DNA Methylation Changes Induced by Cold in Psychrophilic and Psychrotolerant Naganishia Yeast Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Determination of the Optimum Temperature
2.3. Yeasts Growth
2.4. DNA Methylation Analysis
3. Results
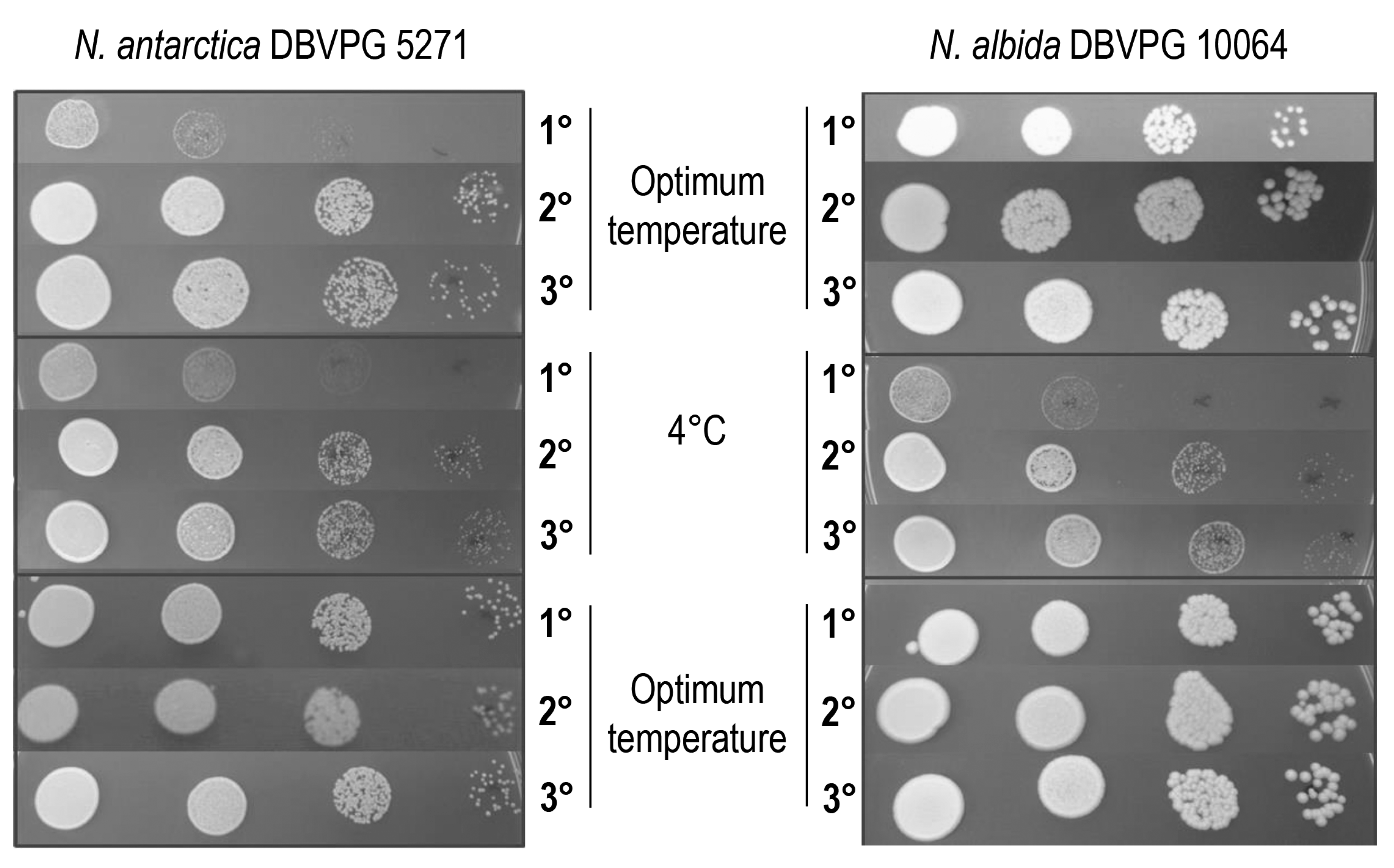
3.1. Yeasts Growth
3.2. Effect of Cold stress on DNA Methylation
3.3. Sequencing of Differentially Methylated DNA Fragments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Begerow, D.; Kemler, M.; Feige, A.; Yurkov, A. Yeasts in Natural Ecosystems: Ecology; Buzzini, P., Lachance, M.A., Yurkov, A., Eds.; Springer: Berlin, Germany, 2017; pp. 179–210. [Google Scholar]
- Inacio, J.; Daniel, H.M. Yeasts in Natural Ecosystems: Ecology; Buzzini, P., Lachance, M.A., Yurkov, A., Eds.; Springer: Berlin, Germany, 2017; pp. 211–228. [Google Scholar]
- Starmer, W.T.; Lachance, M.A. The Yeasts, a Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 65–83. [Google Scholar]
- Yurkov, A. Yeasts in Natural Ecosystems: Ecology; Springer: Berlin, Germany, 2017; pp. 101–130. [Google Scholar]
- Peter, G.; Takashima, M.; Cadež, N. Yeasts in Natural Ecosystems: Ecology; Buzzini, P., Lachance, M.A., Yurkov, A., Eds.; Springer: Berlin, Germany, 2017; pp. 39–72. [Google Scholar]
- Buzzini, P.; Branda, E.; Goretti, M.; Turchetti, B. Psychrophilic yeasts from worldwide glacial habitats: Diversity, adaptation strategies and biotechnological potential. FEMS Microbiol. Ecol. 2012, 82, 217–241. [Google Scholar] [CrossRef]
- Buzzini, P.; Turk, M.; Perini, L.; Turchetti, B.; Gunde-Cimerman, N. Yeasts in Natural Ecosystems: Diversity; Buzzini, P., Lachance, M.A., Yurkov, A., Eds.; Springer: Berlin, Germany, 2017; pp. 331–366. [Google Scholar]
- Sannino, C.; Tasselli, G.; Filippucci, S.; Turchetti, B.; Buzzini, P. Yeasts in Natural Ecosystems: Diversity; Buzzini, P., Lachance, M.A., Yurkov, A., Eds.; Springer: Berlin, Germany, 2017; pp. 367–396. [Google Scholar]
- Buondy-Mills, K.I. Cold-Adapted Yeasts: Biodiversity, Adaptation Strategies and Biotechnological Significance; Springer: Berlin, Germany, 2014; pp. 23–48. [Google Scholar]
- Buzzini, P.; Margesin, R. Cold-Adapted Yeasts: Biodiversity, Adaptation Strategies and Biotechnological Significance; Buzzini, P., Margesin, R., Eds.; Springer: Berlin, Germany, 2014; pp. 3–22. [Google Scholar]
- Barria, C.; Malecki, M.; Arraiano, C.M. Bacterial adaptation to cold. Microbiology 2013, 159, 2437–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliodori, A.M. Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria; de Bruijn Frans, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 859–872. [Google Scholar]
- Yayanos, A.A. Microbiology to 10,500 m in the deep sea. Annu. Rev. Microbiol. 1995, 49, 777–805. [Google Scholar] [CrossRef] [PubMed]
- Vishniac, H.S. Enigmatic Microorganisms and Life in Extreme Environments; Seckbach, J., Ed.; Kluwer Academic Publishers: Berlin, Germany, 1999; pp. 317–324. [Google Scholar]
- Gerday, C.; Aittaleb, M.; Arpigny, J.L.; Baise, E.; Chessa, J.P.; Garsoux, G.; Petrescu, I.; Feller, G. Psychrophilic enzymes: A thermodynamic challenge. Biochim. Biophys. Acta 1997, 1342, 119–131. [Google Scholar] [CrossRef]
- Gerday, C.; Aittaleb, M.; Bentahir, M.; Chessa, J.P.; Claverie, P.; Collins, T.; D’Amico, S.; Dumont, J.; Garsoux, G.; Georlette, D.; et al. Cold-adapted enzymes: From fundamentals to biotechnology. Trend Biotechnol. 2000, 18, 103–107. [Google Scholar] [CrossRef]
- Morgan-Kiss, R.M.; Priscu, J.C.; Pocock, T.; Gudynaite-Savitch, L.; Huner, N.P. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. R. 2006, 70, 222–252. [Google Scholar] [CrossRef] [Green Version]
- Margesin, R.; Schinner, F.; Marx, J.C.; Gerday, C. Psychrophiles. From Biodiversity to Biotechnology; Springer: Berlin, Germany, 2008. [Google Scholar]
- Rossi, M.; Buzzini, P.; Cordisco, L.; Amaretti, A.; Sala, M.; Raimondi, S.; Ponzoni, C.; Pagnoni, U.M.; Matteuzzi, D. Growth, lipid accumulation and fatty acid composition in obligate psychrophilic, facultative psychrophilic, and mesophilic yeasts. FEMS Microbiol. Ecol. 2009, 69, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, M. Cold-stress response in the Antarctic Basidiomycetous yeast Mrakia blollopis. R. Soc. Open Sci. 2016, 3, 160106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Ma, Y.; Zheng, Y.; Zhao, W.; Zhao, X.; Luo, T.; Zhang, J.; Yang, Z. Cold-stressed response of probiotic Lactobacillus plantarum K25 by iTRAQ proteomic analysis. J. Microbiol. Biotechnol. 2019, 29. [Google Scholar] [CrossRef]
- Sinetova, M.A.; Los, D.A. Newinsights in cyanobacterial cold stress responses: Genes, sensors, and molecular triggers. Biochim. Biophys. Acta 2016, 1860, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Sahara, T.; Goda, T.; Ohgiya, S. Comprehensive expression analysis of time-dependent genetic responses in yeast cells to low temperature. J. Biol. Chem. 2002, 277, 50015–50021. [Google Scholar] [CrossRef] [Green Version]
- Tronchoni, J.; Medina, V.; Guillamón, J.M.; Querol, A.; Pérez-Torrado, R. Transcriptomics of cryophilic Saccharomyces kudriavzevii reveals the key role of gene translation efficiency in cold-stress adaptations. BMC Genom. 2014, 15, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvadó, Z.; Ramos-Alonso, L.; Tronchoni, J.; Penacho, V.; García-Ríos, E.; Morales, P.; Gonzalez, R.; Guillamón, J.M. Genome-wide identification of genes involved in growth and fermentation activity at low temperature in Saccharomyces cerevisiae. Int. J. Food Microbiol. 2016, 236, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Vargas, S.; Estruch, F.; Randez-Gil, F. Gene expression analysis of cold and freeze stress in Baker’s yeast. Appl. Environ. Microbiol. 2002, 68, 3024–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thieringer, H.A.; Jones, P.G.; Inouye, M. Cold shock and adaptation. BioEssays 1998, 20, 49–57. [Google Scholar] [CrossRef]
- Aguilera, J.; Randez-Gil, F.; Prieto, J.A. Cold response in Saccharomyces cerevisiae: New functions for old mechanisms. FEMS Microbiol. Rev. 2007, 31, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.M.V.L.; Boo, S.Y.; Voo, C.L.Y.; Zainuddin, N.; Najimudin, N. comparative transcriptomic analysis provides insights into the cold-adaptation mechanisms of a psychrophilic yeast, Glaciozyma antarctica PI12. Polar Biol. 2019, 42, 541–553. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, X.D.; Wang, Y.; Yuan, B.F.; Feng, Y.Q. Widespread existence of cytosine methylation in yeast DNA measured by gas chromatography/mass spectrometry. Anal. Chem. 2012, 84, 7249–7255. [Google Scholar] [CrossRef] [Green Version]
- Iacobazzi, V.; Castegna, A.; Infantino, V.; Andria, G. Mitochondrial DNA methylation as a next-generation biomarker and diagnostic tool. Mol. Genet. Metab. 2013, 110, 25–34. [Google Scholar] [CrossRef]
- Wibowo, A.; Becker, C.; Marconi, G.; Durr, J.; Price, J.; Hagmann, J.; Papareddy, R.; Putra, H.; Kageyama, J.; Becker, J.; et al. Hyperosmotic stress memory in Arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by DNA glycosylase activity. eLife 2016, 5, e13546. [Google Scholar] [CrossRef] [Green Version]
- Simpson, V.J.; Johnson, T.E.; Hammen, R.F. Caenorhabditis elegans DNA does not contain 5-methylcytosine at any time during development or aging. Nucleic Acids Res. 1986, 14, 6711–6719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowher, H.; Leismann, O.; Jeltsch, A. DNA of Drosophila melanogaster contains 5-methylcytosine. EMBO J. 2000, 19, 6918–6923. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, H.; Selker, E.U. A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 2001, 414, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Curk, T.; Xu, Q.; Zupan, B.; Kuspa, A.; Shaulsky, G. Developmentally regulated DNA methylation in Dictyostelium discoideum. Eukaryot. Cell 2006, 5, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Geyer, K.; Rodríguez López, C.M.; Chalmers, I.W.; Munshi, S.E.; Truscott, M.; Heald, J.; Wilkinson, M.J.; Hoffmann, K.F. Cytosine methylation regulates oviposition in the pathogenic blood fluke Schistosoma mansoni. Nat. Commun. 2011, 2, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, S. Birds do it, bees do it, worms and ciliates do it too: DNA methylation from unexpected corners of the tree of life. Genom Biol. 2012, 13, 174. [Google Scholar] [CrossRef] [Green Version]
- Capuano, F.; Mülleder, M.; Kok, R.; Blom, H.J.; Ralser, M. Cytosine DNA methylation is found in Drosophila melanogaster but absent in Saccharomyces cerevisiae, Schizosaccharomyces pombe, and other yeast species. Anal. Chem. 2014, 86, 3697–3702. [Google Scholar] [CrossRef]
- Hattman, S.; Kenny, C.; Berger, L.; Pratt, K. Comparative study of DNA methylation in three unicellular eucaryotes. J. Bacteriol. 1978, 135, 1156–1157. [Google Scholar] [CrossRef] [Green Version]
- Bulkowska, U.; Ishikawa, T.; Kurlandzka, A.; Trzcińska-Danielewicz, J.; Derlacz, R.; Fronk, J. Expression of murine DNA methyltransferases Dnmt1 and Dnmt3a in the yeast Saccharomyces cerevisiae. Yeast 2007, 24, 871–882. [Google Scholar] [CrossRef]
- Baum, M.; Carbon, J. DNA methylation regulates phenotype-dependent transcriptional activity in Candida albicans. Proc. Natl. Acad. Sci. USA 2011, 108, 11965–11970. [Google Scholar]
- Turchetti, B.; Goretti, M.; Branda, E.; Diolaiuti, G.; D’Agata, C.; Smiraglia, C.; Onofri, A.; Buzzini, P. Influence of abiotic variables on culturable yeast diversity in two distinct Alpine glaciers. FEMS Microbiol. Ecol. 2013, 86, 327–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madigan, M.T.; Bender, K.S.; Buckley, D.H.; Sattley, W.M.; Stahl, D.A. Brock Biology of Microorganisms; Pearson: London, UK, 2018. [Google Scholar]
- Turchetti, B.; Buzzini, P.; Goretti, M.; Branda, E.; Diolaiuti, G.; D’Agata, C.; Smiraglia, C.; Vaughan-Martini, A. Psychrophilic yeasts in glacial environments of Alpine glaciers. FEMS Microbiol. Ecol. 2008, 63, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marconi, G.; Pace, R.; Traini, A.; Raggi, L.; Lutts, S.; Chiusano, M.; Guiducci, M.; Falcinelli, M.; Benincasa, P.; Albertini, E. Use of MSAP Markers to Analyse the Effects of Salt Stress on DNA Methylation in Rapeseed (Brassica napus var. oleifera). PLoS ONE 2013, 8, e75597. [Google Scholar] [CrossRef] [Green Version]
- McClelland, M.; Nelson, M.; Raschke, E. Effect of site- specific modification on restriction endonucleases and DNA modification methyltransferases. Nucl. Acids Res. 1994, 22, 3640–3659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karan, R.; DeLeon, T.; Biradar, H.; Subudhi, P.K. Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PLoS ONE 2012, 7, e40203. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, L.; Hamsher, S.; Schubert, Z.; Schmidt, S.K. Growth of high-elevation Cryptococcus sp. during extreme freeze-thaw cycles. Extremophiles 2016, 20, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, S.M. Characterization of yeast and filamentous fungi isolated from cryoconite holes of Svalbard, Arctic. Polar Biol. 2012, 35, 575–583. [Google Scholar] [CrossRef]
- Zalar, P.; Gunde-Cimerman, N. Cold-Adapted Yeasts: Biodiversity, Adaptation Strategies and Biotechnological Significance; Buzzini, P., Margesin, R., Eds.; Springer: Berlin, Germany, 2014; pp. 49–74. [Google Scholar]
- Herrera, C.M.; Pozo, M.I.; Bazaga, P. Jack of all nectars, master of most: DNA methylation and the epigenetic basis of niche width in a flower-living yeast. Molec. Ecol. 2012, 21, 2602–2616. [Google Scholar] [CrossRef]
- Steward, N.; Kusano, T.; Sano, H. Expression of ZmMET1, a gene encoding a DNA methytransferase from maize, is associated not only with DNA replication in actively proliferating cells but also with altered DNA methylation status in cold-stress quiescent cell. Nucleic Acid Res. 2000, 28, 3250–3259. [Google Scholar] [CrossRef] [Green Version]
- Labra, M.; Ghiani, A.; Citterio, S.; Sgorbati, S.; Sala, F.; Vannini, C.; Ruffini-Castiglione, M.; Bracale, M. Analysis of cytosine methylation pattern in response to water deficit in pea roots tips. Plant Biol. 2002, 4, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Sha, A.H.; Lin, X.H.; Huang, J.B.; Zhang, D.P. Analysis of DNA methylation related to rice adult plant resistance to bacterial blight based on methylation-sensitive AFLP (MSAP) analysis. Mol. Genet. Genom. 2005, 273, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.P.; Li, Y.; Song, X.X.; Ou, X.F.; Xing, S.C.; Ma, J.; Von Wettstein, D.; Liu, B. Heritable alteration in DNA methylation induced by nitrogen-deficiency stress accompanies enhanced tolerance by progenies to the stress in rice (Oryza sativa L.). J. Plant Physiol. 2011, 168, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Bocchini, M.; Bartucca, M.L.; Ciancaleoni, S.; Mimmo, T.; Cesco, S.; Pii, Y.; Albertini, E.; Del Buono, D. Iron deficiency in barley plants: Phytosiderophore release, iron translocation and DNA methylation. Front. Plant Sci. 2015, 6, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margesin, R. Effect of temperature on growth parameters of psychrophilic bacteria and yeasts. Extremophiles 2009, 13, 257–262. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [Green Version]
- Pianalto, K.M.; Billmyre, R.B.; Telzrow, C.L.; Alspaugh, J.A. Roles for stress response and cell wall biosynthesis pathways in caspofungin tolerance in Cryptococcus neoformans. Genetics 2019, 213, 213–227. [Google Scholar] [CrossRef] [Green Version]




| N. antarctica DBVPG 5271 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 4 °C | 20 °C | |||||||
| MSAP Band Type | 5271-1 | 5271-2 | 5271-3 | 5271-1 | 5271-2 | 5271-3 | 5271-1 | 5271-2 | 5271-3 |
| I | 175 | 155 | 149 | 164 | 202 | 150 | 157 | 143 | 123 |
| II | 45 | 120 | 71 | 66 | 97 | 63 | 70 | 78 | 89 |
| III | 55 | 31 | 50 | 43 | 58 | 89 | 61 | 67 | 55 |
| IV | 275 | 244 | 280 | 277 | 193 | 248 | 262 | 262 | 283 |
| Tot. Amplified bands | 550 | 550 | 550 | 550 | 550 | 550 | 550 | 550 | 550 |
| Tot. methylated bands | 375 | 395 | 401 | 386 | 348 | 400 | 393 | 407 | 427 |
| Fully methylated bands | 330 | 275 | 330 | 320 | 251 | 337 | 323 | 329 | 338 |
| MSAP (%) a | 68.18 | 71.82 | 72.91 | 70.18 | 63.27 | 72.73 | 71.45 | 74.00 | 77.645 |
| Fully methylated ratio (%) b | 60.00 | 50.003 | 60.00 | 58.18 | 45.64 | 61.27 | 58.73 | 59.82 | 61.45 |
| Hemimethylated ratio (%) c | 8.18 | 21.82 | 12.91 | 12.00 | 17.64 | 11.45 | 12.73 | 14.18 | 16.18 |
| Mean MSAP (%) | 70.97 | 68.73 | 74.36 | ||||||
| N. albida DBVPG 10064 | |||||||||
| 25 °C | 4 °C | 25 °C | |||||||
| MSAP Band Type | 10064-1 | 10064-2 | 10064-3 | 10064-1 | 10064-2 | 10064-3 | 10064-1 | 10064-2 | 10064-3 |
| I | 177 | 173 | 179 | 140 | 142 | 175 | 112 | 137 | 138 |
| II | 82 | 108 | 77 | 94 | 121 | 79 | 78 | 87 | 57 |
| III | 50 | 46 | 77 | 45 | 58 | 54 | 99 | 70 | 104 |
| 134 | 114 | 96 | 90 | 144 | 102 | 115 | 129 | 124 | |
| Tot. Amplified bands | 423 | 423 | 423 | 423 | 423 | 423 | 423 | 423 | 423 |
| Tot. methylated bands | 246 | 250 | 244 | 283 | 281 | 248 | 311 | 286 | 285 |
| Fully methylated bands | 164 | 142 | 167 | 189 | 160 | 169 | 233 | 199 | 228 |
| MSAP (%) a | 58.16 | 59.10 | 57.68 | 66.90 | 66.43 | 58.63 | 75.52 | 67.61 | 67.38 |
| Fully methylated ratio (%) b | 38.77 | 33.57 | 39.48 | 44.68 | 37.83 | 39.95 | 55.08 | 47.04 | 53.90 |
| Hemimethylated ratio (%) c | 19.39 | 25.53 | 18.20 | 22.22 | 28.61 | 18.68 | 18.449 | 20.57 | 13.48 |
| Mean MSAP (%) | 58.31 | 63.99 | 69.50 | ||||||
| N. antarctica DBVPG 5271 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pattern | Class | 20 °C | 4 °C | Rep1 | Rep2 | Rep3 | ||
| HpaII | MspI | HpaII | MspI | |||||
| No change | A | 1 | 0 | 1 | 0 | 34 | 44 | 43 |
| B | 0 | 1 | 0 | 1 | 23 | 14 | 23 | |
| C | 1 | 1 | 1 | 1 | 146 | 118 | 112 | |
| D | 0 | 0 | 0 | 0 | 242 | 144 | 225 | |
| Total | 445 (80.9%) | 320 (58.2%) | 403 (73.3%) | |||||
| Demethylation | E | 1 | 0 | 1 | 1 | 1 | 47 | 12 |
| F | 0 | 1 | 1 | 1 | 13 | 5 | 20 | |
| G | 0 | 0 | 1 | 1 | 4 | 32 | 6 | |
| H | 0 | 1 | 1 | 0 | 3 | 1 | 1 | |
| I | 0 | 0 | 1 | 0 | 21 | 47 | 16 | |
| J | 0 | 0 | 0 | 1 | 8 | 21 | 33 | |
| Total | 50 (9.1%) | 153 (27.9%) | 88 (16.0%) | |||||
| Methylation | K | 1 | 1 | 1 | 0 | 8 | 5 | 3 |
| L | 1 | 1 | 0 | 1 | 10 | 19 | 30 | |
| M | 1 | 1 | 0 | 0 | 11 | 13 | 4 | |
| N | 1 | 0 | 0 | 1 | 2 | 4 | 3 | |
| O | 1 | 0 | 0 | 0 | 8 | 25 | 13 | |
| P | 0 | 1 | 0 | 0 | 16 | 11 | 6 | |
| Total | 55 (10.0%) | 77 (14.0%) | 59 (10.7%) | |||||
| N. albida DBVPG 10064 | ||||||||
| Pattern | Class | 25 °C | 4 °C | Rep1 | Rep2 | Rep3 | ||
| HpaII | MspI | HpaII | MspI | |||||
| No change | A | 1 | 0 | 1 | 0 | 42 | 57 | 37 |
| B | 0 | 1 | 0 | 1 | 11 | 24 | 20 | |
| C | 1 | 1 | 1 | 1 | 112 | 116 | 128 | |
| D | 0 | 0 | 0 | 0 | 77 | 41 | 48 | |
| Total | 242 (57.2%) | 238 (56.3%) | 233 (55.1%) | |||||
| Demethylation | E | 1 | 0 | 1 | 1 | 8 | 19 | 9 |
| F | 0 | 1 | 1 | 1 | 12 | 1 | 27 | |
| G | 0 | 0 | 1 | 1 | 8 | 6 | 11 | |
| H | 0 | 1 | 1 | 0 | 4 | 7 | 9 | |
| I | 0 | 0 | 1 | 0 | 12 | 36 | 22 | |
| J | 0 | 0 | 0 | 1 | 17 | 13 | 9 | |
| Total | 61 (14.4%) | 82 (19.4%) | 87 (20.6%) | |||||
| Methylation | K | 1 | 1 | 1 | 0 | 36 | 21 | 11 |
| L | 1 | 1 | 0 | 1 | 13 | 17 | 23 | |
| M | 1 | 1 | 0 | 0 | 16 | 19 | 17 | |
| N | 1 | 0 | 0 | 1 | 4 | 4 | 2 | |
| O | 1 | 0 | 0 | 0 | 28 | 28 | 29 | |
| P | 0 | 1 | 0 | 0 | 23 | 14 | 21 | |
| Total | 120 (28.4%) | 103 (24.3%) | 103 (24.3%) | |||||
| a | b | c | d | e | f | g | |
|---|---|---|---|---|---|---|---|
| N. antarctica DBVPG 5271 | 32 | 24 | 5 | 24 | 64 | 278 | 123 |
| N. albida DBVPG 10064 | 25 | 16 | 16 | 38 | 58 | 140 | 130 |
| Frag | bp | BLASTX Analysis | e-value | Accession Number | Banding Profile | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Similarity | Species | C1 | C2 | C3 | S1 | S2 | S3 | R1 | R2 | R3 | |||
| B1 | 116 | No similarity found | ---- | ---- | X | X | X | X | X | X | ||||
| B2 | 217 | Elongation factor G, mitochondrial | Cryptococcus neoformans var. grubii c45 | 1 × 10−14 | OWZ31767 | X | X | X | X | X | X | |||
| B3 | 126 | No similarity found | ---- | ---- | X | X | X | X | X | X | ||||
| B4 | 287 | Chitin synthase export chaperone | Cryptococcus amylolentus CBS 6039 | 1 × 10−9 | XM019140565 | X | X | X | ||||||
| B5 | 143 | Low-affinity glucose transporter | Saccharomyces cerevisiae | 3 × 10−1 | CAA47735 | X | X | X | X | X | X | |||
| B6 | 284 | Chitin synthase export chaperone | Cryptococcus amylolentus CBS 6039 | 2 × 10−11 | XM019140565 | X | X | X | ||||||
| B7 | 208 | Elongation factor G, mitochondrial | Cryptococcus neoformans var. grubii c45 | 1 × 10−3 | OWZ31767 | X | X | X | ||||||
| B8 | 210 | AcrB/AcrD/AcrF family protein | Cyanothece sp. ATCC 51142 | 2 × 10−15 | CP000806 | X | X | X | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turchetti, B.; Marconi, G.; Sannino, C.; Buzzini, P.; Albertini, E. DNA Methylation Changes Induced by Cold in Psychrophilic and Psychrotolerant Naganishia Yeast Species. Microorganisms 2020, 8, 296. https://doi.org/10.3390/microorganisms8020296
Turchetti B, Marconi G, Sannino C, Buzzini P, Albertini E. DNA Methylation Changes Induced by Cold in Psychrophilic and Psychrotolerant Naganishia Yeast Species. Microorganisms. 2020; 8(2):296. https://doi.org/10.3390/microorganisms8020296
Chicago/Turabian StyleTurchetti, Benedetta, Gianpiero Marconi, Ciro Sannino, Pietro Buzzini, and Emidio Albertini. 2020. "DNA Methylation Changes Induced by Cold in Psychrophilic and Psychrotolerant Naganishia Yeast Species" Microorganisms 8, no. 2: 296. https://doi.org/10.3390/microorganisms8020296





