Enzymes Catalyzing the TCA- and Urea Cycle Influence the Matrix Composition of Biofilms Formed by Methicillin-Resistant Staphylococcus aureus USA300
Abstract
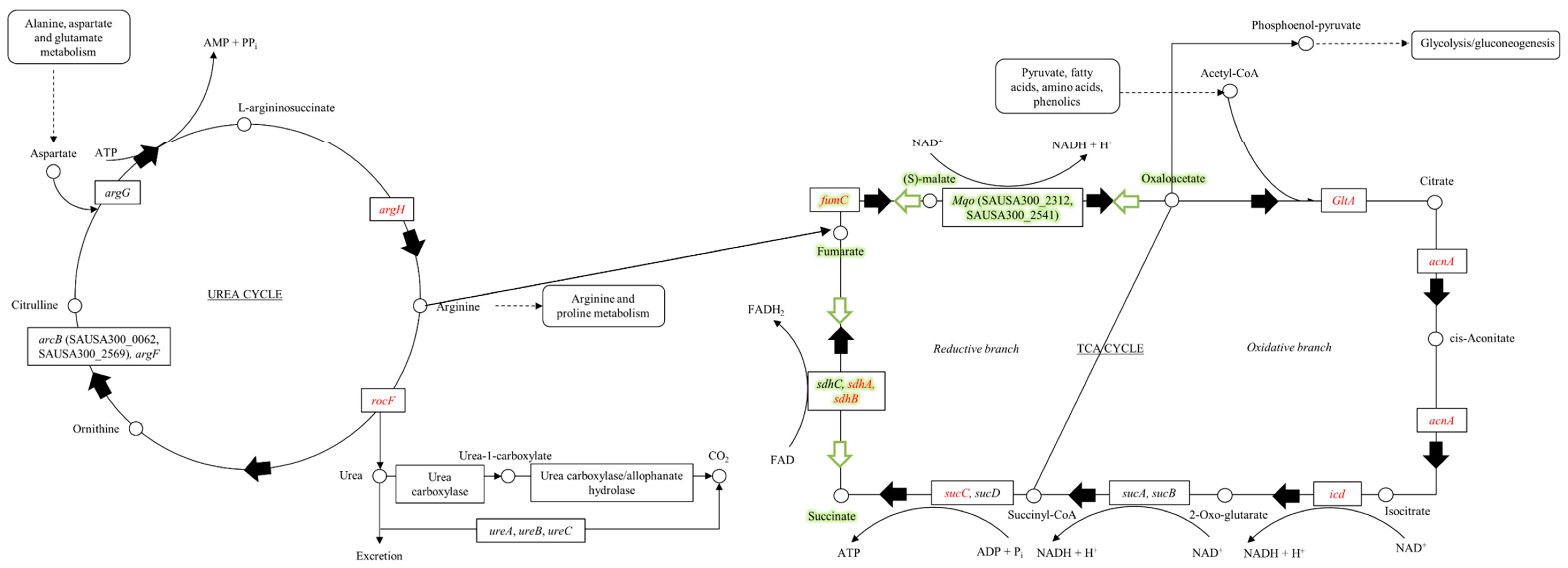
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Transduction Experiments
2.3. Growth Rate Analysis
2.4. Quantitative Biofilm Assay under Static (No Flow) Conditions
2.5. Quantitative Biofilm Assay under Flow (Dynamic) Conditions
2.6. Analysis of Biofilm Matrix Composition
2.7. Relative Gene Expression Analysis
2.8. Complementation Experiments
2.9. Statistical Analysis
3. Results
3.1. Urea Cycle Mutant argH::Tn, but Not rocF::Tn, Demonstrates a Significantly Decreased Capacity for Biofilm Formation
3.2. TCA-Cycle Mutants, fumC::Tn, sdhA::Tn and sdhB::Tn, but not acnA::Tn, icd::Tn, gltA::Tn and sucC::Tn, Demonstrate a Significantly Decreased Capacity for Biofilm Formation
3.3. Fluorescent Staining of the Biofilm Matrix Reveals That the Protein Component Is Decreased in TCA- and Urea Cyle Mutants
3.4. Enzymatic Digest Correlates BIOFILM-Defective Transposon Mutants with a Protein- and eDNA-Based Matrix
4. Discussion
4.1. TCA-Cycle Inactivation Impacts the Protein Component of the Biofilm Matrix of MRSA-USA300
4.2. Inactivation of Specific TCA-Cycle Genes Is Associated with a High Metabolic Fitness Cost
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, F.; Humphreys, H.; O’Gara, J.P. Environmental regulation of biofilm development in methicillin-resistant and methicillin-susceptible Staphylococcus aureus clinical isolates. J. Hosp. Infect. 2006, 62, 120–122. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Pozzi, C.; Houston, P.; Smyth, D.; Humphreys, H.; Robinson, D.A.; O’Gara, J.P. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J. Clin. Microbiol. 2007, 45, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, J.P. Ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 2007, 270, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Waters, E.M.; Rudkin, J.K.; Schaeffer, C.R.; Lohan, A.J.; Tong, P.; Loftus, B.J.; Pier, G.B.; Fey, P.D.; Massey, R.C.; et al. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 2012, 8, e1002626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, E.; Pozzi, C.; Houston, P.; Humphreys, H.; Robinson, D.A.; Loughman, A.; Foster, T.J.; O’Gara, J.P. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 2008, 190, 3835–3850. [Google Scholar] [CrossRef] [PubMed]
- Izano, E.A.; Amarante, M.A.; Kher, W.B.; Kaplan, J.B. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 2008, 74, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Houston, P.; Rowe, S.E.; Pozzi, C.; Waters, E.M.; O’Gara, J.P. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect. Immun. 2011, 79, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Rice, K.C.; Boles, B.R.; Endres, J.L.; Ranjit, D.; Chandramohan, L.; Tsang, L.H.; Smeltzer, M.S.; Horswill, A.R.; Bayles, K.W. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE 2009, 4, e5822. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.C.; Mann, E.E.; Endres, J.L.; Weiss, E.C.; Cassat, J.E.; Smeltzer, M.S.; Bayles, K.W. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2007, 104, 8113–8118. [Google Scholar] [CrossRef] [PubMed]
- Sonenshein, A.L. The Krebs Citric Acid Cycle; ASM Press: Washington, DC, USA, 2002. [Google Scholar]
- Sadykov, M.R.; Olson, M.E.; Halouska, S.; Zhu, Y.; Fey, P.D.; Powers, R.; Somerville, G.A. Tricarboxylic acid cycle-dependent regulation of Staphylococcus epidermidis polysaccharide intercellular adhesin synthesis. J. Bacteriol. 2008, 190, 7621–7632. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Kidder, J.B.; Jacobson, E.R.; Otto, M.; Proctor, R.A.; Somerville, G.A. Staphylococcus epidermidis polysaccharide intercellular adhesin production significantly increases during tricarboxylic acid cycle stress. J. Bacteriol. 2005, 187, 2967–2973. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xiong, Y.Q.; Sadykov, M.R.; Fey, P.D.; Lei, M.G.; Lee, C.Y.; Bayer, A.S.; Somerville, G.A. Tricarboxylic acid cycle-dependent attenuation of Staphylococcus aureus in vivo virulence by selective inhibition of amino acid transport. Infect. Immun. 2009, 77, 4256–4264. [Google Scholar] [CrossRef] [PubMed]
- Fey, P.D.; Endres, J.L.; Yajjala, V.K.; Widhelm, T.J.; Boissy, R.J.; Bose, J.L.; Bayles, K.W. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio 2013, 4, e00537-00512. [Google Scholar] [CrossRef] [PubMed]
- Sabirova, J.S.; Hernalsteens, J.P.; De Backer, S.; Xavier, B.B.; Moons, P.; Turlej-Rogacka, A.; De Greve, H.; Goossens, H.; Malhotra-Kumar, S. Fatty acid kinase A is an important determinant of biofilm formation in Staphylococcus aureus USA300. BMC Genom. 2015, 16, 861. [Google Scholar] [CrossRef] [PubMed]
- Vanhommerig, E.; Moons, P.; Pirici, D.; Lammens, C.; Hernalsteens, J.P.; De Greve, H.; Kumar-Singh, S.; Goossens, H.; Malhotra-Kumar, S. Comparison of biofilm formation between major clonal lineages of methicillin-resistant Staphylococcus aureus. PLoS ONE 2014, 9, e104561. [Google Scholar] [CrossRef] [PubMed]
- Sabirova, J.S.; Xavier, B.B.; Hernalsteens, J.P.; De Greve, H.; Ieven, M.; Goossens, H.; Malhotra-Kumar, S. Complete genome sequences of two prolific biofilm-forming Staphylococcus aureus isolates belonging to USA300 and EMRSA-15 clonal lineages. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Luppens, S.B.I.; Reij, M.W.; van der Heijden, R.W.L.; Rombouts, F.M.; Abee, T. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl. Environ. Microbiol. 2002, 68, 4194–4200. [Google Scholar] [CrossRef] [PubMed]
- Somerville, G.A.; Proctor, R.A. At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol. Mol. Biol. Rev. 2009, 73, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Bateman, B.T.; Donegan, N.P.; Jarry, T.M.; Palma, M.; Cheung, A.L. Evaluation of a tetracycline-inducible promoter in Staphylococcus aureus in vitro and in vivo and its application in demonstrating the role of sigB in microcolony formation. Infect. Immun. 2001, 69, 7851–7857. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G.; Acar, H.; Nandipati, A.; Barlow, M. Growth rates made easy. Mol. Biol. Evol. 2014, 31, 232–238. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.; Lee, J.C. Staphylococcus aureus Capsular Polysaccharides. Clin. Microbiol. Rev. 2004, 17, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, M.R.; Mattes, T.A.; Luong, T.T.; Zhu, Y.; Day, S.R.; Sifri, C.D.; Lee, C.Y.; Somerville, G.A. Tricarboxylic acid cycle-dependent synthesis of Staphylococcus aureus type 5 and 8 capsular polysaccharides. J. Bacteriol. 2010, 192, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Boyle-Vavra, S.; Li, X.; Alam, M.T.; Read, T.D.; Sieth, J.; Cywes-Bentley, C.; Dobbins, G.; David, M.Z.; Kumar, N.; Eells, S.J.; et al. USA300 and USA500 clonal lineages of Staphylococcus aureus do not produce a capsular polysaccharide due to conserved mutations in the cap5 locus. MBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Lauderdale, K.J.; Malone, C.L.; Boles, B.R.; Morcuende, J.; Horswill, A.R. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J. Orthop. Res. 2010, 28, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 207–228. [Google Scholar] [PubMed]
- Shanks, R.M.; Meehl, M.A.; Brothers, K.M.; Martinez, R.M.; Donegan, N.P.; Graber, M.L.; Cheung, A.L.; O’Toole, G.A. Genetic evidence for an alternative citrate-dependent biofilm formation pathway in Staphylococcus aureus that is dependent on fibronectin binding proteins and the GraRS two-component regulatory system. Infect. Immun. 2008, 76, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Mrak, L.N.; Zielinska, A.K.; Beenken, K.E.; Mrak, I.N.; Atwood, D.N.; Griffin, L.M.; Lee, C.Y.; Smeltzer, M.S. saeRS and sarA act synergistically to repress protease production and promote biofilm formation in Staphylococcus aureus. PLoS ONE 2012, 7, e38453. [Google Scholar] [CrossRef] [PubMed]
- Halsey, C.R.; Lei, S.; Wax, J.K.; Lehman, M.K.; Nuxoll, A.S.; Steinke, L.; Sadykov, M.; Powers, R.; Fey, P.D. Amino acid catabolism in Staphylococcus aureus and the function of carbon catabolite repression. MBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]



| Name | Description | Source |
|---|---|---|
| Strains | ||
| UAS391 | Prolific biofilm forming MRSA USA300 strain isolated from a patient with an abscess in a Belgian hospital. | [18,19] |
| UAS391-EryS | Erythromycin-sensitive variant (loss of ermC gene) of S. aureus UAS391 obtained by plasmid curing through growth at 44 °C. | [17] |
| JE2 | Plasmid-cured derivative of MRSA USA300 LAC, isolated from a skin and soft tissue infection in a detainee from the Los Angeles County jail. | [16] |
| RN0450 (NRS135) | MSSA strain derived by successive cycles of UV treatment of S. aureus strain NCTC8325, curing it of phages Φ11, Φ12 and Φ13. | NARSA repository |
| RN0451 (NRS136) | MSSA strain derived from S. aureus strain RN0450, lysogenic for phage Φ11. | NARSA repository |
| RN4220 | Generated through UV and chemical mutagenesis of S. aureus strain RN0450 and selected for transformability with DNA from E. coli (restriction deficient through mutation in sau1 hsdR). | NARSA repository |
| DH5α | Escherichia coli cloning strain with multiple mutations (fhuA2 lacΔU169 phoA glnV44 Φ80’ lacZΔM15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17) that enable efficient transformation. | Thermo Fisher Scientific Inc., Waltham, MA, USA |
| ATCC® 6538™ | Positive quality control for biofilm formation of S. aureus under no flow conditions. | [20] |
| 5374 | Negative quality control for biofilm formation of S. aureus under no flow conditions. | [18] |
| ATCC® 25923™ | PIA/PNAG-dependent biofilm producing strain used as positive quality control during fluorescent staining and enzymatic treatment of biofilm matrix. | [21] |
| NE106 (NR-46649) | JE2 Tn mutant (insertion position: 943092) in argininosuccinate lyase (argH, SAUSA300_0863; 942072-943451). | NARSA repository |
| NE134 (NR-46677) | JE2 Tn mutant (insertion position: 2290057) in arginase (rocF, SAUSA300_2114; 2289284-2290192). | NARSA repository |
| NE427 (NR-46970) | JE2 Tn mutant (insertion position: 1985575) in fumarate hydratase, class II (fumC, SAUSA300_1801; 1984212-1985597). | NARSA repository |
| NE491 (NR-47034) | JE2 Tn mutant (insertion position: 1799416) in isocitrate dehydrogenase, NADP-dependent (icd, SAUSA300_1640; 1798291-1799559). | NARSA repository |
| NE569 | JE2 Tn mutant (insertion position: 1247122) in succinyl-CoA synthetase, beta subunit (sucC, SAUSA300_1138; 1246832-1247998). | NARSA repository |
| NE594 (NR-47137) | JE2 Tn mutant (insertion position: 1800430) in citrate synthase II (gltA, SAUSA300_1641; 1799608-1800729). | NARSA repository |
| NE626 (NR-47169) | JE2 Tn mutant (insertion position: 1145819) in succinate dehydrogenase, flavoprotein subunit (sdhA, SAUSA300_1047; 1145459-1147225). | NARSA repository |
| NE808 (NR-47351) | JE2 Tn mutant (insertion position: 1147490) in succinate dehydrogenase iron-sulfur subunit (sdhB, SAUSA300_1048; 1147225-1148040). | NARSA repository |
| NE861 (NR-47404) | JE2 Tn mutant (insertion position: 1367722) in aconitate hydratase (acnA, SAUSA300_1246; 1367131-1369836). | NARSA repository |
| Plasmids | ||
| pALC2073 | Contains the pSK236 vector, with the tetR-gene and the xyl/tetO promotor, originating from pWH35. | [22] |
| pGV5990 | argH gene amplified with primers ArgH-1 and ArgH-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | This study |
| pGV5992 | gltA gene amplified with primers GltA-1 and GltA-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| pGV5994 | icd gene amplified with primers Icd-1 and Icd-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| pGV5996 | sdhB gene amplified with primers SdhB-1 and SdhB-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| pGV5998 | sucC gene amplified with primers SucC-1 and SucC-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| pGV5999 | gltA & icd genes (operon of two genes; 1st gene gltA & 2nd gene icd) amplified with primers GltA-1 and Icd-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| pGV6000 | sucC genes (operon of two genes) amplified with primers SucC-1 and SucC-3 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| pGV6001 | sdhA gene amplified with primer SdhA-1 and SdhA-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| pGV6002 | sdhA (2nd gene in operon of 3 genes) & sdhB (3rd gene in operon of 3 genes) genes amplified with primers SdhA-1 and SdhB-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| pGV6003 | rocF gene amplified with primers RocF-1 and RocF-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| pGV6005 | fumC gene amplified with primers FumC-1 and FumC-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| pGV6007 | acnA gene amplified with primers AcnA-1 and AcnA-2 and cloned in the EcoRI site of pALC2073 via Gibson cloning. | |
| Strain | Optical Density (OD492) | Integrated Density (Fluorescence in Pixels) | Growth Rate (min−1) | Ratio Live:Dead Cells (%) | Protein Component (%) | PIA/PNAG Component (%) |
|---|---|---|---|---|---|---|
| UAS391-Erys | 0.814 ± 0.14 (100%) | 11,301 ± 61 (100%) | 0.157 ± 0.01 | 61:39 ± 3:1 | 100 ± 3 | 100 ± 6 |
| ATCC® 25923™ | NT | NT | NT | 58:42 ± 13:4 | 34 ± 8 | 835 ± 2 |
| argH::Tn | 0.504 ± 0.07 (62%) | 3653 ± 45 (32%) | 0.161 ± 0.01 | 44:56 ± 2:1 | 48 ± 10 | 110 ± 15 |
| argH::Tn with pGV5990 | 0.828 ± 0.15 (102%) | 9389 ± 66 (83%) | NT | 59:41 ± 16:5 | 83 ± 2 | 106 ± 16 |
| acnA::Tn | 0.782 ± 0.11 (96%) | 651 ± 036 (58%) | 0.163 ± 0.01 | 61:39 ± 2:1 | 64 ± 3 | 77 ± 4 |
| acnA::Tn with pGV6007 | 0.805 ± 0.15 (99%) | 773 ± 57 (68%) | NT | 60:40 ± 10:3 | 92 ± 16 | 101 ± 11 |
| icd::Tn | 0.838 ± 0.13 (103%) | 8264 ± 16 (73%) | 0.166 ± 0.01 | 58:42 ± 13:4 | 67 ± 4 | 110 ± 17 |
| icd::Tn with pGV5994 | 1.143 ± 0.12 (141%) | 8308 ± 163 (74%) | NT | 57:43 ± 15:5 | 91 ± 3 | 100 ± 20 |
| icd::Tn with pGV5999 | 1.248 ± 0.17 (153%) | 8076 ± 133 (72%) | NT | 54:46 ± 19:7 | 96 ± 22 | 101 ± 9 |
| gltA::Tn | 0.789 ± 0.11 (97%) | 7911 ± 11 (70%) | 0.157 ± 0.01 | 57:43 ± 18:6 | 61 ± 10 | 91 ± 5 |
| gltA::Tn with pGV5992 | 1.102 ± 0.12 (135%) | 9616 ± 25 (85%) | NT | 61:39 ± 5:2 | 93 ± 0 | 100 ± 12 |
| gltA::Tn with pGV5999 | 1.055 ± 0.10 (130%) | 8149 ± 27 (72%) | NT | 59:41 ± 7:2 | 89 ± 9 | 100 ± 2 |
| fumC::Tn | 0.374 ± 0.07 (46%) | 2065 ± 42 (18%) | 0.162 ± 0.01 | 43:57 ± 1:0 | 49 ± 0 | 122 ± 7 |
| fumC::Tn with pGV6005 | 0.941 ± 0.14 (116%) | 7662 ± 188 (68%) | NT | 66:34 ± 10:3 | 80 ± 5 | 100 ± 13 |
| sucC::Tn | 0.847 ± 0.17 (104%) | 7506 ± 58 (67%) | 0.150 ± 0.01 | 47:53 ± 19:6 | 54 ± 3 | 103 ± 17 |
| sucC::Tn with pGV5998 | 0.804 ± 0.11 (99%) | 8715 ± 10 (77%) | NT | 65:35 ± 15:5 | 96 ± 7 | 101 ± 16 |
| sucC::Tn with pGV6000 | 1.041 ± 0.13 (128%) | 7599 ± 159 (67%) | NT | 68:32 ± 11:4 | 83 ± 2 | 101 ± 5 |
| sdhA::Tn | 0.537 ± 0.11 (66%) | 3835 ± 60 (34%) | 0.162 ± 0.01 | 47:53 ± 5:2 | 33 ± 3 | 89 ± 8 |
| sdhA::Tn with pGV6001 | 1.002 ± 0.10 (123%) | 9002 ± 11 (80%) | NT | 51:49 ± 6:2 | 78 ± 8 | 101 ± 6 |
| sdhA::Tn with pGV6002 | 1.071 ± 0.11 (132%) | 7252 ± 092 (64%) | NT | 53:47 ± 4:1 | 92 ± 10 | 100 ± 11 |
| sdhB::Tn | 0.667 ± 0.10 (82%) | 4875 ± 056 (43%) | 0.159 ± 0.01 | 48:52 ± 5:2 | 44 ± 18 | 84 ± 7 |
| sdhB::Tn with pGV5996 | 0.908 ± 0.12 (112%) | 11,057 ± 129 (98%) | NT | 66:34 ± 6:2 | 106 ± 21 | 100 ± 13 |
| sdhB::Tn with pGV6002 | 1.140 ± 0.09 (140%) | 8455 ± 65 (75%) | NT | 49:51 ± 5:2 | 92 ± 3 | 100 ± 17 |
| rocF::Tn | 0.765 ± 0.12 (94%) | 7586 ± 15 (67%) | 0.163 ± 0.01 | 49:51 ± 5:2 | 51 ± 11 | 89 ± 19 |
| rocF::Tn with pGV6003 | 0.878 ± 0.09 (108%) | 8342 ± 21 (74%) | NT | 65:35 ± 12:4 | 84 ± 3 | 101 ± 8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Backer, S.; Sabirova, J.; De Pauw, I.; De Greve, H.; Hernalsteens, J.-P.; Goossens, H.; Malhotra-Kumar, S. Enzymes Catalyzing the TCA- and Urea Cycle Influence the Matrix Composition of Biofilms Formed by Methicillin-Resistant Staphylococcus aureus USA300. Microorganisms 2018, 6, 113. https://doi.org/10.3390/microorganisms6040113
De Backer S, Sabirova J, De Pauw I, De Greve H, Hernalsteens J-P, Goossens H, Malhotra-Kumar S. Enzymes Catalyzing the TCA- and Urea Cycle Influence the Matrix Composition of Biofilms Formed by Methicillin-Resistant Staphylococcus aureus USA300. Microorganisms. 2018; 6(4):113. https://doi.org/10.3390/microorganisms6040113
Chicago/Turabian StyleDe Backer, Sarah, Julia Sabirova, Ines De Pauw, Henri De Greve, Jean-Pierre Hernalsteens, Herman Goossens, and Surbhi Malhotra-Kumar. 2018. "Enzymes Catalyzing the TCA- and Urea Cycle Influence the Matrix Composition of Biofilms Formed by Methicillin-Resistant Staphylococcus aureus USA300" Microorganisms 6, no. 4: 113. https://doi.org/10.3390/microorganisms6040113





