The Impact on Antioxidant Enzyme Activity and Related Gene Expression Following Adult Zebrafish (Danio rerio) Exposure to Dimethyl Phthalate
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Exposure Experiments and Sample Collection
2.3. Biochemical Assays
2.4. Molecular Studies
2.5. Statistical Analyses
3. Results
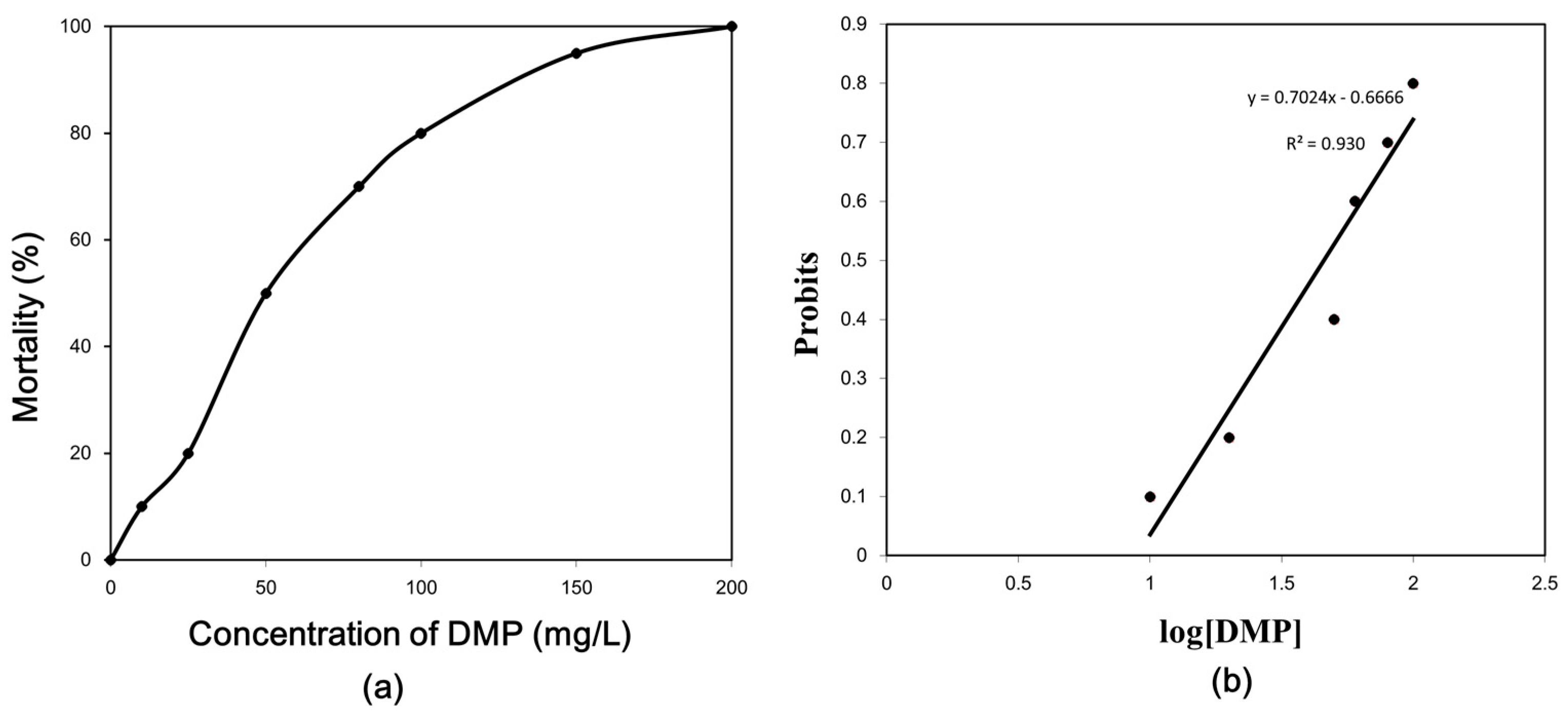
3.1. Effects of DMP on Zebrafish Survival
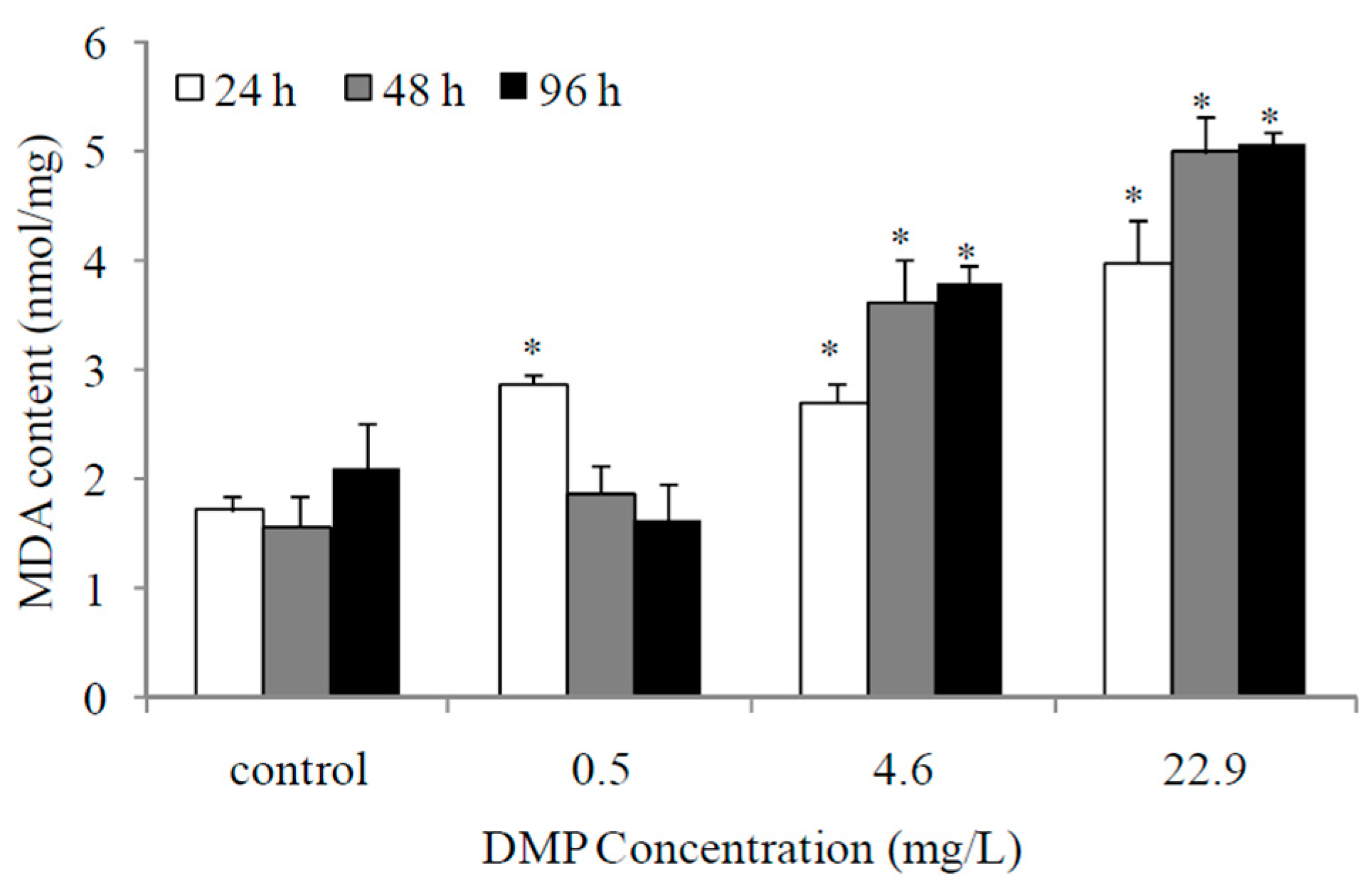
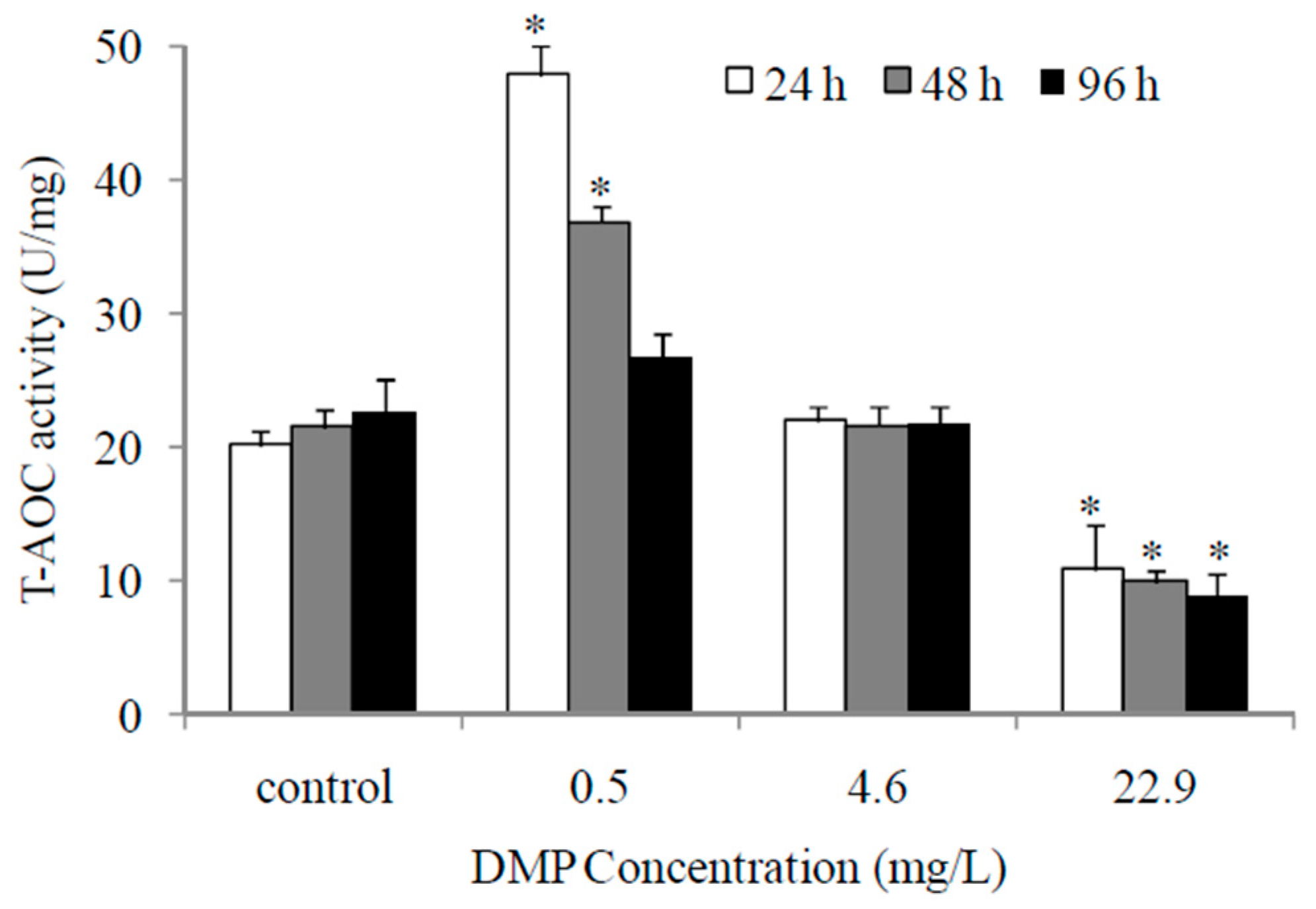
3.2. Effect of DMP on Oxidative Stress in Zebrafish
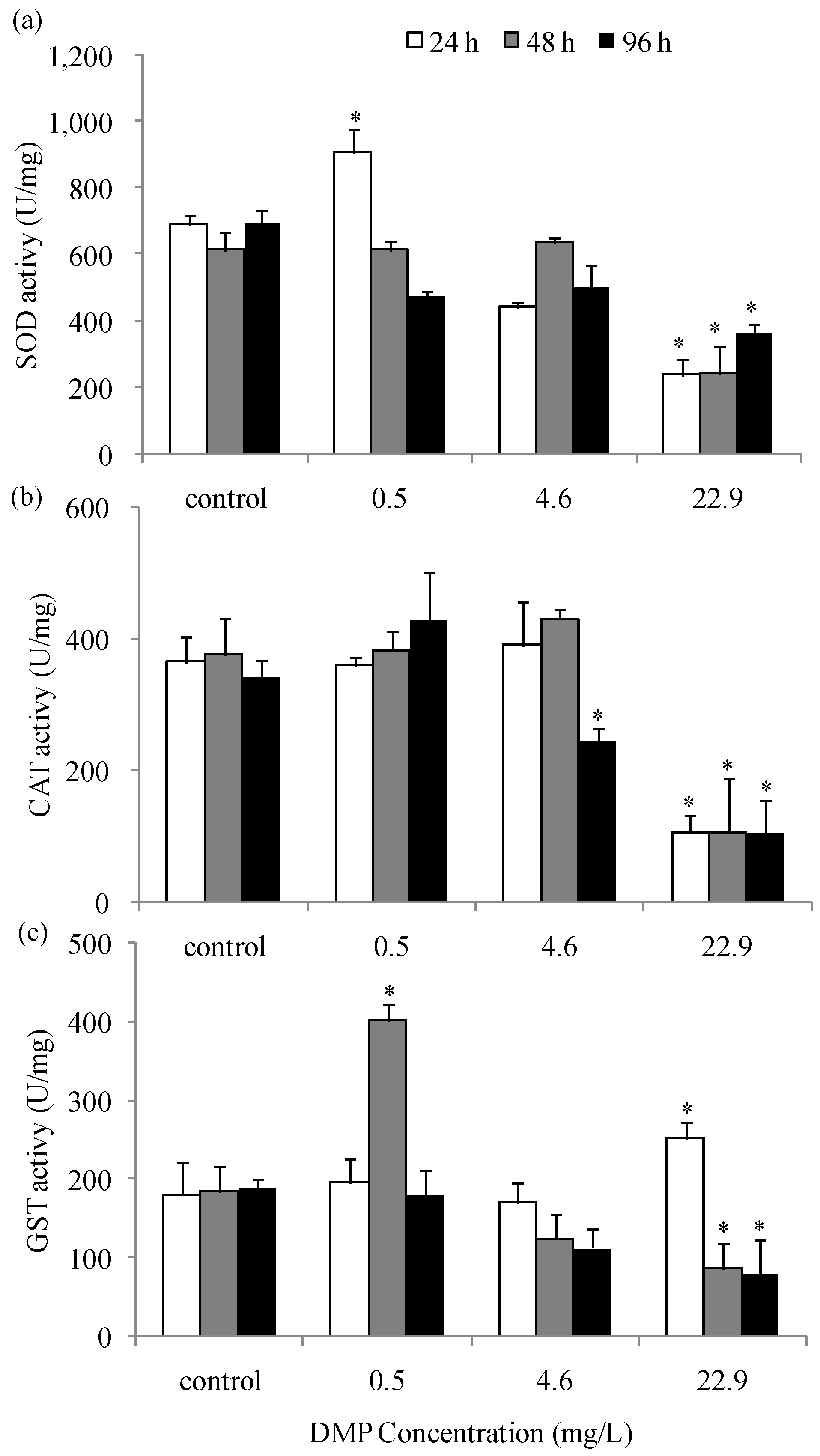
3.3. Effects of DMP on the Transcription of Related Antioxidant Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef] [PubMed]
- Wittassek, M.; Koch, H.M.; Angerer, J.; Bruning, T. Assessing exposure to phthalates—the human biomonitoring approach. Mol. Nutr. Food Res. 2011, 55, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Liu, H.; Wan, G.; Zhang, S. The occurrence and ecological risk assessment of phthalate esters (PAEs) in urban aquatic environments of China. Ecotoxicology 2015, 24, 967–984. [Google Scholar] [CrossRef] [PubMed]
- Osman, B.; Ozer, E.T.; Demirbel, E.; Güçer, S.; Beşirli, N. Synthesis and characterization of L-tryptophan containing microbeads for removal of dimethyl phthalate from aqueous phase. Sep. Purif. Technol. 2013, 109, 40–47. [Google Scholar] [CrossRef]
- Mersiowsky, L. Long-term fate of PVC products and their additives in landfills. Rog. Polym. Sci. 2002, 27, 2227–2277. [Google Scholar] [CrossRef]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D.; et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect. 2013, 121, 473–494. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Leung, A.O.W.; Chu, L.H.; Wong, M.H. Phthalates contamination in China: Status, trends and human exposure-with an emphasis on oral intake. Environ. Pollut. 2018, 238, 771–782. [Google Scholar] [CrossRef]
- European Union. Council Regulation (EC) No. 793/93 on the evaluation and control of the risks of existing substances. Off. J. Eur. Communities. 1993, 36, 1–7. [Google Scholar]
- Appendix A to 40 CFR, Part 423--126 Priority Pollutants. Available online: https://www3.epa.gov/region1/npdes/permits/generic/prioritypollutants.pdf (accessed on 16 December 2019).
- Stales, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The Environmental Fate of Phthalate Esters: A Literature Review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshinaga, J.; Mizumoto, Y.; Serizawa, S.; Shiraishi, H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int. J. Androl. 2012, 35, 236–244. [Google Scholar] [CrossRef]
- Braun, J.M.; Sathyanarayana, S.; Hauser, R. Phthalate exposure and children’s health. Curr. Opin. Pediatr. 2013, 25, 247–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Xu, S.; Tan, T.; Lee, S.T.; Cheng, S.H.; Lee, F.W.F.; Xu, S.J.L.; Ho, K.C. Toxicity and Estrogenic Endocrine Disrupting Activity of Phthalates and Their Mixtures. Int. J. Environ. Res. Public Health. 2014, 11, 3156–3168. [Google Scholar] [CrossRef] [PubMed]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Call, D.J.; Markee, T.P.; Geiger, D.L.; Brooke, L.T.; VandeVenter, F.A.; Cox, D.A.; Genisot, K.I.; Robillard, K.A.; Gorsuch, J.W.; Parkerton, T.F. An assessment of the toxicity of phthalate esters to freshwater benthos. 1. Aqueous exposures. Environ. Toxicol. Chem. 2001, 20, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Gutleb, A.C.; Bergman, A.; Eriksen, G.S.; Murk, A.J.; Ropstad, E.; Saunders, M.; Skaare, J.U. Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health B. Crit. Rev. 2009, 12, 225–249. [Google Scholar] [CrossRef]
- Kumar, P. Role of Plastics on Human Health. Indian J. Pediatr. 2018, 85, 384–389. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, Y.; Qi, M. Toxicity of phthalate esters exposure to carp (Cyprinus carpio) and antioxidant response by biomarker. Ecotoxicology. 2014, 23, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Qu, R.; Feng, M.; Sun, P.; Wang, Z. A comparative study on antioxidant status combined with integrated biomarker response in Carassius auratus fish exposed to nine phthalates. Environ. Toxicol. 2015, 30, 1125–1134. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Zhu, L.; Wang, J.; Li, H.; Zhang, Y.; Liu, W.; Gao, J. Oxidative Damage and Genetic Toxicity Induced by DBP in Earthworms (Eisenia fetida). Arch. Environ. Contam. Toxicol. 2018, 74, 527–538. [Google Scholar] [CrossRef]
- Kondolot, M.; Ozmert, E.N.; Ascl, A.; Erkekoglu, P.n.; Oztop, D.B.; Gumus, H.; Kocer-Gumusel, B.; Yurdakok, K. Plasma phthalate and bisphenol a levels and oxidant-antioxidant status in autistic children. Environ. Toxicol. Pharmacol. 2016, 43, 149–158. [Google Scholar] [CrossRef]
- Kang, J.C.; Jee, J.-H.; Koo, J.-G.; Keum, Y.-H.; Jo, S.-G.; Park, K.H. Anti-oxidative status and hepatic enzymes following acute administration of diethyl phthalate in olive flounder Paralichthys olivaceus, a marine culture fish. Ecotoxicol. Environ. Saf. 2010, 73, 1149–1455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, X.; He, Z.; Zhong, Q.; Guo, J.; Hu, X.J.; Xiong, L.; Liu, D. Influence of BBP exposure on nervous system and antioxidant system in zebrafish. Ecotoxicology 2014, 23, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.K.; Chiang, L.F.; Tan, S.W.; Chen, P.J. Environmentally relevant concentrations of di(2-ethylhexyl) phthalate exposure alter larval growth and locomotion in medaka fish via multiple pathways. Sci. Total. Environ. 2018, 640–641, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Feng, M.; Dai, Y. Comparative antioxidant responses in liver of Carassius auratus exposed to phthalates: An integrated biomarker approach. Environ. Toxicol. Pharmacol. 2013, 36, 741–749. [Google Scholar] [CrossRef]
- Pecoraro, R.; Marino, F.; Salvaggio, A.; Capparucci, F.; Di Caro, G.; Iaria, C.; Salvo, A.; Rotondo, A.; Tibullo, D.; Guerriero, G.; et al. Evaluation of chronic nanosilver toxicity to adult zebrafish. Front. Physiol. 2017, 8, 1011. [Google Scholar] [CrossRef] [Green Version]
- Pecoraro, R.; D’Angelo, D.; Filice, S.; Scalese, S.; Capparucci, F.; Marino, F.; Iaria, C.; Guerriero, G.; Tibullo, D.; Scalisi, E.M.; et al. Toxicity evaluation of graphene oxide and titania loaded nafion membranes in zebrafish. Front. Physiol. 2018, 8, 1039. [Google Scholar] [CrossRef]
- Iaria, C.; Migliore, S.; Macri, D.; Bivona, M.; Capparucci, F.; Gaglio, G.; Marino, F. Evidence of Centrocestus formosanus (Nishigori, 1924) in Zebrafish (Danio rerio). Zebrafish 2019, 16, 522–526. [Google Scholar] [CrossRef]
- Critchfield, F.E.; Bishop, F.E. Water Determination by Reaction with 2,2-Dimethoxypropane. Anal. Chem. 1961, 33, 1034–1035. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals. Methods Mol. Biol. 2004, 947, 37–56. [Google Scholar]
- Hallare, A.; Nagel, K.; Köhler, H.R.; Triebskorn, R. Comparative embryotoxicity and proteotoxicity of three carrier solvents to zebrafish (Danio rerio) embryos. Ecotoxicol. Environ. Saf. 2006, 63, 378–388. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: London, UK; New York, NY, USA, 1971; pp. 20–47. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Livingstone, D.R. Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue de Medecine Veterinaire 2003, 154, 427–430. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Adams, W.J.; Biddinger, G.R.; Robillard, K.A.; Gorsuch, J.W. A summary of the acute toxicity of 14 phthalate esters to representative aquatic organisms. Ecotoxicol. Environ. Saf. 1995, 14, 1569–1574. [Google Scholar] [CrossRef]
- Buccafusco, R.J.; Ells, S.J.; Leblanc, G.A. Acute Toxicity of Priority Pollutants to Bluegill (Lepomis macrochirus). Bull. Environ. Contam. Toxicol. 1981, 26, 446–452. [Google Scholar] [CrossRef]
- Rana, K.; Patel, J.; Prasad, B. Effect of an Endocrine-disrupting Chemical Dimethyl Phthalate on Poecilia sphenops. Nat. Environ. Pollut. Technol. 2018, 17, 71–76. [Google Scholar]
- Brannen, K.C.; Panzica-Kelly, J.M.; Danberry, T.L.; Augustine-Rauch, K.A. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defects Res. B. Dev. Reprod. Toxicol. 2010, 89, 66–77. [Google Scholar] [CrossRef]
- Burridge, L.E.; Haya, K. A Review of Di-n-butylphthalate in the Aquatic Environment: Concerns Regarding Its Use in Salmonid Aquaculture. J. World Aquacult. Soc. 2007, 26, 1–13. [Google Scholar] [CrossRef]
- Staples, C.A.; Adams, W.J.; Parkerton, T.F.; Gorsuch, J.W.; Biddinger, G.R.; Reinert, K.H. Aquatic toxicity of eighteen phthalate esters. Environ. Toxicol. Chem. 1997, 16, 875–891. [Google Scholar] [CrossRef]
- Martínez-Alvarez, R.M.; Hidalgo, M.C.; Domezain, A.; Morales, A.E.; García-Gallego, M.; Sanz, A. Physiological changes of sturgeon Acipenser nacarii caused by increasing environmental salinity. J. Exp. Biol. 2003, 205 (Pt 23), 3699–3706. [Google Scholar]
- Sies, H. Biochemistry of oxidative stress. Angew. Chem. Int. Edit. 1986, 23, 1798. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide Radical and Superoxide Dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Arun, S.; Subramanian, P. Antioxidant enzymes activity in subcellular fraction of freshwater prawns M. malcolmsonii and M. lamarrei lamarrei. Appl. Biochem. Biotechnol. 1998, 75, 187–192. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.S.; Lee, Y.M. Changes in activity and transcription of antioxidant enzymes and heat shock protein 90 in the water flea, Daphnia magna exposed to mercury. Toxicol. Environ. Health. Sci. 2017, 9, 300–308. [Google Scholar] [CrossRef]
- Yokota, H.; Satoh, Y.; Ono, Y.; Kaneko, M.; Ikeda, H.; Tsuji, S.; Yatomi, Y. Establishment of a pharmacogenomic testing system for the realization of individual pharmacotherapy. Rinsho Byori 2008, 56, 772–780. [Google Scholar]
- Zhang, C.; Hu, J.; Wang, P. Effects of B(a)P on T-AOC in liver of Carassius auratu. J. Environ. Health. 2004, 5, 324–326. (In Chinese) [Google Scholar]
- Bernanke, J.; Köhler, H.R. The Impact of Environmental Chemicals on Wildlife Vertebrates. Rev. Environ. Contam. Toxicol. 2009, 198, 1–47. [Google Scholar]
- Mather-Mihaich, E.; Di Giulio, R.T. Oxidant, mixed-function oxidase and peroxisomal responses in channel catfish exposed to a bleached kraft mill effluent. Arch. Environ. Contam. Toxicol. 1991, 20, 391–397. [Google Scholar] [CrossRef]
- Livingstone, D.R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef]
- Benedetti, M.; Giuliani, M.E.; Regoli, F. Oxidative metabolism of chemical pollutants in marine organisms: Molecular and biochemical biomarkers in environmental toxicology. Ann. N. Y. Acad. Sci. 2015, 1340, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Pedrajas, J.R.; Peinado, J.; Lopez-Barea, J. Purification of Cu, Zn-superoxide dismutase isoenzymes from fish liver: Appearance of new isoforms as a consequence of pollution. Free Radic. Res. Commun. 1993, 19, 29–41. [Google Scholar] [CrossRef]
- Pedrajas, J.R.; Peinado, J.; Lopez-Barea, J. Oxidative stress in fish exposed to model xenobiotics. Oxidatively modified forms of Cu, Zn-superoxide dismutase as potential biomarkers. Chem. Biol. Interact. 1995, 98, 267–282. [Google Scholar] [CrossRef]
- Dorval, J.; Hontela, A. Role of glutathione redox cycle and catalase in defense against oxidative stress induced by endosulfan in adrenocortical cells of rainbow trout (Oncorhynchus mykiss). Toxicol. Appl. Pharmacol. 2003, 192, 191–200. [Google Scholar] [CrossRef]
- Dinu, D.; Marinescu, D.; Munteanu, M.C.; Staicu, A.C.; Costache, M.; Dinischiotu, A. Modulatory Effects of Deltamethrin on Antioxidant Defense Mechanisms and Lipid Peroxidation in Carassius auratus gibelio Liver and Intestine. Arch. Environ. Contam. Toxicol. 2010, 58, 757–764. [Google Scholar] [CrossRef]

| Primer | Primer Sequence (5′-3′) | Annealing Temperature (Tm) | Product Length (bp) | Amplification Efficiency |
|---|---|---|---|---|
| superoxide dismutase (SOD) primer-F | TCCGCACTTCAACCCTCA | 58.44 | 215 | 97.0% |
| SOD primer-R | CCTCATTGCCACCCTTCC | 57.66 | ||
| catalase (CAT) primer-F | TACCAGTCAACTGCCCGTAC | 59.40 | 145 | 96.5% |
| CAT primer-R | GACTCAAGGAAGCGTGGC | 58.43 | ||
| glutathione S-transferase (GST) primer-F | CCAACCACCTCAAATGCT | 55.09 | 150 | 98.1% |
| GST primer-R | ACGGGAAAGAGTCCAGACAG | 59.03 | ||
| Beta-actin primer-F | CGAGCAGGAGATGGGAACC | 59.86 | 214 | 99.5% |
| Beta-actin primer-R | CAACGGAAACGCTCATTGC | 58.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, B.; Liu, C.; Wang, L.; Chai, Y. The Impact on Antioxidant Enzyme Activity and Related Gene Expression Following Adult Zebrafish (Danio rerio) Exposure to Dimethyl Phthalate. Animals 2020, 10, 717. https://doi.org/10.3390/ani10040717
Cong B, Liu C, Wang L, Chai Y. The Impact on Antioxidant Enzyme Activity and Related Gene Expression Following Adult Zebrafish (Danio rerio) Exposure to Dimethyl Phthalate. Animals. 2020; 10(4):717. https://doi.org/10.3390/ani10040717
Chicago/Turabian StyleCong, Bailin, Cong Liu, Lujie Wang, and Yingmei Chai. 2020. "The Impact on Antioxidant Enzyme Activity and Related Gene Expression Following Adult Zebrafish (Danio rerio) Exposure to Dimethyl Phthalate" Animals 10, no. 4: 717. https://doi.org/10.3390/ani10040717




