Transcriptome Analysis of Durusdinium Associated with the Transition from Free-Living to Symbiotic
Abstract
:1. Introduction
2. Materials and Methods
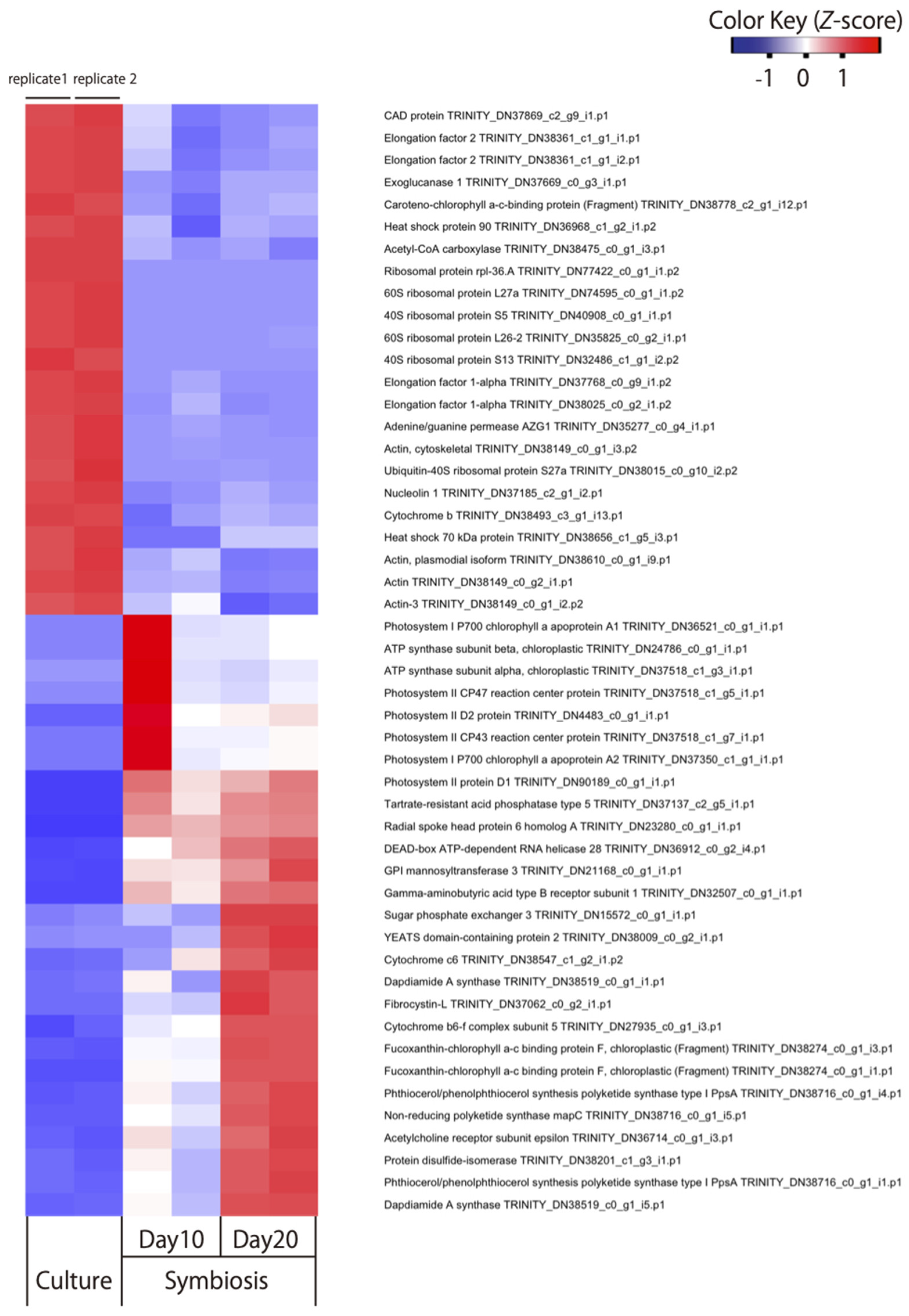
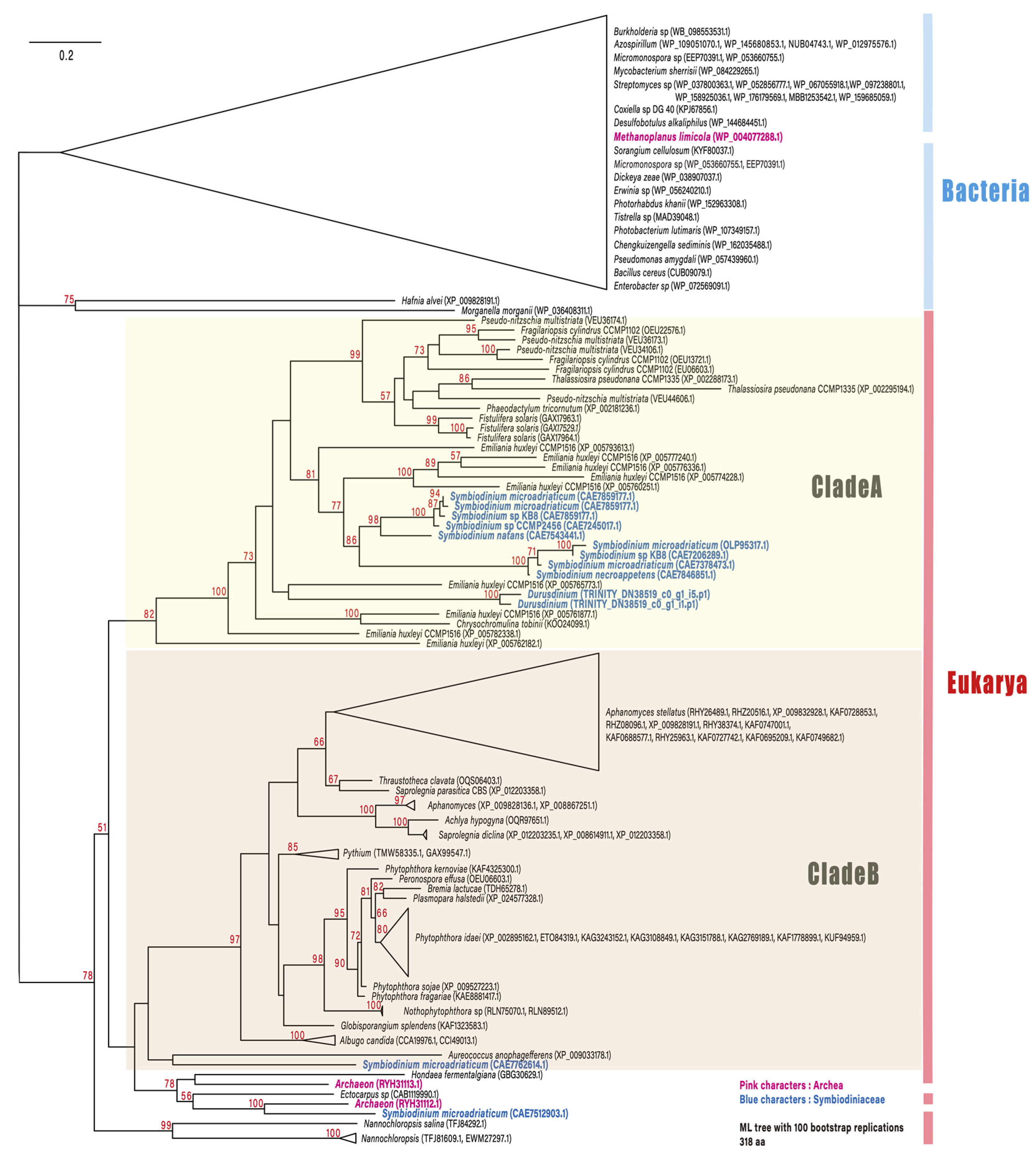
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 2018, 28, 2570–2580.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pochon, X.; Gates, R.D. A New Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai’i. Mol. Phylogenet. Evol. 2010, 56, 492–497. [Google Scholar] [CrossRef]
- Pochon, X.; Montoya-Burgos, J.I.; Stadelmann, B.; Pawlowski, J. Molecular phylogeny, evolutionary rates, and divergence timing of the symbiotic dinoflagellate genus Symbiodinium. Mol. Phylogenet. Evol. 2006, 38, 20–30. [Google Scholar] [CrossRef]
- Tanaka, Y.; Miyajima, T.; Koike, I.; Hayashibara, T.; Ogawa, H. Translocation and conservation of organic nitrogen within the coral-zooxanthella symbiotic system of Acropora pulchra, as demonstrated by dual isotope-labeling techniques. J. Exp. Mar. Biol. Ecol. 2006, 336, 110–119. [Google Scholar] [CrossRef]
- Whitehead, L.F.; Douglas, A.E. Metabolite comparisons and the identity of nutrients translocated from symbiotic algae to an animal host. J. Exp. Biol. 2003, 206, 3149–3157. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.T.; Douglas, A.E. Essential amino acid synthesis and nitrogen recycling in an alga-invertebrate symbiosis. Mar. Biol. 1999, 135, 219–222. [Google Scholar] [CrossRef]
- Barott, K.L.; Venn, A.A.; Perez, S.O.; Tambutté, S.; Tresguerres, M. Coral host cells acidify symbiotic algal microenvironment to promote photosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, R.; Jimbo, M.; Tanimoto, F.; Iijima, M.; Yamashita, H.; Suzuki, G.; Harii, S.; Nakano, Y.; Yasumoto, K.; Watabe, S. N-Acetyl-d-Glucosamine-binding lectin in Acropora tenuis attracts specific Symbiodiniaceae cell culture strains. Mar. Drugs 2021, 19, 146. [Google Scholar] [CrossRef]
- Kuniya, N.; Jimbo, M.; Tanimoto, F.; Yamashita, H.; Koike, K.; Harii, S.; Nakano, Y.; Iwao, K.; Yasumoto, K.; Watabe, S. Possible involvement of Tachylectin-2-like lectin from Acropora tenuis in the process of Symbiodinium Acquisition. Fish. Sci. 2015, 81, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Maor-Landaw, K.; van Oppen, M.J.H.; McFadden, G.I. Symbiotic lifestyle triggers drastic changes in the gene expression of the algal endosymbiont Breviolum minutum (Symbiodiniaceae). Ecol. Evol. 2020, 10, 451–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.R.; Andrade, N.; Moya, A.; Chan, C.X.; Negri, A.P.; Bourne, D.G.; Ying, H.; Ball, E.E.; Miller, D.J. Dual RNA-sequencing analyses of a coral and its native symbiont during the establishment of symbiosis. Mol. Ecol. 2020, 29, 3921–3937. [Google Scholar] [CrossRef]
- Bertucci, A.; Tambutté, É.; Tambutté, S.; Allemand, D.; Zoccola, D. Symbiosis-dependent gene expression in coral–dinoflagellate association: Cloning and characterization of a P-Type H+-ATPase gene. Proc. R. Soc. B Biol. Sci. 2010, 277, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Kii, S.; Tanaka, J.; Takishita, K.; Maruyama, T. cDNA cloning and phylogenetic and expression analyses of actin in symbiotic dinoflagellates (Symbiodinium spp.). J. Appl. Phycol. 2006, 18, 219–225. [Google Scholar] [CrossRef]
- Yuyama, I.; Ishikawa, M.; Nozawa, M.; Yoshida, M.; Ikeo, K. Transcriptomic changes with increasing algal symbiont reveal the detailed process underlying establishment of coral-algal symbiosis. Sci. Rep. 2018, 8, 16802. [Google Scholar] [CrossRef]
- Yuyama, I.; Higuchi, T. Comparing the effects of symbiotic algae (Symbiodinium) clades C1 and D on Early growth stages of Acropora tenuis. PLoS ONE 2014, 9, e98999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, Y.; Yamashita, H.; Suzuki, G.; Zayasu, Y.; Tada, I.; Kanda, M.; Satoh, N.; Shoguchi, E.; Shinzato, C. Whole-genome transcriptome analyses of native symbionts reveal host coral genomic novelties for establishing coral–algae symbioses. Genome Biol. Evol. 2021, 13, evaa240. [Google Scholar] [CrossRef]
- Al Seesi, S.; Tiagueu, Y.T.; Zelikovsky, A.; Măndoiu, I.I. Bootstrap-based differential gene expression analysis for RNA-Seq data with and without replicates. BMC Genom. 2014, 15, S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camaya, A.P. Stages of the symbiotic zooxanthellae–host cell division and the dynamic role of coral nucleus in the partitioning process: A novel observation elucidated by electron microscopy. Coral Reefs 2020, 39, 929–938. [Google Scholar] [CrossRef]
- Hollenhorst, M.A.; Clardy, J.; Walsh, C.T. The ATP-dependent amide aigases DdaG and DdaF assemble the fumaramoyl-dipeptide scaffold of the Dapdiamide antibiotics. Biochemistry 2009, 48, 10467–10472. [Google Scholar] [CrossRef] [Green Version]
- Silva Lima, A.W.; Leomil, L.; Oliveira, L.; Varasteh, T.; Thompson, J.R.; Medina, M.; Thompson, C.C.; Thompson, F.L. Insights on the genetic repertoire of the coral Mussismilia braziliensis endosymbiont Symbiodinium. Symbiosis 2020, 80, 183–193. [Google Scholar] [CrossRef]
- Stat, M.; Gates, R.D. Clade D Symbiodinium in Scleractinian Corals: A “Nugget” of Hope, a Selfish Opportunist, an Ominous Sign, or All of the Above? J. Mar. Biol. 2010, 2011, e730715. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuyama, I.; Ugawa, N.; Hashimoto, T. Transcriptome Analysis of Durusdinium Associated with the Transition from Free-Living to Symbiotic. Microorganisms 2021, 9, 1560. https://doi.org/10.3390/microorganisms9081560
Yuyama I, Ugawa N, Hashimoto T. Transcriptome Analysis of Durusdinium Associated with the Transition from Free-Living to Symbiotic. Microorganisms. 2021; 9(8):1560. https://doi.org/10.3390/microorganisms9081560
Chicago/Turabian StyleYuyama, Ikuko, Naoto Ugawa, and Tetsuo Hashimoto. 2021. "Transcriptome Analysis of Durusdinium Associated with the Transition from Free-Living to Symbiotic" Microorganisms 9, no. 8: 1560. https://doi.org/10.3390/microorganisms9081560




