Effect of GABA-T on Reproductive Function in Female Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.2.1. Distribution of GABA-T protein and Abat mRNA Level in The HPG Axis at Different Developmental Stages
2.2.2. Effect of GABA-T inhibitor on The Expression of Reproduction-related Genes in Hypothalamus Cells
2.2.3. Effect of GABA-T inhibitor on Puberty Onset, Reproduction-related Genes, Hormones and Ovarian Morphology in Female Rat
2.2.4. Effect of GABA-T inhibitor on Estrous Cycle and Reproductive Performance in Adult Female Rats
2.2.5. The Short-term Effect of GABA-T inhibitor for 4 h on Reproduction-related Genes and Hormones in Rats
2.3. Lateral Ventricle Injection
2.4. Vaginal Smear and Wright’s Giemsa Staining
2.5. Serum Preparation and ELISA
2.6. Immunofluorescence Staining
2.7. Hematoxylin and Eosin (H&E) Staining
2.8. RNA Extractions and Reverse Transcription
2.9. RT-qPCR
2.10. Statistical Analysis
3. Results
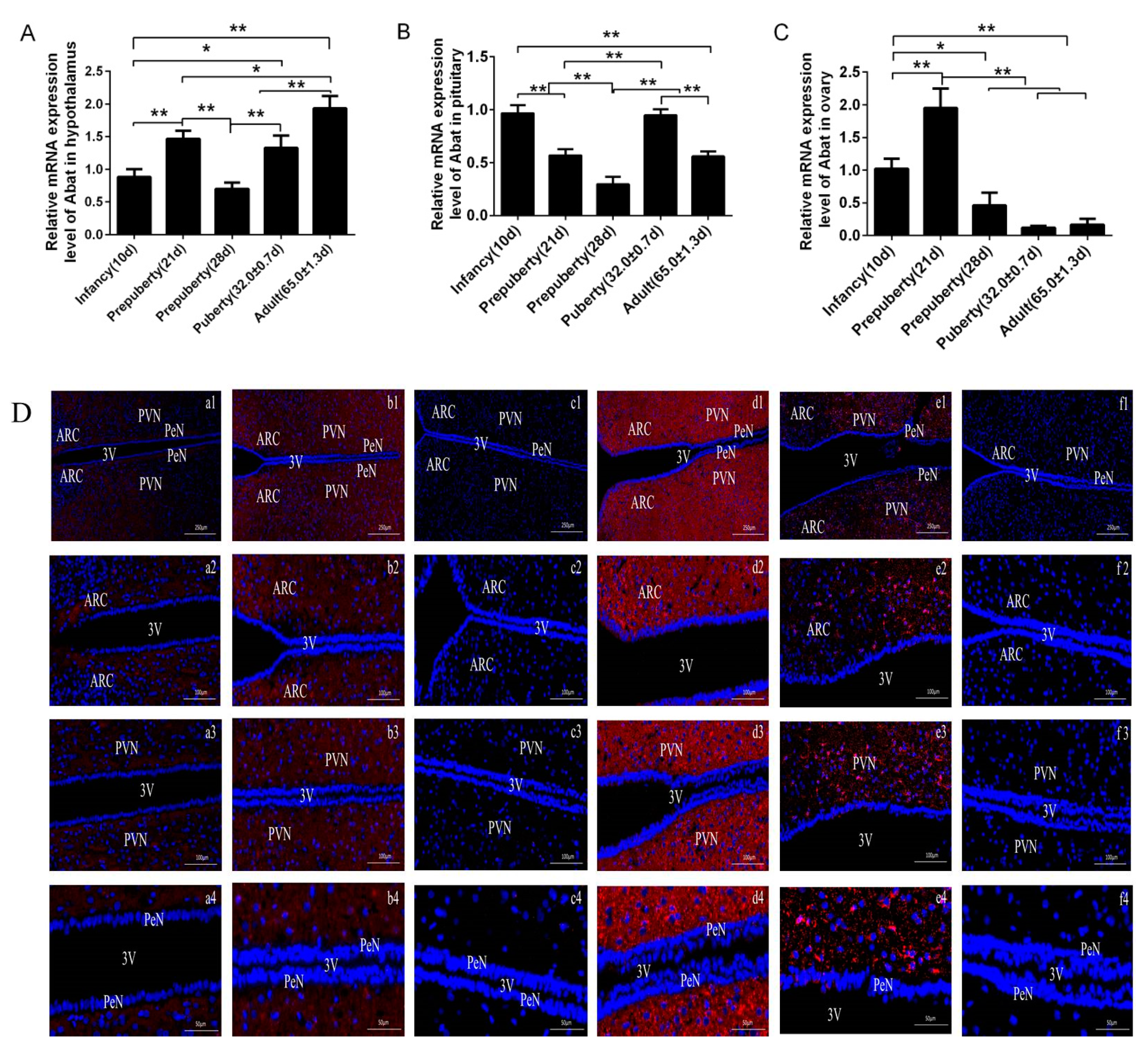
3.1. Level of Abat mRNA and Distribution of GABA-T Protein on The Reproductive Axis of Female Rats
3.2. Effects of GABA-T inhibitor on Reproduction-related Genes in Rat Hypothalamic Cells
3.3. Effects of GABA-T Inhibitor on Puberty in Rats
3.3.1. Changes in Time of Vaginal Opening
3.3.2. The Expression of Reproduction-related Genes
3.3.3. Concentrations of Serum Hormones
3.3.4. The Structure of Ovarian Histology
3.4. Effects of GABA-T Inhibitors on Estrous Cycle and Reproductive Performance in Adult Rats
3.4.1. Changes in The Estrous Cycle
3.4.2. Mating Time and Rate of Pregnancy
3.4.3. Number and Growth of The Offspring
3.5. The Short-term Effects of GABA-T Inhibitor on Reproduction-related Genes and Hormones
3.5.1. Expression of Reproduction-related Genes in The Reproductive Axis After 4 h of GABT-T Inhibitor Treatment
3.5.2. Concentration of serum hormone after 4 h with GABT-T inhibitor injection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parent, A.S.; Teilmann, G.; Juul, A.; Skakkebaek, N.E.; Toppari, J.; Bourguignon, J.P. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr. Rev. 2003, 24, 668–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinilla, L.; Aguilar, E.; Dieguez, C.; Millar, R.P.; Tenasempere, M. Kisspeptins and reproduction: Physiological roles and regulatory mechanisms. Physiol. Rev. 2012, 92, 1235–1316. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.R.; Dunkel, L. The genetic basis of delayed puberty. Neuroendocrinology 2018, 106, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.; et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef] [Green Version]
- Herbison, A.E.; Moenter, S.M. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: Towards an emerging consensus. J. Neuroendocrinol. 2011, 23, 557–569. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chua, S., Jr. Leptin function and regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar]
- Di Fiore, M.M.; Santillo, A.; Falvo, S.; Chieffi Baccari, G.; Venditti, M.; Di Giacomo Russo, F.; Lispi, M.; D’Aniello, A. Sex hormone levels in the brain of d-aspartate-treated rats. Comptes Rendus Biol. 2018, 341, 9–15. [Google Scholar] [CrossRef]
- Ancel, C.; Inglis, M.A.; Anderson, G.M. Central RFRP-3 stimulates LH secretion in male mice and has cycle stage-dependent inhibitory effects in females. Endocrinology 2017, 158, 2873–2883. [Google Scholar] [CrossRef]
- Hu, K.L.; Chang, H.M.; Li, R.; Yu, Y.; Qiao, J. Regulation of LH secretion by RFRP-3—From the hypothalamus to the pituitary. Front. Neuroendocrinol. 2019, 52, 12–21. [Google Scholar] [CrossRef]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 2014, 20, 485–500. [Google Scholar] [CrossRef] [Green Version]
- Camille Melon, L.; Maguire, J. GABAergic regulation of the HPA and HPG axes and the impact of stress on reproductive function. J. Steroid Biochem. Mol. Biol. 2016, 160, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Tao, B.; Chen, J.; Jia, S.; Zhu, Z.; Trudeau, V.L.; Hu, W. GABAergic neurons and their modulatory effects on GnRH3 in zebrafish. Endocrinology 2017, 158, 874–886. [Google Scholar] [CrossRef] [Green Version]
- Gilhotra, N.; Dhingra, D. Thymoquinone produced antianxiety-like effects in mice through modulation of GABA and NO levels. Pharmacol. Rep. 2011, 63, 660–669. [Google Scholar] [CrossRef]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Grachev, P.; Li, X.F.; Goffin, V.; O‘Byrne, K.T. Hypothalamic prolactin regulation of luteinizing hormone secretion in the female rat. Endocrinology 2015, 156, 2880–2892. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Hao, C.; Shen, X.; Liu, X.; Shan, Y.; Zhang, Y.; Chen, L. Differences in the transcriptional profiles of human cumulus cells isolated from MI and MII oocytes of patients with polycystic ovary syndrome. Reproduction 2013, 145, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Burrello, N.; Vicari, E.; D’Amico, L.; Satta, A.; D’Agata, R.; Calogero, A.E. Human follicular fluid stimulates the sperm acrosome reaction by interacting with the gamma-aminobutyric acid receptors. Fertil. Steril. 2004, 82 (Suppl. 3), 1086–1090. [Google Scholar] [CrossRef]
- Berg, T.; Silveira, M.A.; Moenter, S.M. Prepubertal development of GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons and postsynaptic response are altered by prenatal androgenization. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 2304–2317. [Google Scholar] [CrossRef]
- Kurian, J.R.; Keen, K.L.; Guerriero, K.A.; Terasawa, E. Tonic control of kisspeptin release in prepubertal monkeys: Implications to the mechanism of puberty onset. Endocrinology 2012, 153, 3331–3336. [Google Scholar] [CrossRef]
- Koenig, M.K.; Hodgeman, R.; Riviello, J.J.; Chung, W.; Bain, J.; Chiriboga, C.A.; Ichikawa, K.; Osaka, H.; Tsuji, M.; Gibson, K.M.; et al. Phenotype of GABA-transaminase deficiency. Neurology 2017, 88, 1919–1924. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, W.; Richerson, G.B. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 2630. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.J.; Lippert, B.; Metcalf, B.W.; Bohlen, P.; Schechter, P.J. Gamma-Vinyl GABA (4-amino-hex-5-enoic acid), a new selective irreversible inhibitor of GABA-T: Effects on brain GABA metabolism in mice. J. Neurochem. 1977, 29, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Gey, L.; Gernert, M.; Loscher, W. Continuous bilateral infusion of vigabatrin into the subthalamic nucleus: Effects on seizure threshold and GABA metabolism in two rat models. Neurobiol. Dis. 2016, 91, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Errante, L.D.; Williamson, A.; Spencer, D.D.; Petroff, O.A. Gabapentin and vigabatrin increase GABA in the human neocortical slice. Epilepsy Res. 2002, 49, 203–210. [Google Scholar] [CrossRef]
- Yang, C.; Ye, J.; Liu, Y.; Ding, J.; Liu, H.; Gao, X.; Li, X.; Zhang, Y.; Zhou, J.; Zhang, X.; et al. Methylation pattern variation between goats and rats during the onset of puberty. Reprod. Domest. Anim. Zuchthyg. 2018, 53, 793–800. [Google Scholar] [CrossRef]
- Yang, C.; Ye, J.; Li, X.; Gao, X.; Zhang, K.; Luo, L.; Ding, J.; Zhang, Y.; Li, Y.; Cao, H.; et al. DNA methylation patterns in the hypothalamus of female pubertal goats. PLoS ONE 2016, 11, e0165327. [Google Scholar] [CrossRef] [Green Version]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal cytology of the laboratory rat and mouse: Review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [Green Version]
- Guerra-Crespo, M.; Charli, J.L.; Rosales-Garcia, V.H.; Pedraza-Alva, G.; Perez-Martinez, L. Polyethylenimine improves the transfection efficiency of primary cultures of post-mitotic rat fetal hypothalamic neurons. J. Neurosci. Methods 2003, 127, 179–192. [Google Scholar] [CrossRef]
- Lin, K.C.; Lin, H.J.; Chang, C.P.; Lin, M.T. Decreasing or increasing heat shock protein 72 exacerbates or attenuates heat-induced cell death, respectively, in rat hypothalamic cells. FEBS Open Bio 2015, 5, 724–730. [Google Scholar] [CrossRef] [Green Version]
- Boivin, G.P.; Bottomley, M.A.; Schiml, P.A.; Goss, L.; Grobe, N. Physiologic, behavioral, and histologic responses to various euthanasia methods in C57BL/6NTac male mice. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2017, 56, 69–78. [Google Scholar]
- Gao, X.; Zhang, K.; Song, M.; Li, X.; Luo, L.; Tian, Y.; Zhang, Y.; Li, Y.; Zhang, X.; Ling, Y. Role of nesfatin-1 in the reproductive axis of male rat. Sci. Rep. 2016, 6, 32877. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, P. Enhanced at puberty-1 (Eap1) expression critically regulates the onset of puberty independent of hypothalamic kiss1 expression. Cell. Physiol. Biochem. 2017, 43, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Z.; Manthari, R.K.; Li, M.; Guo, Q.; Wang, J. Effect of gestational exposure to arsenic on puberty in offspring female mice. Chemosphere 2018, 202, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Divall, S.A.; Williams, T.R.; Carver, S.E.; Koch, L.; Brüning, J.C.; Kahn, C.R.; Wondisford, F.; Radovick, S.; Wolfe, A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J. Clin. Investig. 2010, 120, 2900–2909. [Google Scholar] [CrossRef] [Green Version]
- Robertson, D.; Savage, K.; Reis-Filho, J.S.; Isacke, C. Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol. 2008, 9, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods A Companion Methods Enzymol. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Okamura, H.; Tsukamura, H.; Ohkura, S.; Uenoyama, Y.; Wakabayashi, Y.; Maeda, K. Kisspeptin and GnRH pulse generation. Adv. Exp. Med. Biol. 2013, 784, 297–323. [Google Scholar]
- Voigt, C.; Bennett, N.C. Gnrh mRNA expression in the brain of cooperatively breeding female Damaraland mole-rats. Reproduction 2017, 153, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Dufourny, L.; Lomet, D. Crosstalks between kisspeptin neurons and somatostatin neurons are not photoperiod dependent in the ewe hypothalamus. Gen. Comp. Endocrinol. 2017, 254, 68–74. [Google Scholar] [CrossRef]
- Plant, T.M. The neurobiological mechanism underlying hypothalamic GnRH pulse generation: The role of kisspeptin neurons in the arcuate nucleus. F1000Research 2019, 8, 982–999. [Google Scholar] [CrossRef]
- Piet, R.; Kalil, B.; McLennan, T.; Porteous, R.; Czieselsky, K.; Herbison, A.E. Dominant neuropeptide cotransmission in kisspeptin-GABA regulation of gnrh neuron firing driving ovulation. J. Neurosci. 2018, 38, 6310–6322. [Google Scholar] [CrossRef]
- Cottrell, E.C.; Campbell, R.E.; Han, S.K.; Herbison, A.E. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology 2006, 147, 3652–3661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uenoyama, Y.; Inoue, N.; Maeda, K.I.; Tsukamura, H. The roles of kisspeptin in the mechanism underlying reproductive functions in mammals. J. Reprod. Dev. 2018, 64, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, A.; Langlet, F.; Chachlaki, K.; Roa, J.; Rasika, S.; Jouy, N.; Gallet, S.; Gaytan, F.; Parkash, J.; Tena-Sempere, M.; et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat. Neurosci. 2016, 19, 835–844. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Li, Z.; Wang, D.; Li, N.; Song, Y.; Guo, C.; Liu, X. Molecular cloning and characterization of kiss1 in Brandt’s voles (Lasiopodomys brandtii). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 208–209, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Uenoyama, Y.; Tomikawa, J.; Inoue, N.; Goto, T.; Minabe, S.; Ieda, N.; Nakamura, S.; Watanabe, Y.; Ikegami, K.; Matsuda, F.; et al. Molecular and epigenetic mechanism regulating hypothalamic kiss1 gene expression in mammals. Neuroendocrinology 2016, 103, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Rachdaoui, N.; Sarkar, D.K. Pathophysiology of the effects of alcohol abuse on the endocrine system. Alcohol Res. Curr. Rev. 2017, 38, 255–276. [Google Scholar]
- Duvilanski, B.H.; Seilicovich, A.; Diaz, M.C.; Munoz Maines, V.; Lasaga, M.; Debeljuk, L. Effect of GABA-T inhibitors on prolactin secretion in vitro. Eur. J. Pharmacol. 1985, 115, 65–69. [Google Scholar] [CrossRef]
- Wroe, S.J.; Rimmer, E.; Kongola, G.; John, R.; Henley, R.; Richens, A. An enhanced serum prolactin response to TRH in the presence of GABA transaminase inhibition. Horm. Metab. Res. 1987, 19, 278–279. [Google Scholar] [CrossRef]
- Cabrera-Reyes, E.A.; Limon-Morales, O.; Rivero-Segura, N.A.; Camacho-Arroyo, I.; Cerbon, M. Prolactin function and putative expression in the brain. Endocrine 2017, 57, 199–213. [Google Scholar] [CrossRef]
- Donato, J., Jr.; Frazao, R. Interactions between prolactin and kisspeptin to control reproduction. Arch. Endocrinol. Metab. 2016, 60, 587–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, A.M.; Campbell, R.E. The neuroendocrine genesis of polycystic ovary syndrome: A role for arcuate nucleus GABA neurons. J. Steroid Biochem. Mol. Biol. 2016, 160, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Boller, M.; Schmidt, M. Postnatal maturation of GABA(A) and GABA(C) receptor function in the mammalian superior colliculus. Eur. J. Neurosci. 2001, 14, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Gaytan, M.; Roa, J.; Vigo, E.; Navarro, V.M.; Bellido, C.; Dieguez, C.; Aguilar, E.; Sanchez-Criado, J.E.; Pellicer, A.; et al. Expression of KiSS-1 in rat ovary: Putative local regulator of ovulation? Endocrinology 2006, 147, 4852–4862. [Google Scholar] [CrossRef] [PubMed]
- Peragine, D.E.; Pokarowski, M.; Mendoza-Viveros, L.; Swift-Gallant, A.; Cheng, H.M.; Bentley, G.E.; Holmes, M.M. RFamide-related peptide-3 (RFRP-3) suppresses sexual maturation in a eusocial mammal. Proc. Natl. Acad. Sci. USA 2017, 114, 1207–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Fukuda, A.; Nabekura, J. The role of GABA in the regulation of GnRH neurons. Front. Neurosci. 2014, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.D.; Kafitz, K.W.; Rose, C.R. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia 2008, 56, 1127–1137. [Google Scholar] [CrossRef]
- Root, A.R.; Sanford, J.D.; Kavanaugh, S.I.; Sower, S.A. In vitro and in vivo effects of GABA, muscimol, and bicuculline on lamprey GnRH concentration in the brain of the sea lamprey (Petromyzon marinus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 138, 493–501. [Google Scholar] [CrossRef]
- Han, X.; He, Y.; Zeng, G.; Wang, Y.; Sun, W.; Liu, J.; Sun, Y.; Yu, J. Intracerebroventricular injection of RFRP-3 delays puberty onset and stimulates growth hormone secretion in female rats. Reprod. Biol. Endocrinol. RBE 2017, 15, 35. [Google Scholar] [CrossRef] [Green Version]
- Loscher, W.; Fassbender, C.P.; Gram, L.; Gramer, M.; Horstermann, D.; Zahner, B.; Stefan, H. Determination of GABA and vigabatrin in human plasma by a rapid and simple HPLC method: Correlation between clinical response to vigabatrin and increase in plasma GABA. Epilepsy Res. 1993, 14, 245–255. [Google Scholar] [CrossRef]
- Katsikis, I.; Karkanaki, A.; Misichronis, G.; Delkos, D.; Kandaraki, E.A.; Panidis, D. Phenotypic expression, body mass index and insulin resistance in relation to LH levels in women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 156, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Heykants, M.; Mahabir, E. Estrous cycle staging before mating led to increased efficiency in the production of pseudopregnant recipients without negatively affecting embryo transfer in mice. Theriogenology 2016, 85, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G. Determining the stage of the estrous cycle in female mice by vaginal smear. Cold Spring Harb. Protoc. 2016, 2016, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Yie, S.; Zhang, Z.; Wang, J.; Cai, Z.; Luo, L.; Liu, Y.; Wang, H.; Huang, H.; Wang, C.; et al. Urinary profiles of luteinizing hormone, estrogen and progestagen during the estrous and gestational periods in giant pandas (Ailuropda melanoleuca). Sci. Rep. 2017, 7, 40749. [Google Scholar] [CrossRef] [Green Version]
- Soede, N.M.; Langendijk, P.; Kemp, B. Reproductive cycles in pigs. Anim. Reprod. Sci. 2011, 124, 251–258. [Google Scholar] [CrossRef]






| Gene | Forward primer, 5′–3′ | Reverse primer, 5′–3′ | Product Length, (bp) |
|---|---|---|---|
| Abat | GGTCATCAACATCATCAAG | TTATTCCGTATGGCTTCG | 174 |
| Gnrh | GCCGCTGTTGTTCTGTTGAC | CTGGGGTTCTGCCATTTGA | 134 |
| Rfrp-3 | CCAAAGGTTTGGGAGAACAA | GGGTCATGGCATAGAGCAAT | 127 |
| Kiss1 | TGCTGCTTCTCCTCTGTG | CCAGGCATTAACGAGTTCC | 116 |
| Gabbr1 | CACGAAGAAGGAGGAGAAG | CAGATGGCAAGAGTCAGG | 108 |
| Gapdh | TCAACGGCACAGTCAAGG | CTCAGCACCAGCATCACC | 113 |
| β-actin | CGTGACATCAAGGAGAAG | GAAGGAAGGCTGGAAGAG | 171 |
| Group | Weight (mg) | Transverse Diameter (cm) | Longitudinal Diameter (cm) | Transverse Perimeter (cm) | Longitudinal Perimeter (cm) | Ovary Index |
|---|---|---|---|---|---|---|
| Control | 20.83 ± 1.913 | 0.486 ± 0.014 | 0.326 ± 0.016 | 1.238 ± 0.072 | 0.936 ± 0.017 | 0.216 ± 0.163 |
| Inhibitor (1μg/g) | 20.23 ± 1.356 | 0.504 ± 0.024 | 0.317 ± 0.009 | 1.231 ± 0.055 | 0.913 ± 0.022 | 0.172 ± 0.011 * |
| Time (d) | Number of Pregnant Rat and Precent (%) | |
|---|---|---|
| Control (n = 10) | Inhibitor (n = 10) | |
| 1 | 1 (10%) | 3 (30%) |
| 2 | 3 (30%) | 2 (20%) |
| 3 | 1 (10%) | 1 (10%) |
| 4 | 4 (40%) | 4 (40%) |
| 5~12 | 0 (0%) | 0 (0%) |
| 13 | 1 (10%) | 0 (0%) |
| 14~21 | 0 (0%) | 0 (0%) |
| Total | 10 (100%) | 10 (100%) |
| Period (d) | Number of Pregnant Rat and Precent (%) | |
|---|---|---|
| Control (n = 10) | Inhibitor (n = 10) | |
| 22 | 4 (40%) | 4 (40%) |
| 23 | 6 (60%) | 6 (60%) |
| Total | 10 (100%) | 10 (100%) |
| Age (d) | Control (n = 10) | Inhibitor (n = 10) |
|---|---|---|
| 0 | 17.30 ± 2.71 | 15.50 ± 2.50 |
| 4 | 16.30 ± 2.71 | 15.10 ± 2.51 |
| 7 | 8.00 ± 0.00 | 8.00 ± 0.00 |
| 14 | 7.70 ± 0.15 | 8.00 ± 0.00 |
| 21 | 7.70 ± 0.15 | 8.00 ± 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, W.; Li, H.; Kang, T.; Ye, J.; Yao, Z.; Liu, Y.; Yu, T.; Zhang, Y.; Ling, Y.; Cao, H.; et al. Effect of GABA-T on Reproductive Function in Female Rats. Animals 2020, 10, 567. https://doi.org/10.3390/ani10040567
Si W, Li H, Kang T, Ye J, Yao Z, Liu Y, Yu T, Zhang Y, Ling Y, Cao H, et al. Effect of GABA-T on Reproductive Function in Female Rats. Animals. 2020; 10(4):567. https://doi.org/10.3390/ani10040567
Chicago/Turabian StyleSi, Wenyu, Hailing Li, Tiezhu Kang, Jing Ye, Zhiqiu Yao, Ya Liu, Tong Yu, Yunhai Zhang, Yinghui Ling, Hongguo Cao, and et al. 2020. "Effect of GABA-T on Reproductive Function in Female Rats" Animals 10, no. 4: 567. https://doi.org/10.3390/ani10040567





