed]
- Royan, S.V.; Jones, R.M.; Koutsouris, A.; Roxas, J.L.; Falzari, K.; Weflen, A.W.; Kim, A.; Bellmeyer, A.; Turner, J.R.; Neish, A.S.; et al. Enteropathogenic E. coli non-LEE encoded effectors NleH1 and NleH2 attenuate NF-κB activation. Mol. Microbiol. 2010, 78, 1232–1245. [Google Scholar] [CrossRef] [PubMed]
- Quitard, S.; Dean, P.; Maresca, M.; Kenny, B. The enteropathogenic Escherichia coli EspF effector molecule inhibits PI-3 kinase-mediated uptake independently of mitochondrial targeting. Cell Microbiol. 2006, 8, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Liu, L.; Shao, F. A bacterial effector targets host DH-PH domain RhoGEFs and antagonizes macrophage phagocytosis. EMBO J. 2010, 29, 1363–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchès, O.; Covarelli, V.; Dahan, S.; Cougoule, C.; Bhatta, P.; Frankel, G.; Caron, E. EspJ of enteropathogenic and enterohaemorrhagic Escherichia coli inhibits opsono-phagocytosis. Cell Microbiol. 2008, 10, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Kenny, B.; DeVinney, R.; Stein, M.; Reinscheid, D.J.; Frey, E.A.; Finlay, B.B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 1997, 91, 511–520. [Google Scholar] [CrossRef]
- Jerse, A.E.; Yu, J.; Tall, B.D.; Kaper, J.B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7839–7843. [Google Scholar] [CrossRef] [PubMed]
- Tobe, T.; Beatson, S.A.; Taniguchi, H.; Abe, H.; Bailey, C.M.; Fivian, A.; Younis, R.; Matthews, S.; Marches, O.; Frankel, G.; et al. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc. Natl. Acad. Sci. USA 2006, 103, 14941–14946. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.A.; Miller, S.I. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 2000, 97, 7539–7544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumell, J.H.; Kujat-Choy, S.; Brown, N.F.; Vallance, B.A.; Knodler, L.A.; Finlay, B.B. SopD2 is a novel type III secreted effector of Salmonella typhimurium that targets late endocytic compartments upon delivery into host cells. Traffic 2003, 4, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Lawley, T.D.; Chan, K.; Thompson, L.J.; Kim, C.C.; Govoni, G.R.; Monack, D.M. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2006, 2, e11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Rossanese, O.W.; Brown, N.F.; Kujat-Choy, S.; Galán, J.E.; Finlay, B.B.; Brumell, J.H. The related effector proteins SopD and SopD2 from Salmonella enterica serovar Typhimurium contribute to virulence during systemic infection of mice. Mol Microbiol 2004, 54, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Bakowski, M.A.; Cirulis, J.T.; Brown, N.F.; Finlay, B.B.; Brumell, J.H. SopD acts cooperatively with SopB during Salmonella enterica serovar Typhimurium invasion. Cell Microbiol. 2007, 9, 2839–2855. [Google Scholar] [CrossRef] [PubMed]
- Blasche, S.; Arens, S.; Ceol, A.; Siszler, G.; Schmidt, M.A.; Häuser, R.; Schwarz, F.; Wuchty, S.; Aloy, P.; Uetz, P.; et al. The EHEC-host interactome reveals novel targets for the translocated intimin receptor. Sci. Rep. 2015, 4, 7531. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.; Couillault, C.; Rockenfeller, P.; Boucrot, E.; Dumont, A.; Schroeder, N.; Hermant, A.; Knodler, L.A.; Lecine, P.; Steele-Mortimer, O.; et al. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc. Natl. Acad. Sci. USA 2006, 103, 13497–13502. [Google Scholar] [CrossRef] [PubMed]
- Knodler, L.A.; Vallance, B.A.; Hensel, M.; Jäckel, D.; Finlay, B.B.; Steele-Mortimer, O. Salmonella type III effectors PipB and PipB2 are targeted to detergent-resistant microdomains on internal host cell membranes. Mol. Microbiol. 2004, 49, 685–704. [Google Scholar] [CrossRef]
- Bateman, A.; Murzin, A.G.; Teichmann, S.A. Structure and distribution of pentapeptide repeats in bacteria. Protein Sci. 1998, 7, 1477–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosqvist, R.; Håkansson, S.; Forsberg, A.; Wolf-Watz, H. Functional conservation of the secretion and translocation machinery for virulence proteins of Yersiniae, Salmonellae and Shigellae. EMBO J. 1995, 14, 4187–4195. [Google Scholar] [CrossRef]
- Frithz-Lindsten, E.; Du, Y.; Rosqvist, R.; Forsberg, A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol. Microbiol. 1997, 25, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.H.; Bauer, D.W.; Fouts, D.E.; Collmer, A. A cloned Erwinia chrysanthemi Hrp (type III protein secretion) system functions in Escherichia coli to deliver Pseudomonas syringae Avr signals to plant cells and to secrete Avr proteins in culture. Proc. Natl. Acad. Sci. USA 1998, 95, 10206–10211. [Google Scholar] [CrossRef] [PubMed]
- Subtil, A.; Parsot, C.; Dautry-Varsat, A. Secretion of predicted Inc proteins of Chlamydia pneumoniae by a heterologous type III machinery. Mol. Microbiol. 2001, 39, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Fouts, D.E.; Collmer, A.; Schneewind, O. Reciprocal secretion of proteins by the bacterial type III machines of plant and animal pathogens suggests universal recognition of mRNA targeting signals. Proc. Natl. Acad. Sci. USA 1999, 96, 12839–12843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.M.; Schneewind, O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 1997, 278, 1140–1143. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larzábal, M.; Marques Da Silva, W.; Riviere, N.A.; Cataldi, Á.A. Novel Effector Protein EspY3 of Type III Secretion System from Enterohemorrhagic Escherichia coli Is Localized in Actin Pedestals. Microorganisms 2018, 6, 112. https://doi.org/10.3390/microorganisms6040112
Larzábal M, Marques Da Silva W, Riviere NA, Cataldi ÁA. Novel Effector Protein EspY3 of Type III Secretion System from Enterohemorrhagic Escherichia coli Is Localized in Actin Pedestals. Microorganisms. 2018; 6(4):112. https://doi.org/10.3390/microorganisms6040112
Chicago/Turabian StyleLarzábal, Mariano, Wanderson Marques Da Silva, Nahuel A. Riviere, and Ángel A. Cataldi. 2018. "Novel Effector Protein EspY3 of Type III Secretion System from Enterohemorrhagic Escherichia coli Is Localized in Actin Pedestals" Microorganisms 6, no. 4: 112. https://doi.org/10.3390/microorganisms6040112



